Management of Coagulopathy in Bleeding Patients
Abstract
:1. Introduction
2. Management of Acquired Bleeding Disorders
3. Bleeding in Patients on Oral Anticoagulants
3.1. Treatment of VKA-Associated Bleeding
3.2. Treatment of DOAC-Associated Bleeding
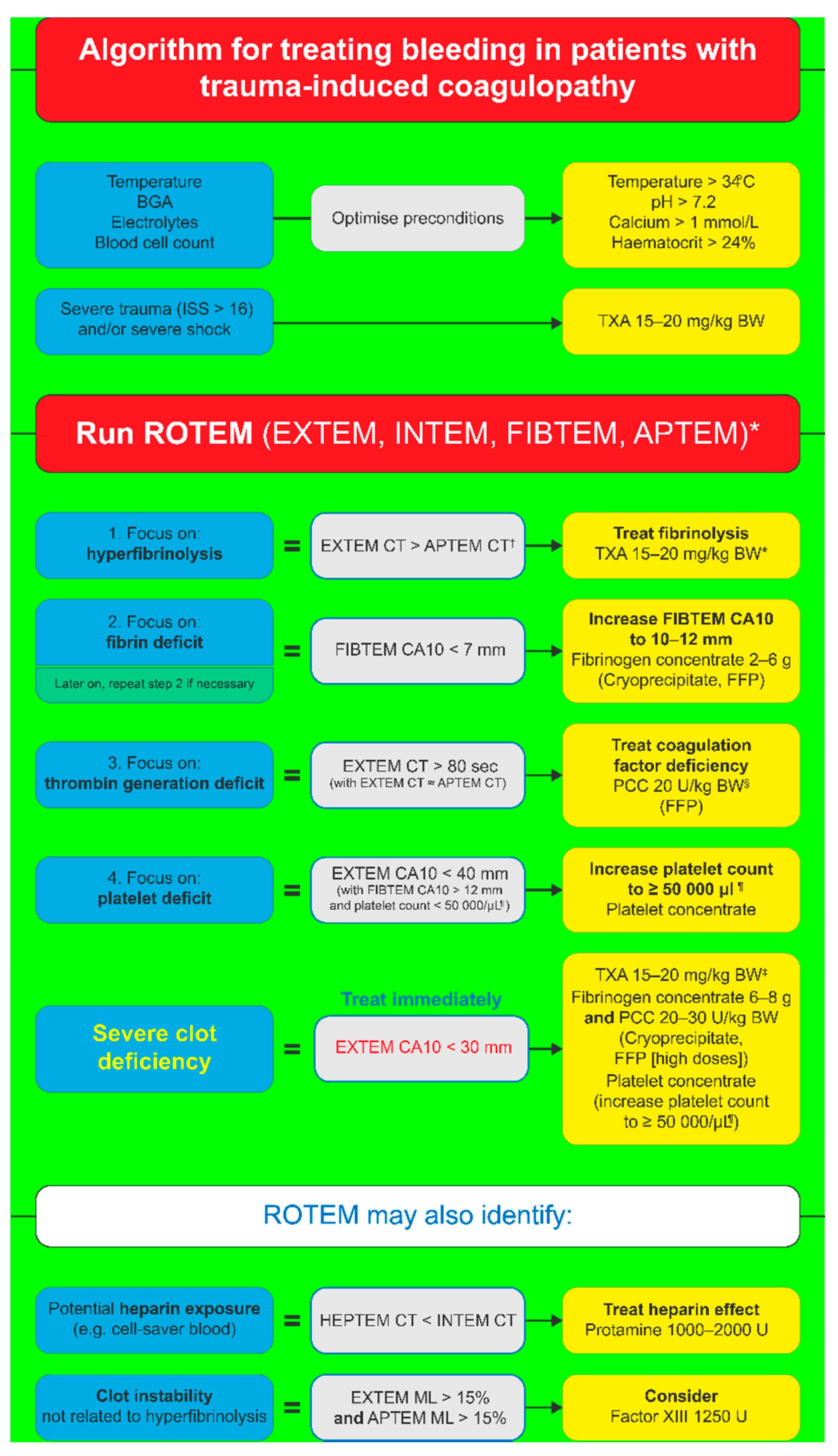
4. Management of Trauma-Induced Coagulopathy
5. Goal-Directed Coagulation Management for TIC Patients
5.1. Fibrinogen Supplementation
5.2. Thrombin Generation
5.3. Platelet Supplementation
5.4. Clot Stability and FXIII
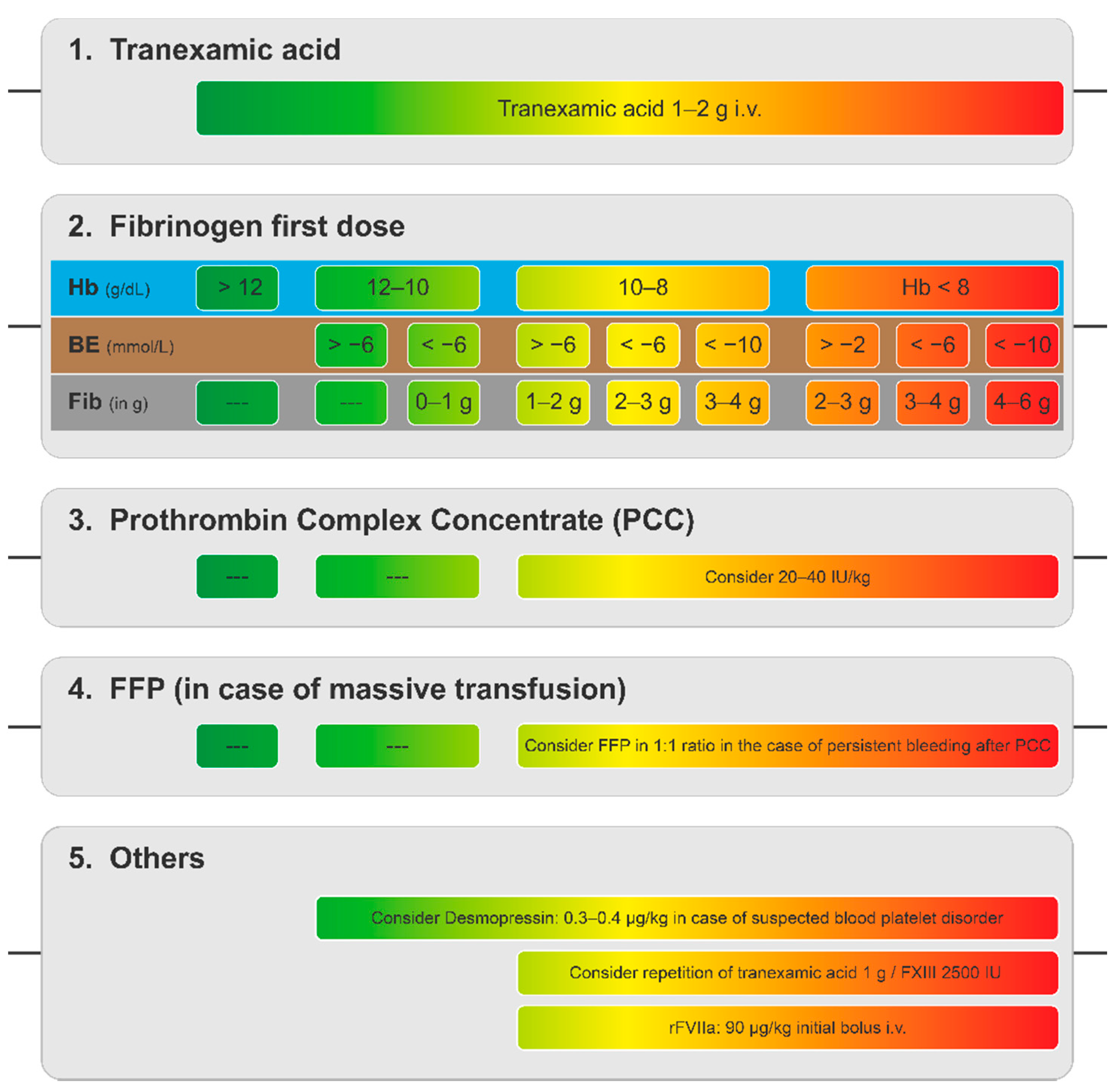
6. Goal-Directed Coagulation Management without POC Testing
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hurwitz, A.; Massone, R.; Lopez, B.L. Acquired bleeding disorders. Emerg. Med. Clin. N. Am. 2014, 32, 691–713. [Google Scholar] [CrossRef]
- Grottke, O.; Fries, D.; Nascimento, B. Perioperatively acquired disorders of coagulation. Curr. Opin. Anaesthesiol. 2015, 28, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Bolliger, D.; Görlinger, K.; Tanaka, K.A. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology 2010, 113, 1205–1219. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Bolliger, D. Acquired coagulopathy. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Hardy, J.F.; De Moerloose, P.; Samama, M. Massive transfusion and coagulopathy: Pathophysiology and implications for clinical management. Can. J. Anaesth. 2004, 51, 293–310. [Google Scholar] [CrossRef] [Green Version]
- Schochl, H.; Maegele, M.; Solomon, C.; Gorlinger, K.; Voelckel, W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 15. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, S.T.; Myllylä, G.J.; Vahtera, E.M. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth. Analg. 1995, 81, 360–365. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Pieters, M.; de Lange-Loots, Z.; Weisel, J.W. Fibrinogen and fibrin. Subcell. Biochem. 2021, 96, 471–501. [Google Scholar] [CrossRef]
- Lowe, G.D.; Rumley, A.; Woodward, M.; Morrison, C.E.; Philippou, H.; Lane, D.A.; Tunstall-Pedoe, H. Epidemiology of coagulation factors, inhibitors and activation markers: The Third Glasgow MONICA Survey. I. Illustrative reference ranges by age, sex and hormone use. Br. J. Haematol. 1997, 97, 775–784. [Google Scholar] [CrossRef]
- Fenger-Eriksen, C.; Ingerslev, J.; Sorensen, B. Fibrinogen concentrate--a potential universal hemostatic agent. Expert Opin. Biol. Ther. 2009, 9, 1325–1333. [Google Scholar] [CrossRef]
- Kreuz, W.; Meili, E.; Peter-Salonen, K.; Haertel, S.; Devay, J.; Krzensk, U.; Egbring, R. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfus. Apher. Sci. 2005, 32, 247–253. [Google Scholar] [CrossRef]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology: First update 2016. Eur. J. Anaesthesiol. 2017, 34, 332–395. [Google Scholar] [CrossRef] [Green Version]
- McQuilten, Z.K.; Bailey, M.; Cameron, P.A.; Stanworth, S.J.; Venardos, K.; Wood, E.M.; Cooper, D.J. Fibrinogen concentration and use of fibrinogen supplementation with cryoprecipitate in patients with critical bleeding receiving massive transfusion: A bi-national cohort study. Br. J. Haematol. 2017, 179, 131–141. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Cerneca, F.; Ricci, G.; Simeone, R.; Malisano, M.; Alberico, S.; Guaschino, S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 73, 31–36. [Google Scholar] [CrossRef]
- Bremme, K.A. Haemostatic changes in pregnancy. Best Pract. Res. Clin. Haematol. 2003, 16, 153–168. [Google Scholar] [CrossRef]
- Collins, P.W.; Lilley, G.; Bruynseels, D.; Laurent, D.B.; Cannings-John, R.; Precious, E.; Hamlyn, V.; Sanders, J.; Alikhan, R.; Rayment, R.; et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: A prospective study. Blood 2014, 124, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Charbit, B.; Mandelbrot, L.; Samain, E.; Baron, G.; Haddaoui, B.; Keita, H.; Sibony, O.; Mahieu-Caputo, D.; Hurtaud-Roux, M.F.; Huisse, M.G.; et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J. Thromb. Haemost. 2007, 5, 266–273. [Google Scholar] [CrossRef]
- Patil, V.; Shetmahajan, M. Massive transfusion and massive transfusion protocol. Indian J. Anaesth. 2014, 58, 590–595. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Baulig, W.; Seifert, B.; Emmert, M.Y.; Spahn, D.R.; Asmis, L.M. Relative concentrations of haemostatic factors and cytokines in solvent/detergent-treated and fresh-frozen plasma. Br. J. Anaesth. 2011, 106, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.A.; Esper, S.; Bolliger, D. Perioperative factor concentrate therapy. Br. J. Anaesth. 2013, 111 (Suppl. 1), i35–i49. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.W.; Solomon, C.; Sutor, K.; Crispin, D.; Hochleitner, G.; Rizoli, S.; Schochl, H.; Schreiber, M.; Ranucci, M. Theoretical modelling of fibrinogen supplementation with therapeutic plasma, cryoprecipitate, or fibrinogen concentrate. Br. J. Anaesth. 2014, 113, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Kozek-Langenecker, S.; Sørensen, B.; Hess, J.R.; Spahn, D.R. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: A systematic review. Crit. Care 2011, 15, R239. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Brohi, K.; Chana, M.; Raza, I.; Stanworth, S.; Gaarder, C.; Davenport, R. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J. Trauma Acute Care Surg. 2014, 76, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Innerhofer, P.; Fries, D.; Mittermayr, M.; Innerhofer, N.; von Langen, D.; Hell, T.; Gruber, G.; Schmid, S.; Friesenecker, B.; Lorenz, I.H.; et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): A single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017, 4, e258–e271. [Google Scholar] [CrossRef]
- Morrison, G.A.; Koch, J.; Royds, M.; McGee, D.; Chalmers, R.T.A.; Anderson, J.; Nimmo, A.F. Fibrinogen concentrate vs. fresh frozen plasma for the management of coagulopathy during thoraco-abdominal aortic aneurysm surgery: A pilot randomised controlled trial. Anaesthesia 2019, 74, 180–189. [Google Scholar] [CrossRef]
- Weitz, J.I.; Eikelboom, J.W.; Samama, M.M. New antithrombotic drugs: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e120S–e151S. [Google Scholar] [CrossRef] [Green Version]
- Loo, S.Y.; Dell’Aniello, S.; Huiart, L.; Renoux, C. Trends in the prescription of novel oral anticoagulants in UK primary care. Br. J. Clin. Pharmacol. 2017, 83, 2096–2106. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.H.; van Hove, M.; Leng, G. Trends in anticoagulant prescribing: A review of local policies in English primary care. BMC Health Serv. Res. 2020, 20, 279. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, D.J.; Eikelboom, J.W.; Weitz, J.I. Four-factor prothrombin complex concentrate for urgent reversal of vitamin K antagonists in patients with major bleeding. Circulation 2013, 128, 1179–1181. [Google Scholar] [CrossRef] [Green Version]
- Watson, H.G.; Baglin, T.; Laidlaw, S.L.; Makris, M.; Preston, F.E. A comparison of the efficacy and rate of response to oral and intravenous Vitamin K in reversal of over-anticoagulation with warfarin. Br. J. Haematol. 2001, 115, 145–149. [Google Scholar] [CrossRef]
- Narick, C.; Triulzi, D.J.; Yazer, M.H. Transfusion-associated circulatory overload after plasma transfusion. Transfusion 2012, 52, 160–165. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. Prothrombin complex concentrates: An update. Blood Transfus. 2010, 8, 149–154. [Google Scholar] [CrossRef]
- Brekelmans, M.P.A.; Ginkel, K.V.; Daams, J.G.; Hutten, B.A.; Middeldorp, S.; Coppens, M. Benefits and harms of 4-factor prothrombin complex concentrate for reversal of vitamin K antagonist associated bleeding: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2017, 44, 118–129. [Google Scholar] [CrossRef]
- Chai-Adisaksopha, C.; Hillis, C.; Siegal, D.M.; Movilla, R.; Heddle, N.; Iorio, A.; Crowther, M. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal. A systematic review and meta-analysis. Thromb. Haemost. 2016, 116, 879–890. [Google Scholar] [CrossRef]
- Hill, R.; Han, T.S.; Lubomirova, I.; Math, N.; Bentley, P.; Sharma, P. Prothrombin complex concentrates are superior to fresh frozen plasma for emergency reversal of vitamin K antagonists: A meta-analysis in 2606 subjects. Drugs 2019, 79, 1557–1565. [Google Scholar] [CrossRef]
- Zapata-Wainberg, G.; Ximénez-Carrillo Rico, Á.; Benavente Fernández, L.; Masjuan Vallejo, J.; Gállego Culleré, J.; Freijó Guerrero, M.D.M.; Egido, J.; Gómez Sánchez, J.C.; Martínez Domeño, A.; Purroy García, F.; et al. Epidemiology of intracranial haemorrhages associated with vitamin K antagonist oral anticoagulants in Spain: TAC Registry. Interv. Neurol. 2015, 4, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Flibotte, J.J.; Hagan, N.; O’Donnell, J.; Greenberg, S.M.; Rosand, J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004, 63, 1059–1064. [Google Scholar] [CrossRef]
- Parry-Jones, A.R.; Di Napoli, M.; Goldstein, J.N.; Schreuder, F.H.; Tetri, S.; Tatlisumak, T.; Yan, B.; van Nieuwenhuizen, K.M.; Dequatre-Ponchelle, N.; Lee-Archer, M.; et al. Reversal strategies for vitamin K antagonists in acute intracerebral hemorrhage. Ann. Neurol. 2015, 78, 54–62. [Google Scholar] [CrossRef]
- Steiner, T.; Poli, S.; Griebe, M.; Hüsing, J.; Hajda, J.; Freiberger, A.; Bendszus, M.; Bösel, J.; Christensen, H.; Dohmen, C.; et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): A randomised trial. Lancet Neurol. 2016, 15, 566–573. [Google Scholar] [CrossRef]
- Christensen, H.; Cordonnier, C.; Korv, J.; Lal, A.; Ovesen, C.; Purrucker, J.C.; Toni, D.; Steiner, T. European Stroke Organisation guideline on reversal of oral anticoagulants in acute intracerebral haemorrhage. Eur. Stroke J. 2019, 4, 294–306. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Stanley, A.J.; Morris, A.J.; Camus, M.; Lau, J.; Lanas, A.; Laursen, S.B.; Radaelli, F.; Papanikolaou, I.S.; Curdia Goncalves, T.; et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 300–332. [Google Scholar] [CrossRef]
- Maegele, M. The European perspective on the management of acute major hemorrhage and coagulopathy after trauma: Summary of the 2019 updated European guideline. J. Clin. Med. 2021, 10, 362. [Google Scholar] [CrossRef]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H. Evidence-based management of anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141, e152S–e184S. [Google Scholar] [CrossRef] [Green Version]
- Pernod, G.; Godier, A.; Gozalo, C.; Tremey, B.; Sie, P.; French National Authority for Haematology. French clinical practice guidelines on the management of patients on vitamin K antagonists in at-risk situations (overdose, risk of bleeding, and active bleeding). Thromb. Res. 2010, 126, e167–e174. [Google Scholar] [CrossRef]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Gluckman, T.J.; Hucker, W.J.; Mehran, R.; et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 594–622. [Google Scholar] [CrossRef]
- Frontera, J.A.; Lewin, J.J., 3rd; Rabinstein, A.A.; Aisiku, I.P.; Alexandrov, A.W.; Cook, A.M.; del Zoppo, G.J.; Kumar, M.A.; Peerschke, E.I.; Stiefel, M.F.; et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: A statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit. Care 2016, 24, 6–46. [Google Scholar] [CrossRef]
- Acosta, R.D.; Abraham, N.S.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fisher, D.A.; Fonkalsrud, L.; et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest. Endosc. 2016, 83, 3–16. [Google Scholar] [CrossRef]
- ASA. Practice guidelines for perioperative blood management: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015, 122, 241–275. [Google Scholar] [CrossRef]
- Witt, D.M.; Nieuwlaat, R.; Clark, N.P.; Ansell, J.; Holbrook, A.; Skov, J.; Shehab, N.; Mock, J.; Myers, T.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018, 2, 3257–3291. [Google Scholar] [CrossRef]
- Baugh, C.W.; Levine, M.; Cornutt, D.; Wilson, J.W.; Kwun, R.; Mahan, C.E.; Pollack, C.V., Jr.; Marcolini, E.G.; Milling, T.J., Jr.; Peacock, W.F.; et al. Anticoagulant reversal strategies in the emergency department setting: Recommendations of a multidisciplinary expert panel. Ann. Emerg. Med. 2020, 76, 470–485. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Patrice Lindsay, M.; Castellucci, L.A.; Cayley, A.; Crowther, M.; de Wit, K.; English, S.W.; Hoosein, S.; Huynh, T.; Kelly, M.; et al. Canadian stroke best practice recommendations: Management of spontaneous intracerebral hemorrhage, 7th Edition Update 2020. Int. J. Stroke 2021, 16, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Keeling, D.; Baglin, T.; Tait, C.; Watson, H.; Perry, D.; Baglin, C.; Kitchen, S.; Makris, M.; British Committee for Standards in Haematology. Guidelines on oral anticoagulation with warfarin—Fourth edition. Br. J. Haematol. 2011, 154, 311–324. [Google Scholar] [CrossRef]
- Erdoes, G.; Koster, A.; Ortmann, E.; Meesters, M.I.; Bolliger, D.; Baryshnikova, E.; Martinez Lopez De Arroyabe, B.; Ahmed, A.; Lance, M.D.; Ranucci, M.; et al. A European consensus statement on the use of four-factor prothrombin complex concentrate for cardiac and non-cardiac surgical patients. Anaesthesia 2021, 76, 381–392. [Google Scholar] [CrossRef]
- Marano, G.; Vaglio, S.; Pupella, S.; Liumbruno, G.M.; Franchini, M. How we treat bleeding associated with direct oral anticoagulants. Blood Transfus. 2016, 14, 465–473. [Google Scholar] [CrossRef]
- Kustos, S.A.; Fasinu, P.S. Direct-acting oral anticoagulants and their reversal agents-An update. Medicines 2019, 6, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grottke, O.; Schulman, S. Four-factor prothrombin complex concentrate for the management of patients receiving direct oral activated factor X inhibitors. Anesthesiology 2019, 131, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Burnett, A.; Triller, D.; Crowther, M.; Ansell, J.; Van Cott, E.M.; Wirth, D.; Kaatz, S. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am. J. Hematol. 2019, 94, 697–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, M.; Goldstein, J.N.; Levy, J.H. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: A review. Int. J. Emerg. Med. 2018, 11, 55. [Google Scholar] [CrossRef]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Hucker, W.; Mehran, R.; Messé, S.R.; et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: A report of the American College of Cardiology Task Force on expert consensus decision pathways. J. Am. Coll. Cardiol. 2017, 70, 3042–3067. [Google Scholar] [CrossRef]
- Schulman, S.; Gross, P.L.; Ritchie, B.; Nahirniak, S.; Lin, Y.; Lieberman, L.; Carrier, M.; Crowther, M.A.; Ghosh, I.; Lazo-Langner, A.; et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: A prospective cohort study. Thromb. Haemost. 2018, 118, 842–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, A.; Ågren, A.; Holmström, M.; Bruzelius, M.; Chaireti, R.; Odeberg, J.; Hempel, E.L.; Magnusson, M.; Frisk, T.; Schulman, S. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: A cohort study. Blood 2017, 130, 1706–1712. [Google Scholar] [CrossRef]
- Lipari, L.; Yang, S.; Milligan, B.; Blunck, J. Emergent reversal of oral factor Xa inhibitors with four-factor prothrombin complex concentrate. Am. J. Emerg. Med. 2020, 38, 2641–2645. [Google Scholar] [CrossRef]
- Connolly, S.J.; Crowther, M.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Lawrence, J.H.; Yue, P.; Bronson, M.D.; Lu, G.; Conley, P.B.; et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N. Engl. J. Med. 2019, 380, 1326–1335. [Google Scholar] [CrossRef]
- Allison, T.A.; Lin, P.J.; Gass, J.A.; Chong, K.; Prater, S.J.; Escobar, M.A.; Hartman, H.D. Evaluation of the use of low-dose 4-factor prothrombin complex concentrate in the reversal of direct oral anticoagulants in bleeding patients. J. Intensive Care Med. 2020, 35, 903–908. [Google Scholar] [CrossRef]
- Berger, K.; Santibañez, M.; Lin, L.; Lesch, C.A. A low-dose 4F-PCC protocol for DOAC-associated intracranial hemorrhage. J. Intensive Care Med. 2020, 35, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.A.; Ammar, M.A.; Owusu, K.A.; Brown, S.C.; Kaddouh, F.; Elsamadicy, A.A.; Acosta, J.N.; Falcone, G.J. Andexanet alfa versus 4-factor prothrombin complex concentrate for reversal of factor Xa inhibitors in intracranial hemorrhage. Neurocrit. Care 2021. [Google Scholar] [CrossRef] [PubMed]
- Barra, M.E.; Das, A.S.; Hayes, B.D.; Rosenthal, E.S.; Rosovsky, R.P.; Fuh, L.; Patel, A.B.; Goldstein, J.N.; Roberts, R.J. Evaluation of andexanet alfa and four-factor prothrombin complex concentrate (4F-PCC) for reversal of rivaroxaban- and apixaban-associated intracranial hemorrhages. J. Thromb. Haemost. 2020, 18, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Bhatt, P.; Lalchan, R.; Yaghi, S.; Ahuja, T.; Papadopoulos, J.; Joset, D. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J. Thromb. Thrombolysis 2020, 49, 121–131. [Google Scholar] [CrossRef]
- Costa, O.S.; Baker, W.L.; Roman-Morillo, Y.; McNeil-Posey, K.; Lovelace, B.; White, C.M.; Coleman, C.I. Quality evaluation of case series describing four-factor prothrombin complex concentrate in oral factor Xa inhibitor-associated bleeding: A systematic review. BMJ Open 2020, 10, e040499. [Google Scholar] [CrossRef]
- Luo, C.; Chen, F.; Chen, Y.H.; Zhao, C.F.; Feng, C.Z.; Liu, H.X.; Luo, D.Z.Q. Prothrombin complex concentrates and andexanet for management of direct factor Xa inhibitor related bleeding: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2637–2653. [Google Scholar] [CrossRef]
- Nederpelt, C.J.; Naar, L.; Krijnen, P.; le Cessie, S.; Kaafarani, H.M.A.; Huisman, M.V.; Velmahos, G.C.; Schipper, I.B. Andexanet alfa or prothrombin complex concentrate for factor Xa inhibitor reversal in acute major bleeding: A systematic review and meta-analysis. Crit. Care Med. 2021. [Google Scholar] [CrossRef]
- Jaspers, T.; Shudofsky, K.; Huisman, M.V.; Meijer, K.; Khorsand, N. A meta-analysis of andexanet alfa and prothrombin complex concentrate in the treatment of factor Xa inhibitor-related major bleeding. Res. Pract. Thromb. Haemost. 2021, 5, e12518. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute traumatic coagulopathy. J. Trauma Acute Care Surg. 2003, 54, 1127–1130. [Google Scholar] [CrossRef] [Green Version]
- Floccard, B.; Rugeri, L.; Faure, A.; Saint Denis, M.; Boyle, E.M.; Peguet, O.; Levrat, A.; Guillaume, C.; Marcotte, G.; Vulliez, A.; et al. Early coagulopathy in trauma patients: An on-scene and hospital admission study. Injury 2012, 43, 26–32. [Google Scholar] [CrossRef]
- Khan, S.; Davenport, R.; Raza, I.; Glasgow, S.; De’Ath, H.D.; Johansson, P.I.; Curry, N.; Stanworth, S.; Gaarder, C.; Brohi, K. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med. 2015, 41, 239–247. [Google Scholar] [CrossRef]
- Shaz, B.H.; Winkler, A.M.; James, A.B.; Hillyer, C.D.; MacLeod, J.B. Pathophysiology of early trauma-induced coagulopathy: Emerging evidence for hemodilution and coagulation factor depletion. J. Trauma 2011, 70, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- Haas, T.; Fries, D.; Tanaka, K.A.; Asmis, L.; Curry, N.S.; Schöchl, H. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: Is there any evidence? Br. J. Anaesth. 2015, 114, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Schochl, H.; Voelckel, W.; Schlimp, C.J. Management of traumatic haemorrhage—The European perspective. Anaesthesia 2015, 70 (Suppl. 1), 102–107. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: A pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann. Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Wool, G.D. Benefits and pitfalls of point-of-care coagulation testing for anticoagulation management: An ACLPS critical review. Am. J. Clin. Pathol. 2018, 151, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, L.M., Jr.; Demiral, H.; Schaap, N.P.; Kamphuisen, P.W.; Pompe, J.C.; Frolke, J.P. ‘Blind’ transfusion of blood products in exsanguinating trauma patients. Resuscitation 2007, 73, 382–388. [Google Scholar] [CrossRef]
- Ponschab, M.; Schochl, H.; Gabriel, C.; Sussner, S.; Cadamuro, J.; Haschke-Becher, E.; Gratz, J.; Zipperle, J.; Redl, H.; Schlimp, C.J. Haemostatic profile of reconstituted blood in a proposed 1:1:1 ratio of packed red blood cells, platelet concentrate and four different plasma preparations. Anaesthesia 2015, 70, 528–536. [Google Scholar] [CrossRef]
- Desborough, M.; Sandu, R.; Brunskill, S.J.; Doree, C.; Trivella, M.; Montedori, A.; Abraha, I.; Stanworth, S. Fresh frozen plasma for cardiovascular surgery. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Schöchl, H.; Nienaber, U.; Hofer, G.; Voelckel, W.; Jambor, C.; Scharbert, G.; Kozek-Langenecker, S.; Solomon, C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit. Care 2010, 14, R55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Stanworth, S.; Hopewell, S.; Doree, C.; Murphy, M. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 2012, 52, 1673–1686. [Google Scholar] [CrossRef]
- Stein, P.; Kaserer, A.; Sprengel, K.; Wanner, G.A.; Seifert, B.; Theusinger, O.M.; Spahn, D.R. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia 2017, 72, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardi, G.; Agostini, V.; Rondinelli, B.; Russo, E.; Bastianini, B.; Bini, G.; Bulgarelli, S.; Cingolani, E.; Donato, A.; Gambale, G.; et al. Trauma-induced coagulopathy: Impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit. Care 2015, 19, 83. [Google Scholar] [CrossRef] [Green Version]
- Hagemo, J.S.; Stanworth, S.; Juffermans, N.P.; Brohi, K.; Cohen, M.; Johansson, P.I.; Røislien, J.; Eken, T.; Næss, P.A.; Gaarder, C. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: A multicentre observational study. Crit. Care 2014, 18, R52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlimp, C.J.; Ponschab, M.; Voelckel, W.; Treichl, B.; Maegele, M.; Schochl, H. Fibrinogen levels in trauma patients during the first seven days after fibrinogen concentrate therapy: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 29. [Google Scholar] [CrossRef] [Green Version]
- Schöchl, H.; Cotton, B.; Inaba, K.; Nienaber, U.; Fischer, H.; Voelckel, W.; Solomon, C. FIBTEM provides early prediction of massive transfusion in trauma. Crit. Care 2011, 15, R265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.A.S.; Ostrowski, S.R.; Sørensen, A.M.; Meyer, A.S.P.; Holcomb, J.B.; Wade, C.E.; Johansson, P.I.; Stensballe, J. Fibrinogen in trauma, an evaluation of thrombelastography and rotational thromboelastometry fibrinogen assays. J. Surg. Res. 2015, 194, 581–590. [Google Scholar] [CrossRef]
- Erdoes, G.; Gerster, G.; Colucci, G.; Kaiser, H.; Alberio, L.; Eberle, B. Prediction of post-weaning fibrinogen status during cardiopulmonary bypass: An observational study in 110 patients. PLoS ONE 2015, 10, e0126692. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, B.; Callum, J.; Tien, H.; Peng, H.; Rizoli, S.; Karanicolas, P.; Alam, A.; Xiong, W.; Selby, R.; Garzon, A.M.; et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): A randomized feasibility trial. Br. J. Anaesth. 2016, 117, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Itagaki, Y.; Hayakawa, M.; Maekawa, K.; Saito, T.; Kodate, A.; Honma, Y.; Mizugaki, A.; Yoshida, T.; Ohyasu, T.; Katabami, K.; et al. Early administration of fibrinogen concentrate is associated with improved survival among severe trauma patients: A single-centre propensity score-matched analysis. World J. Emerg. Surg. 2020, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Gratz, J.; Schlimp, C.J.; Honickel, M.; Hochhausen, N.; Schöchl, H.; Grottke, O. Sufficient thrombin generation despite 95% hemodilution: An iIn vitro experimental study. J. Clin. Med. 2020, 9, 3805. [Google Scholar] [CrossRef]
- Dunbar, N.M.; Chandler, W.L. TRANSFUSION PRACTICE: Thrombin generation in trauma patients. Transfusion 2009, 49, 2652–2660. [Google Scholar] [CrossRef]
- Ponschab, M.; Voelckel, W.; Pavelka, M.; Schlimp, C.J.; Schöchl, H. Effect of coagulation factor concentrate administration on ROTEM® parameters in major trauma. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schochl, H.; Voelckel, W.; Maegele, M.; Kirchmair, L.; Schlimp, C.J. Endogenous thrombin potential following hemostatic therapy with 4-factor prothrombin complex concentrate: A 7-day observational study of trauma patients. Crit. Care 2014, 18, R147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.R.; Lindell, A.L.; Stansbury, L.G.; Dutton, R.P.; Scalea, T.M. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion 2009, 49, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Adány, R.; Mikkola, H. Novel aspects of blood coagulation factor XIII. I. Structure, distribution, activation, and function. Crit. Rev. Clin. Lab. Sci. 1996, 33, 357–421. [Google Scholar] [CrossRef] [PubMed]
- Raspé, C.; Besch, M.; Charitos, E.I.; Flöther, L.; Bucher, M.; Rückert, F.; Treede, H. Rotational thromboelastometry for assessing bleeding complications and factor XIII deficiency in cardiac surgery patients. Clin. Appl. Thromb. Hemost. 2018, 24, 136s–144s. [Google Scholar] [CrossRef] [Green Version]
- Bedreli, S.; Sowa, J.P.; Malek, S.; Blomeyer, S.; Katsounas, A.; Gerken, G.; Saner, F.H.; Canbay, A. Rotational thromboelastometry can detect factor XIII deficiency and bleeding diathesis in patients with cirrhosis. Liver Int. 2017, 37, 562–568. [Google Scholar] [CrossRef]
- Gerlach, R.; Raabe, A.; Zimmermann, M.; Siegemund, A.; Seifert, V. Factor XIII deficiency and postoperative hemorrhage after neurosurgical procedures. Surg. Neurol. 2000, 54, 260–264; discussion 264–265. [Google Scholar] [CrossRef]
- Maegele, M. The Diagnosis and treatment of acute traumatic bleeding and coagulopathy. Dtsch. Arztebl. Int. 2019, 116, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Casu, S. Simplified treatment algorithm for the management of trauma-induced hemorrhage without viscoelastic testing. Trauma Surg. Acute Care Open 2021, 6, e000779. [Google Scholar] [CrossRef]
- Schlimp, C.J.; Voelckel, W.; Inaba, K.; Maegele, M.; Ponschab, M.; Schochl, H. Estimation of plasma fibrinogen levels based on hemoglobin, base excess and Injury Severity Score upon emergency room admission. Crit. Care 2013, 17, R137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauss, T.; Campion, S.; Kerever, S.; Eurin, M.; Raux, M.; Harrois, A.; Paugam-Burtz, C.; Hamada, S.; Traumabase, G. Fibrinogen on Admission in Trauma score: Early prediction of low plasma fibrinogen concentrations in trauma patients. Eur. J. Anaesthesiol. 2018, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dunham, M.P.; Sartorius, B.; Laing, G.L.; Bruce, J.L.; Clarke, D.L. A comparison of base deficit and vital signs in the early assessment of patients with penetrating trauma in a high burden setting. Injury 2017, 48, 1972–1977. [Google Scholar] [CrossRef]

| Organisation | Recommendation | Grade |
|---|---|---|
| A. Recommendations for the treatment of VKA-associated bleeding | ||
| American College of Cardiology [46] | 4F-PCC (FFP only if PCC not available) | |
| American College of Chest Physicians [44] | vit K + 4F-PCC over plasma | 2C |
| Neurocritical Care Society [47] | vit K + 4F-PCC over FFP | strong recommendation, moderate quality |
| American Society for Gastrointestinal Endoscopy [48] | vit K + 4F-PCC over FFP | moderate quality |
| American Society of Anesthesiologists [49] | vit K + 4F-PCC or FFP | |
| American Society of Hematology [50] | vit K + 4F PCC over FFP | conditional recommendation |
| American College of Emergency Physicians [51] | vit K + 4F-PCC over FFP | |
| Canadian stroke best practice recommendations [52] | vit K + PCC | evidence level B |
| European Guidelines [14] | vit K + PCC | 1A |
| European Society of Anaesthesiology [12] | vit K + PCC | 1B |
| British Committee for Standards in Haematology [53] | vit K + 4F-PCC (FFP only if PCC not available) | 1B |
| French clinical practice [45] | vit K + PCC (FFP only if PCC not available) | |
| European Society of Gastrointestinal Endoscopy [42] | vit K + PCC (FFP if PCC not available) | strong recommendation, low quality evidence |
| European Stroke Organisation [41] | vit K + PCC over FFP | strong recommendation, moderate quality evidence |
| European Association of Cardiothoracic Anaesthesiology [54] | 4F-PCC | |
| B. Recommendations for the treatment of DOAC-associated bleeding | ||
| American College of Cardiology [46] | FXa-i: 1st line andexanet alfa, 2nd line PCC/aPCC | |
| FIIa: 1st line idarucizumab, 2nd line PCC/aPCC | ||
| American Society for Gastrointestinal Endoscopy [48] | FXa-i: PCC/aPCC | |
| FIIa: PCC/aPCC | ||
| American Society of Hematology [50] | FXa-i: 4F-PCC or andexanet alfa | conditional recommendation |
| FIIa: idarucizumab | ||
| American College of Emergency Physicians [51] | FXa-i: 1st line andexanet alfa, 2nd line 4F-PCC | |
| FIIa: 1st line idarucizumab, 2nd line 4F-PCC over 3F-PCC | ||
| Canadian stroke best practice recommendations [52] | FXa-i: PCC | evidence level C |
| FIIa: 1st line idarucizumab, 2nd line PCC/aPCC | ||
| European Guidelines [14] | FXa-i: TXA and PCC, until specific antidotes available | 2C |
| FIIa: idarucizumab | 1B | |
| European Society of Anaesthesiology [12] | FXa-i: N/A | 2C |
| FIIa: idarucizumab | ||
| European Society of Gastrointestinal Endoscopy [42] | DOAC reversal agent or PCC | strong recommendation, low quality evidence |
| European Stroke Organisation [41] | 4F-PCC, if specific reversal agents not available | weak recommendation, very low-quality evidence |
| European Association of Cardiothoracic Anaesthesiology [54] | 4F-PCC, if specific reversal agents not available | – |
| Standard Laboratory Tests | Point-of-Care Testing | |
|---|---|---|
| Tests |
|
|
| Advantages |
|
|
| Disadvantages |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofer, S.; Schlimp, C.J.; Casu, S.; Grouzi, E. Management of Coagulopathy in Bleeding Patients. J. Clin. Med. 2022, 11, 1. https://doi.org/10.3390/jcm11010001
Hofer S, Schlimp CJ, Casu S, Grouzi E. Management of Coagulopathy in Bleeding Patients. Journal of Clinical Medicine. 2022; 11(1):1. https://doi.org/10.3390/jcm11010001
Chicago/Turabian StyleHofer, Stefan, Christoph J. Schlimp, Sebastian Casu, and Elisavet Grouzi. 2022. "Management of Coagulopathy in Bleeding Patients" Journal of Clinical Medicine 11, no. 1: 1. https://doi.org/10.3390/jcm11010001






