The Risk of Osteoporosis and Osteoporotic Fracture Following the Use of Irritable Bowel Syndrome Medical Treatment: An Analysis Using the OMOP CDM Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Network and Tools
2.2. Data Source and Study Population
2.3. Exposure
2.4. Outcomes
2.5. Statistical Analysis
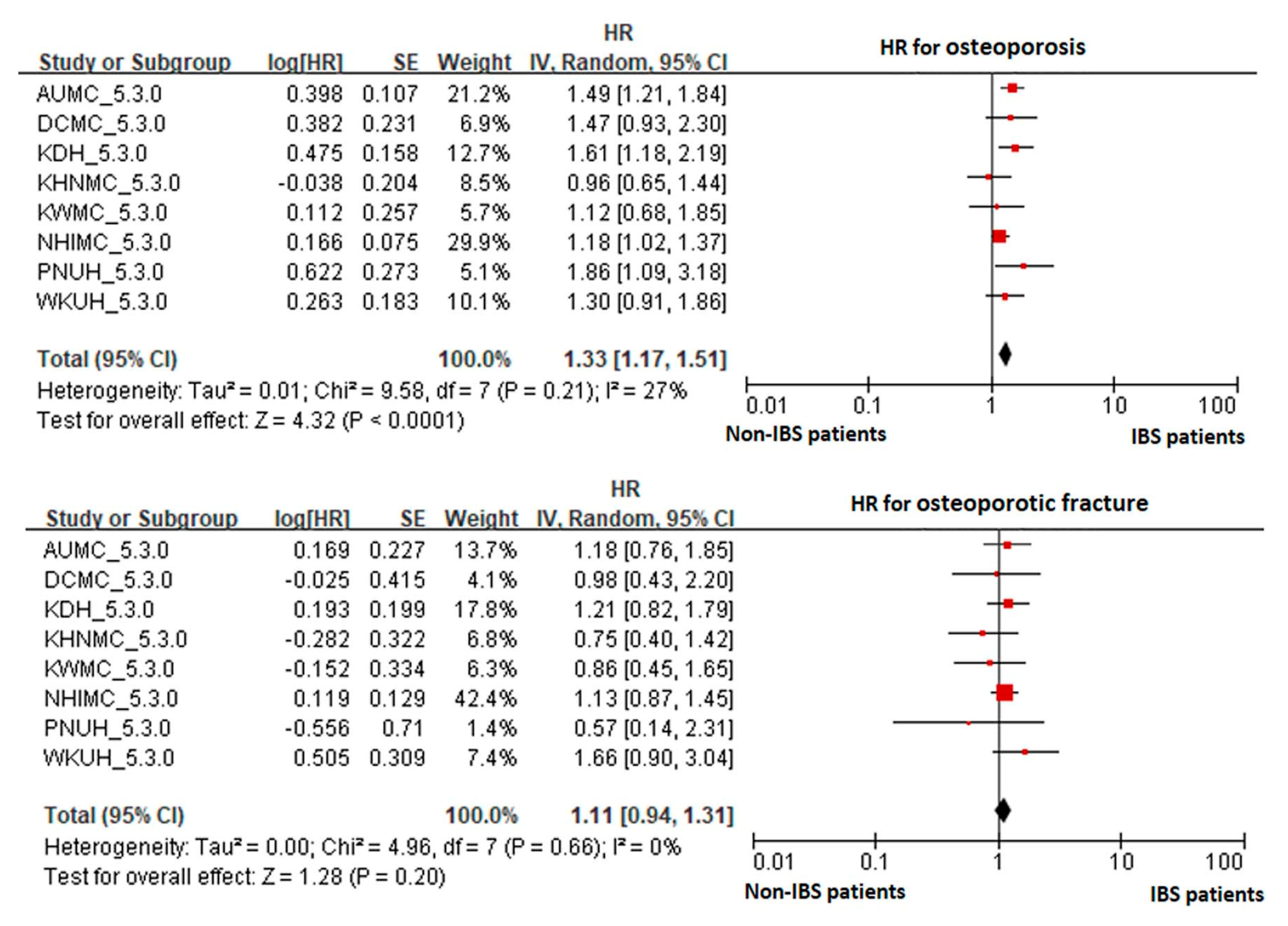
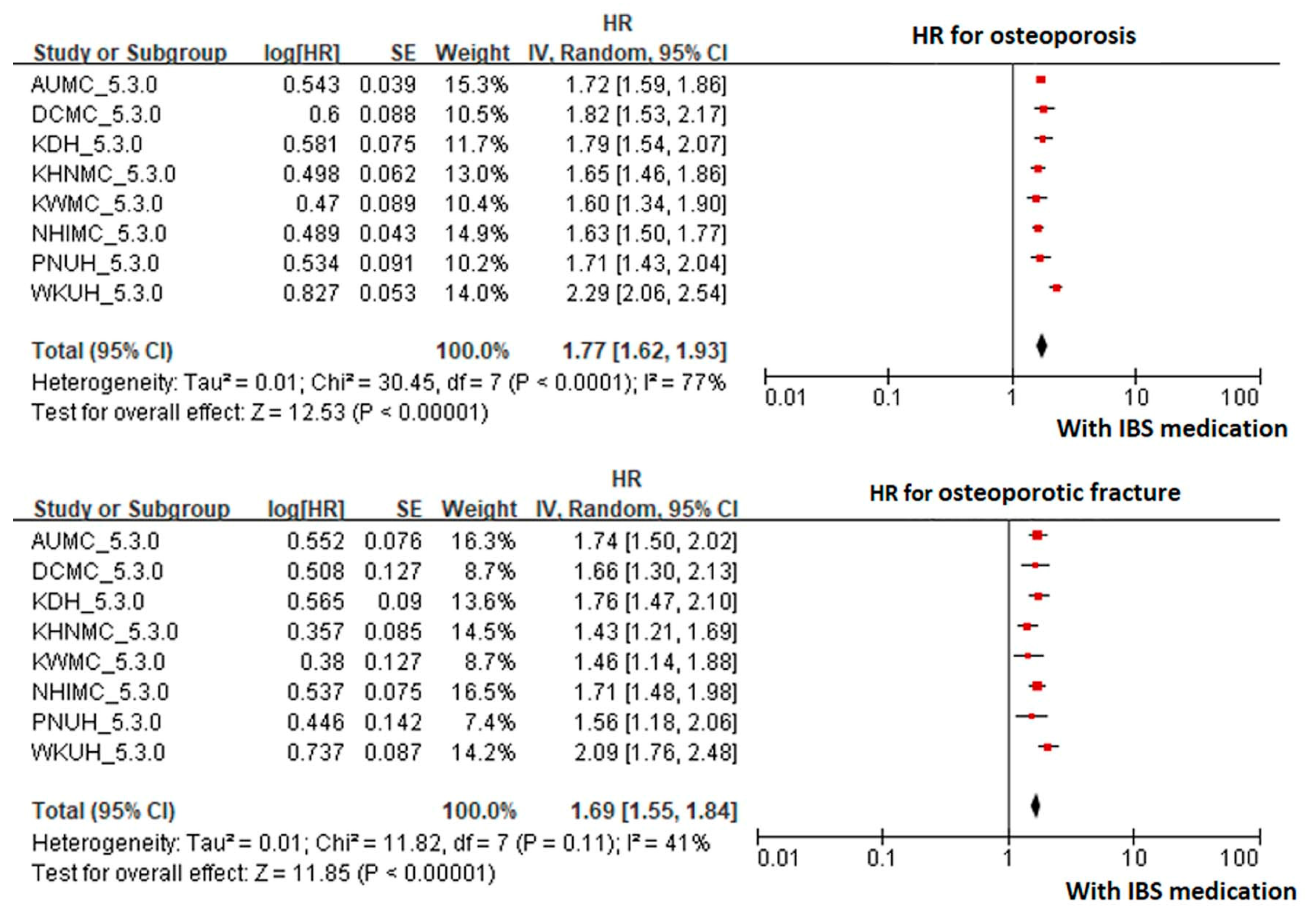
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wongtrakul, W.; Charoenngam, N.; Ungprasert, P. The association between irritable bowel syndrome and osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1049–1057. [Google Scholar] [CrossRef]
- Yen, C.M.; Muo, C.H.; Lin, M.C.; Chang, S.N.; Chang, Y.J.; Kao, C.H. A nationwide population cohort study: Irritable bowel syndrome is a risk factor of osteoporosis. Eur. J. Intern. Med. 2014, 25, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, K.J.; Kim, S.J.; Cho, S.W. Prevalence and risk factors for overlaps between gastroesophageal reflux disease, dyspepsia, and irritable bowel syndrome: A population-based study. Digestion 2009, 79, 196–201. [Google Scholar] [CrossRef]
- Özbaş, H.; Tutgun Onrat, S.; Özdamar, K. Genetic and environment factors in human osteoporosis. Mol. Biol. Rep. 2012, 39, 11289–11296. [Google Scholar] [CrossRef]
- Lima, C.A.; Lyra, A.C.; Rocha, R.; Santana, G.O. Risk factors for osteoporosis in inflammatory bowel disease patients. World J. Gastrointest. Pathophysiol. 2015, 6, 210–218. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef]
- Barau, E.; Dupont, C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef]
- Moayyedi, P.; Mearin, F.; Azpiroz, F.; Andresen, V.; Barbara, G.; Corsetti, M.; Emmanuel, A.; Hungin, A.P.S.; Layer, P.; Stanhellini, V.; et al. Irritable bowel syndrome diagnosis and management: A simplified algorithm for clinical practice. United Eur. Gastroenterol. J. 2017, 5, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, D.J.; Lacy, B.E. Management of irritable bowel syndrome with diarrhea: A review of nonpharmacological and pharmacological interventions. Ther. Adv. Gastroenterol. 2019, 12, 1–19. [Google Scholar] [CrossRef]
- Jung, H.K.; Kim, Y.H.; Park, J.Y.; Jang, B.H.; Park, S.Y.; Nam, M.H.; Choi, M.G. Estimating the burden of irritable bowel syndrome: Analysis of a nationwide Korean database. J. Neurogastroenterol. Motil. 2014, 20, 242–252. [Google Scholar] [CrossRef]
- Panday, K.; Gona, A.; Humphrey, M.B. Medication-induced osteoporosis: Screening and treatment strategies. Ther. Adv. Musculoskel. Dis. 2014, 6, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Miao, S.; Xu, H.; Yin, Y.; Zhu, Y.; Dai, Z.; Shan, T.; Jing, S.; Wang, J.; et al. Analysis of treatment pathways for three chronic diseases using OMOP CDM. J. Med. Syst. 2018, 42, 260. [Google Scholar] [CrossRef] [PubMed]
- FitzHenry, F.; Resnic, F.S.; Robbins, S.L.; Denton, J. Creating a common data model for comparative effectiveness with the observational medical outcomes partnership. Appl. Clin. Inform. 2015, 6, 536–547. [Google Scholar]
- Suchard, M.A.; Schuemie, M.J.; Krumholz, H.M.; You, S.C.; Chen, R.; Pratt, N.; Reich, C.G.; Duke, J.; Madigan, D.; Hripcsak, G.; et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: A systematic, multinational, large-scale analysis. Lancet 2019, 394, 1816–1826. [Google Scholar] [CrossRef]
- Farmer, A.D.; Wood, E.; Ruffle, J.K. An approach to the care of patients with irritable bowel syndrome. CMAJ 2020, 192, E275–E282. [Google Scholar] [CrossRef]
- Park, C.; Ha, Y.C.; Jang, S.; Jang, S.; Yoon, H.K.; Lee, Y.K. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J. Bone Miner. Metab. 2011, 29, 744–751. [Google Scholar] [CrossRef]
- Warriner, A.H.; Patkar, N.M.; Curtis, J.R.; Delzell, E.; Gary, L.; Kilgore, M.; Saag, K. Which fractures are most attributable to osteoporosis? J. Clin. Epidemiol. 2011, 64, 46–53. [Google Scholar] [CrossRef]
- OMOP Common Data Model. Available online: https://www.ohdsi.org/data-standardization/the-common-data-model/ (accessed on 10 January 2018).
- Shin, D.W.; Suh, B.; Lim, H.; Yun, J.M.; Song, S.O.; Park, Y. J-shaped association between postoperative levothyroxine dosage and fracture risk in thyroid cancer patients: A retrospective cohort study. J. Bone Miner. Res. 2018, 33, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Schuemie, M.J.; Suchard, M.A. Evaluating large-scale propensity score performance through realworld and synthetic data experiments. Int. J. Epidemiol. 2018, 47, 2005–2014. [Google Scholar] [CrossRef]
- Suchard, M.A.; Simpson, S.E.; Zorych, I.; Ryan, P.; Madigan, D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans. Model Comput. Simul. 2013, 23. [Google Scholar] [CrossRef]
- Lipsitch, M.; Tchetgen, E.; Cohen, T. Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 2010, 21, 383–388. [Google Scholar] [CrossRef]
- Voss, E.A.; Boyce, R.D.; Ryan, P.B.; van der Lei, J.; Rijnbeek, P.R.; Schuemie, M.J. Accuracy of an automated knowledge base for identifying drug adverse reactions. J. Biomed. Inform. 2017, 66, 72–81. [Google Scholar] [CrossRef]
- Schuemie, M.J.; Hripcsak, G.; Ryan, P.B.; Madigan, D.; Suchard, M.A. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc. Natl. Acad. Sci. USA 2018, 115, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Chen, C.Y.; Huang, W.T.; Chang, L.J.; Chen, S.C.C.; Yang, H.Y. Risk of fractures at different anatomic sites in patients with irritable bowel syndrome: A nationwide population-based cohort study. Arch. Osteoporos. 2018, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yi, Y.H.; Kim, Y.J.; Lee, J.G.; Tak, Y.J.; Hwang, H.R.; Lee, S.H.; Cho, Y.H.; Park, E.J.; Kim, J.M. Irritable Bowel Syndrome Is a Risk of Osteoporosis: A Population Based Nationwide 1 Cohort Study (29 April 2019). SSRN. Available online: https://ssrn.com/abstract=3379819 (accessed on 24 September 2020).
- Stobaugh, D.J.; Deepak, P.; Ehrenpreis, E.D. Increased risk of osteoporosis-related fractures in patients with irritable bowel syndrome. Osteoporos. Int. 2013, 24, 1169–1175. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. SSRIs: Bad to the bone? Innov. Clin. Neurosci. 2012, 9, 42–47. [Google Scholar]
- Bruyère, O.; Reginster, J.-Y. Osteoporosis in patients taking selective serotonin reuptake inhibitors: A focus on fracture outcome. Endocrine 2015, 48, 65–68. [Google Scholar] [CrossRef]
- Crowell, M.D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 2004, 141, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Kumar, M.; Talegaonkar, S.; Vohora, D. Serotonin reuptake inhibitors and bone health: A review of clinical studies and plausible mechanisms. Osteoporos. Sarcopenia 2017, 3, 75–81. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, T.; Tang, Y.; Xiong, W.; Shen, X.; Jiang, L.; Lin, L. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0172846. [Google Scholar]
- Choi, Y.J.; Kim, N.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, J.-W.; Kim, Y.S.; Lee, D.H.; Jung, H.C. Overlap between irritable bowel syndrome and functional dyspepsia including subtype analyses. J. Gastroenterol. Hepatol. 2017, 32, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A. Microbiome and its role in irritable bowel syndrome. Dig. Dis. Sci. 2020, 65, 829–839. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Gkolfakis, P.; Dimitriadis, G.; Triantafyllou, K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis. Int. 2015, 14, 572–581. [Google Scholar] [CrossRef]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Gobert, A.P.; Sagrestani, G.; Delmas, E.; Wilson, K.T.; Verriere, T.G.; Dapoigny, M.; Del’homme, C.; Bernalier-Donadille, A. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci. Rep. 2016, 6, 39399. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Sjögren, K. Osteomicrobiology: A new cross-disciplinary research field. Calcif. Tissue Int. 2018, 102, 426–432. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Röth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.-R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int. J. Mol. Sci 2020, 21, 5169. [Google Scholar] [CrossRef]
- Katz, S.; Weinerman, S. Osteoporosis and gastrointestinal disease. Gastroenterol. Hepatol. 2010, 6, 506–517. [Google Scholar]
- Wedlake, L.; A’Hern, R.; Russell, D.; Thomas, K.; Walters, J.R.; Andreyev, H.J. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 30, 707–717. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Amani, R.; Hasanvand, A.; Yousefi, R.E.; Alipour, M.; Saboori, S.; Choghakhori, R. Association of serum vitamin D concentration with clinical symptoms and quality of life in patients with irritable bowel syndrome. J. Am. Coll. Nutr. 2019, 38, 327–333. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef]
- Hayes, P.; Corish, C.; O’Mahony, E.; Quigley, E.M. A dietary survey of patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2014, 27, 36–47. [Google Scholar] [CrossRef] [PubMed]
| IBS | Non IBS | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Count | Mean, SD or % | Count | Mean, SD or % | |||||
| Age | AUMC_5.3.0 | 4555 | 47.25, | 14.42 | 178,649 | 44.78, | 15.43 | <0.001 |
| DCMC_5.3.0 | 2012 | 55.09, | 14.83 | 30,893 | 49.35, | 16.25 | <0.001 | |
| KDH_5.3.0 | 4003 | 49.45, | 16.24 | 78,039 | 45.94, | 16.54 | <0.001 | |
| KHNMC_5.3.0 | 2323 | 48.53, | 16.10 | 68,398 | 50.36, | 15.35 | <0.001 | |
| KWMC_5.3.0 | 1276 | 52.61, | 16.93 | 53,601 | 47.73, | 17.64 | <0.001 | |
| NHIMC_5.3.0 | 10,737 | 50.26, | 16.05 | 131,941 | 47.41, | 16.28 | <0.001 | |
| PNUH_5.3.0 | 1466 | 57.79, | 14.49 | 33,587 | 51.87, | 16.81 | <0.001 | |
| WKUH_5.3.0 | 1901 | 51.37, | 15.06 | 52,063 | 48.09, | 17.27 | <0.001 | |
| Female | AUMC_5.3.0 | 2277 | 50.0 | 102,909 | 57.6 | <0.001 | ||
| DCMC_5.3.0 | 1142 | 56.8 | 19,417 | 62.9 | <0.001 | |||
| KDH_5.3.0 | 2308 | 57.7 | 44,015 | 56.4 | 0.117 | |||
| KHNMC_5.3.0 | 1288 | 55.4 | 41,709 | 61.0 | <0.001 | |||
| KWMC_5.3.0 | 667 | 52.3 | 29,055 | 54.2 | 0.172 | |||
| NHIMC_5.3.0 | 6046 | 56.3 | 79,071 | 59.9 | <0.001 | |||
| PNUH_5.3.0 | 795 | 54.2 | 19,558 | 58.2 | 0.003 | |||
| WKUH_5.3.0 | 1084 | 57.0 | 28,720 | 55.2 | 0.108 | |||
| Charlson comorbidity index | AUMC_5.3.0 | 721 | 0.254 | 1.272 | 16,894 | 0.134 | 0.833 | <0.001 |
| DCMC_5.3.0 | 493 | 0.421 | 1.165 | 3681 | 0.178 | 0.865 | <0.001 | |
| KDH_5.3.0 | 1184 | 0.439 | 0.988 | 6134 | 0.111 | 0.740 | <0.001 | |
| KHNMC_5.3.0 | 414 | 0.267 | 1.003 | 5414 | 0.120 | 0.895 | 0.001 | |
| KWMC_5.3.0 | 391 | 0.560 | 1.163 | 4411 | 0.125 | 0.775 | <0.001 | |
| NHIMC_5.3.0 | 1951 | 0.275 | 1.026 | 13,726 | 0.140 | 0.719 | <0.001 | |
| PNUH_5.3.0 | 412 | 0.404 | 0.973 | 4540 | 0.216 | 1.107 | 0.001 | |
| WKUH_5.3.0 | 477 | 0.400 | 1.105 | 6702 | 0.196 | 0.890 | <0.001 | |
| 1:4 Matching | ||||||||
|---|---|---|---|---|---|---|---|---|
| IBS | Non-IBS | |||||||
| Patients | Person-Years | Events | Rate | Patients | Person-Years | Events | Rate | |
| Osteoporosis | ||||||||
| AUMC_5.3.0 | 4687 | 25,988 | 166 | 6.39 | 17,636 | 84,401 | 366 | 4.34 |
| DCMC_5.3.0 | 1701 | 7445 | 51 | 6.85 | 4619 | 15,428 | 68 | 4.41 |
| KDH_5.3.0 | 3039 | 18,096 | 100 | 5.53 | 8999 | 46,847 | 155 | 3.31 |
| KHNMC_5.3.0 | 2301 | 8861 | 49 | 5.53 | 7423 | 23,047 | 124 | 5.38 |
| KWMC_5.3.0 | 1162 | 5653 | 37 | 6.55 | 3678 | 13,984 | 66 | 4.72 |
| NHIMC_5.3.0 | 9242 | 50,562 | 323 | 6.39 | 29,261 | 135,289 | 759 | 5.61 |
| PNUH_5.3.0 | 1144 | 4124 | 35 | 8.49 | 3143 | 10,881 | 55 | 5.05 |
| WKUH_5.3.0 | 1447 | 8034 | 85 | 10.58 | 3559 | 15,256 | 116 | 7.60 |
| Total | 24,723 | 128,763 | 846 | 6.57 | 78,318 | 345,133 | 1709 | 4.95 |
| Osteoporotic fracture | ||||||||
| AUMC_5.3.0 | 4687 | 26,644 | 33 | 1.24 | 17,636 | 85,715 | 97 | 1.13 |
| DCMC_5.3.0 | 1701 | 7612 | 12 | 1.58 | 4619 | 15,582 | 35 | 2.25 |
| KDH_5.3.0 | 3039 | 18,275 | 51 | 2.79 | 8999 | 47,029 | 122 | 2.59 |
| KHNMC_5.3.0 | 2301 | 8951 | 23 | 2.57 | 7423 | 23,357 | 65 | 2.78 |
| KWMC_5.3.0 | 1162 | 5725 | 19 | 3.32 | 3678 | 14,109 | 41 | 2.91 |
| NHIMC_5.3.0 | 9242 | 51,622 | 128 | 2.48 | 29,261 | 137,798 | 258 | 1.87 |
| PNUH_5.3.0 | 1144 | 4221 | 7 | 1.66 | 3143 | 11,032 | 21 | 1.90 |
| WKUH_5.3.0 | 1447 | 8303 | 33 | 3.97 | 3559 | 15,586 | 33 | 2.12 |
| Total | 24,723 | 131,353 | 306 | 2.33 | 78,318 | 350,208 | 672 | 1.92 |
| 1:4 Matching | ||||||||
|---|---|---|---|---|---|---|---|---|
| with IBS Medication | without IBS Medication | |||||||
| Patients | Person-Years | Events | Rate | Patients | Person-Years | Events | Rate | |
| Osteoporosis | ||||||||
| AUMC_5.3.0 | 130,159 | 539,339 | 2779 | 5.15 | 242,780 | 641,442 | 1860 | 2.90 |
| DCMC_5.3.0 | 17,180 | 68,044 | 317 | 4.66 | 56,505 | 175,890 | 473 | 2.69 |
| KDH_5.3.0 | 48,861 | 201,544 | 751 | 3.73 | 101,494 | 255,885 | 552 | 2.16 |
| KHNMC_5.3.0 | 43,657 | 156,387 | 1080 | 6.91 | 74,940 | 159,894 | 730 | 4.57 |
| KWMC_5.3.0 | 32,105 | 128,286 | 570 | 4.44 | 42,957 | 108,329 | 336 | 3.10 |
| NHIMC_5.3.0 | 91,027 | 396,647 | 2258 | 5.69 | 151,062 | 417,136 | 1511 | 3.62 |
| PNUH_5.3.0 | 24,002 | 74,428 | 336 | 4.51 | 74,019 | 174,303 | 485 | 2.78 |
| WKUH_5.3.0 | 40,649 | 192,960 | 1442 | 7.47 | 84,197 | 260,602 | 873 | 3.35 |
| Total | 427,640 | 1,757,635 | 9533 | 5.42 | 827,954 | 2,193,481 | 6820 | 3.11 |
| Osteoporotic fracture | ||||||||
| AUMC_5.3.0 | 130,159 | 548,294 | 745 | 1.36 | 242,780 | 645,937 | 511 | 0.79 |
| DCMC_5.3.0 | 17,180 | 68,631 | 162 | 2.36 | 56,505 | 176,838 | 253 | 1.43 |
| KDH_5.3.0 | 48,861 | 202,219 | 571 | 2.82 | 101,494 | 256,277 | 401 | 1.56 |
| KHNMC_5.3.0 | 43,657 | 158,565 | 611 | 3.85 | 74,940 | 160,900 | 445 | 2.77 |
| KWMC_5.3.0 | 32,105 | 129,315 | 342 | 2.64 | 42,957 | 108,818 | 192 | 1.76 |
| NHIMC_5.3.0 | 91,027 | 404,082 | 804 | 1.99 | 151,062 | 420,771 | 516 | 1.23 |
| PNUH_5.3.0 | 24,002 | 75,107 | 136 | 1.81 | 74,019 | 175,270 | 189 | 1.08 |
| WKUH_5.3.0 | 40,649 | 196,736 | 600 | 3.05 | 84,197 | 262,626 | 357 | 1.36 |
| Total | 427,640 | 1,782,949 | 3971 | 2.23 | 827,954 | 2,207,437 | 2864 | 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.L.; Yi, Y.H.; Hwang, H.R.; Kim, J.; Park, Y.; Kim, Y.J.; Lee, J.G.; Tak, Y.J.; Lee, S.H.; Lee, S.Y.; et al. The Risk of Osteoporosis and Osteoporotic Fracture Following the Use of Irritable Bowel Syndrome Medical Treatment: An Analysis Using the OMOP CDM Database. J. Clin. Med. 2021, 10, 2044. https://doi.org/10.3390/jcm10092044
Kim GL, Yi YH, Hwang HR, Kim J, Park Y, Kim YJ, Lee JG, Tak YJ, Lee SH, Lee SY, et al. The Risk of Osteoporosis and Osteoporotic Fracture Following the Use of Irritable Bowel Syndrome Medical Treatment: An Analysis Using the OMOP CDM Database. Journal of Clinical Medicine. 2021; 10(9):2044. https://doi.org/10.3390/jcm10092044
Chicago/Turabian StyleKim, Gyu Lee, Yu Hyeon Yi, Hye Rim Hwang, Jinmi Kim, Youngmin Park, Yun Jin Kim, Jeong Gyu Lee, Young Jin Tak, Seung Hun Lee, Sang Yeoup Lee, and et al. 2021. "The Risk of Osteoporosis and Osteoporotic Fracture Following the Use of Irritable Bowel Syndrome Medical Treatment: An Analysis Using the OMOP CDM Database" Journal of Clinical Medicine 10, no. 9: 2044. https://doi.org/10.3390/jcm10092044
APA StyleKim, G. L., Yi, Y. H., Hwang, H. R., Kim, J., Park, Y., Kim, Y. J., Lee, J. G., Tak, Y. J., Lee, S. H., Lee, S. Y., Cho, Y. H., Park, E. J., & Lee, Y. (2021). The Risk of Osteoporosis and Osteoporotic Fracture Following the Use of Irritable Bowel Syndrome Medical Treatment: An Analysis Using the OMOP CDM Database. Journal of Clinical Medicine, 10(9), 2044. https://doi.org/10.3390/jcm10092044








