Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate?
Abstract
:1. Introduction
2. Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease
3. Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease
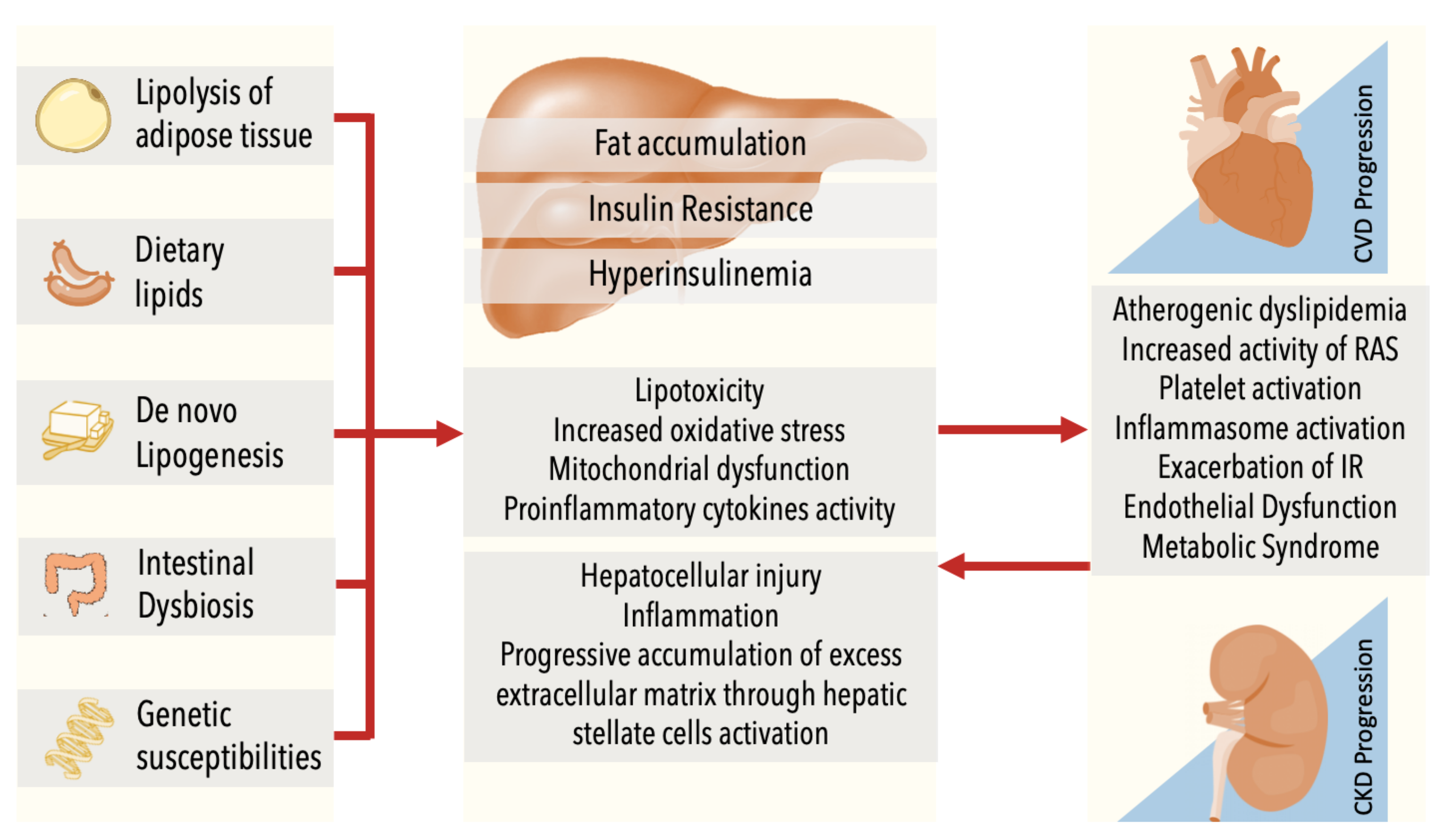
4. Common Pathophysiology
5. Clinical Implications: Assessment and Treatment
5.1. Lifestyle Intervention
5.2. Specific Management of Arterial Hypertension in NAFLD
5.3. Specific Lipid Control in NAFLD
5.4. Specific T2D Treatment in NAFLD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E. Nonalcoholic fatty liver disease a systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Ruissen, M.M.; Mak, A.L.; Beuers, U.; Tushuizen, M.E.; Holleboom, A.G. Non-alcoholic fatty liver disease: A multidisciplinary approach towards a cardiometabolic liver disease. Eur. J. Endocrinol. 2020, 183, R57–R73. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Kaps, L.; Labenz, C.; Galle, P.R.; Weinmann-Menke, J.; Kostev, K.; Schattenberg, J.M. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United Eur. Gastroenterol. J. 2020, 8, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Cho, Y.; Lee, B.W.; Park, C.Y.; Lee, D.H.; Cha, B.S.; Rhee, E.J. Nonalcoholic Fatty Liver Disease in Diabetes. Part I: Epidemiology and Diagnosis. Diabetes Metab. J. 2019, 43, 31–45. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; S Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Ampuero, J.; Pais, R.; Aller, R.; Gallego-Durán, R.; Crespo, J.; García-Monzón, C.; Boursier, J.; Vilar, E.; Petta, S.; Zheng, M.H. HEPAmet Registry. Development and Validation of Hepamet Fibrosis Scoring System-A Simple, Noninvasive Test to Identify Patients With Nonalcoholic Fatty Liver Disease With Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2020, 18, 216–225.e5. [Google Scholar] [CrossRef]
- Foucher, J.; Chanteloup, E.; Vergniol, J.; Castéra, L.; Le Bail, B.; Adhoute, X.; Bertet, J.; Couzigou, P.; de Lédinghen, V. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut 2006, 55, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Schonmann, Y.; Yeshua, H.; Bentov, I.; Zelber-Sagi, S. Liver fibrosis marker is an independent predictor of cardiovascular morbidity and mortality in the general population. Dig. Liver Dis. 2021, 53, 79–85. [Google Scholar] [CrossRef]
- Ballestri, S.; Mantovani, A.; Baldelli, E.; Lugari, S.; Maurantonio, M.; Nascimbeni, F.; Marrazzo, A.; Romagnoli, D.; Targher, G.; Lonardo, A. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis and Are Associated with Higher Cardiovascular Risk Scores in Patients with NAFLD or Viral Chronic Liver Disease. Diagnostics 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Magalhães, R.; Xavier, S.; Magalhães, J.; Rosa, B.; Marinho, C.; Cotter, J. Transient elastography through controlled attenuated parameter assisting the stratification of cardiovascular disease risk in NAFLD patients. Clin. Res. Hepatol. Gastroenterol. 2020, 101580. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, K.D.; Choi, E.K.; Oh, S.; Lip, G. Nonalcoholic fatty liver disease and the risk of atrial fibrillation stratified by body mass index: A nationwide population-based study. Sci. Rep. 2021, 11, 3737. [Google Scholar] [CrossRef]
- Saokaew, S.; Kanchanasurakit, S.; Thawichai, K.; Duangprom, P.; Wannasri, M.; Khankham, S.; Kositamongkol, C.; Chaiyakunapruk, N.; Phisalprapa, P. Association of non-alcoholic fatty liver disease and all-cause mortality in hospitalized cardiovascular disease patients: A systematic review and meta-analysis. Medicine 2021, 100, e24557. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Toda, H.; Ejiri, K.; Yoshida, M.; Nanba, Y.; Yoshida, M.; Nakamura, K.; et al. Prognostic value of non-alcoholic fatty liver disease for predicting cardiovascular events in patients with diabetes mellitus with suspected coronary artery disease: A prospective cohort study. Cardiovasc. Diabetol. 2021, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.S.; Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, Y.H.; Kim, Y.D.; Kim, S.U. Association between the severity of liver fibrosis and cardiovascular outcomes in patients with type 2 diabetes. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Fan, N.; Ding, X.; Zhen, Q.; Gu, L.; Zhang, A.; Shen, T.; Wang, Y.; Peng, Y. Association of the Non-Alcoholic Fatty Liver Disease Fibrosis Score with subclinical myocardial remodeling in patients with type 2 diabetes: A cross-sectional study in China. J. Diabetes Investig. 2020. [Google Scholar] [CrossRef]
- Kim, B.J.; Cheong, E.S.; Kang, J.G.; Kim, B.S.; Kang, J.H. Relationship of epicardial fat thickness and nonalcoholic fatty liver disease to coronary artery calcification: From the CAESAR study. J. Clin. Lipidol. 2016, 10, 619–626.e1. [Google Scholar] [CrossRef] [PubMed]
- Brouha, S.S.; Nguyen, P.; Bettencourt, R.; Sirlin, C.B.; Loomba, R. Increased severity of liver fat content and liver fibrosis in non-alcoholic fatty liver disease correlate with epicardial fat volume in type 2 diabetes: A prospective study. Eur. Radiol. 2018, 28, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Li, Y.; Liu, Y.; Yan, Y.; Luo, A.; Ren, H.; She, Q. Association of epicardial adipose tissue with non-alcoholic fatty liver disease: A meta-analysis. Hepatol. Int. 2019, 13, 757–765. [Google Scholar]
- Musso, G.; Gambino, R.; Tabibian, J.H.; Ekstedt, M.; Kechagias, S.; Hamaguchi, M.; Hultcrantz, R.; Hagström, H.; Yoon, S.K.; Charatcharoenwitthaya, P.; et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Zaza, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism 2018, 79, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Boonpheng, B.; Panjawatanan, P.; Sharma, K.; Ungprasert, P.; Pungpapong, S.; Cheungpasitporn, W. Nonalcoholic fatty liver disease and albuminuria: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Dawwas, G.K.; Liu, X.; Nguyen, M.H. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: A propensity-matched cohort study. J. Intern. Med. 2019, 286, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Ye, F.Z.; Kani, H.T.; Yang, J.R.; Zheng, K.I.; Zhang, H.Y.; Targher, G.; Byrne, C.D.; Chen, Y.P.; Yuan, W.-J.; et al. Higher liver stiffness scores are associated with early kidney dysfunction in patients with histologically proven non-cirrhotic NAFLD. Diabetes Metab. 2020, 46, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.W.; Hsu, Y.C.; Chang, C.H.; Wei, K.L.; Lin, C.L. High FIB-4 index as an independent risk factor of prevalent chronic kidney disease in patients with nonalcoholic fatty liver disease. Hepatol. Int. 2016, 10, 340–346. [Google Scholar] [CrossRef]
- Önnerhag, K.; Dreja, K.; Nilsson, P.M.; Lindgren, S. Increased mortality in non-alcoholic fatty liver disease with chronic kidney disease is explained by metabolic comorbidities. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 542–550. [Google Scholar] [CrossRef]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.I.; Stevens, R.J.; Manley, S.E.; Bilous, R.W.; Cull, C.A.; Holman, R.R. UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003, 63, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Lippi, G.; Day, C.; Muggeo, M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008, 51, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Yeung, M.W.; Wong, G.L.; Choi, K.C.; Luk, A.O.; Kwok, R.; Shu, S.S.; Chan, A.W.; Lau, E.S.H.; Ma, R.C.W.; Chan, H.L.; et al. Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J. Hepatol. 2018, 68, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Muraca, E.; Perra, S.; Bianconi, E.; Zerbini, F.; Oltolini, A.; Cannistraci, R.; Parmeggiani, P.; Manzoni, G.; Gastaldelli, A.; et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res. Care 2020, 8, e000904. [Google Scholar] [CrossRef] [Green Version]
- Han, E.; Cho, Y.; Kim, K.W.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. Hepatic fibrosis is associated with total proteinuria in Korean patients with type 2 diabetes. Medicine 2020, 99, e21038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Wan, H.; Chen, Y.; Xia, F.; Zhang, W.; Zhang, K.; Gu, X.; Zhang, Y.; Lin, Z.; et al. Lower eGFR is associated with increased probability of liver fibrosis in Chinese diabetic patients. Diabetes Metab. Res. Rev. 2020, 36, e3294. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Friedman, S.L.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Villa-Jimenez, O.; Lazo-Del Vallin, S.; Diago, M.; Adams, L.A.; Romero-Gomez, M.; et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2017, 45, 332–344. [Google Scholar] [CrossRef]
- Mantovani, A.; Turino, T.; Lando, M.G.; Gjini, K.; Byrne, C.D.; Zusi, C.; Ravaioli, F.; Colecchia, A.; Maffeis, C.; Salvagno, G. Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab. 2020, 46, 296–303. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD as a driver of chronic kidney disease. J. Hepatol. 2020, 72, 785–801. [Google Scholar] [CrossRef] [Green Version]
- Bril, F.; Cusi, K. Management of Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Call to Action. Diabetes Care 2017, 40, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Budd, J.; Cusi, K. Nonalcoholic Fatty Liver Disease: What Does the Primary Care Physician Need to Know? Am. J. Med. 2020, 133, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Jin, Y.; Wang, T.Y.; Zheng, K.I.; Rios, R.S.; Zhang, H.Y.; Targher, G.; Byrne, C.D.; Yuan, W.J.; Zheng, M.H. MAFLD and risk of CKD. Metabolism 2021, 115, 154433. [Google Scholar] [CrossRef]
- Marchesini, G.; Petta, S.; Dalle Grave, R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundertmark, J.; Krenkel, O.; Tacke, F. Adapted Immune Responses of Myeloid-Derived Cells in Fatty Liver Disease. Front. Immunol. 2018, 9, 2418. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.H.; Lee, M.; Park, S.Y.; Kim, J.H.; Lee, B.W. Glycated Albumin Is a More Useful Glycation Index than HbA1c for Reflecting Renal Tubulopathy in Subjects with Early Diabetic Kidney Disease. Diabetes Metab. J. 2018, 42, 215–223. [Google Scholar] [CrossRef]
- Duseja, A.; Chawla, Y.K. Obesity and NAFLD: The role of bacteria and microbiota. Clin. Liver Dis. 2014, 18, 59–71. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Li, L.; Yu, C.H.; Shen, Z.; Chen, L.H.; Li, Y.M. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. 2013, 19, 6911–6918. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, I.; Rider, S.; Mullins, J.; Hughes, J.; Péault, B. Pericytes in the renal vasculature: Roles in health and disease. Nat. Rev. Nephrol. 2018, 14, 521–534. [Google Scholar] [CrossRef]
- Sun, D.Q.; Zheng, K.I.; Xu, G.; Ma, H.L.; Zhang, H.Y.; Pan, X.Y.; Zhu, P.W.; Wang, X.D.; Targher, G.; Byrne, C.D.; et al. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020, 40, 107–119. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Alisi, A.; Mosca, A.; Panera, N.; Byrne, C.D.; Nobili, V. Relationship Between PNPLA3 rs738409 Polymorphism and Decreased Kidney Function in Children With NAFLD. Hepatology 2019, 70, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Zusi, C.; Sani, E.; Colecchia, A.; Lippi, G.; Zaza, G.L.; Valenti, L.; Byrne, C.D.; Maffeis, C.; Bonora, E.; et al. Association between PNPLA3rs738409 polymorphism decreased kidney function in postmenopausal type 2 diabetic women with or without non-alcoholic fatty liver disease. Diabetes Metab. 2019, 45, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampuero, J.; Aller, R.; Gallego-Durán, R.; Crespo, J.; Calleja, J.L.; García-Monzón, C.; Gómez-Camarero, J.; Caballería, J.; Lo Iacono, O.; Ibañez, L.; et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J. Hepatol. 2020, 73, 17–25. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Mann, J.F.; Yi, Q.; Zinman, B.; Dinneen, S.F.; Hoogwerf, B.; Hallé, J.P.; Young, J.; Rashkow, A.; Joyce, C.; et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001, 286, 421–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glen, J.; Floros, L.; Day, C.; Pryke, R.; Guideline Development Group. Non-alcoholic fatty liver disease (NAFLD): Summary of NICE guidance. BMJ 2016, 354, i4428. [Google Scholar] [CrossRef]
- American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S37–S47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S89–S97. [Google Scholar] [CrossRef] [Green Version]
- Radaelli, M.G.; Martucci, F.; Perra, S.; Accornero, S.; Castoldi, G.; Lattuada, G.; Manzoni, G.; Perseghin, G. NAFLD/NASH in patients with type 2 diabetes and related treatment options. J. Endocrinol. Invest. 2018, 41, 509–521. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Arrese, M.; Barrera, F.; Triantafilo, N.; Arab, J.P. Concurrent nonalcoholic fatty liver disease and type 2 diabetes: Diagnostic and therapeutic considerations. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Hollis-Hansen, K.; Wan, X.Y.; Fei, S.J.; Pang, X.L.; Meng, F.D.; Yu, C.H.; Li, Y.M. Clinical guidelines of non-alcoholic fatty liver disease: A systematic review. World J. Gastroenterol. 2016, 22, 8226–8233. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, E.; Negri, C.; Targher, G.; Faccioli, N.; Lanza, M.; Zoppini, G.; Zanolin, E.; Schena, F.; Bonora, E.; Moghetti, P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013, 58, 1287–1295. [Google Scholar] [CrossRef]
- Dickinson, H.O.; Mason, J.M.; Nicolson, D.J.; Campbell, F.; Beyer, F.R.; Cook, J.V.; Williams, B.; Ford, G.A. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J. Hypertens. 2006, 24, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doménech, M.; Roman, P.; Lapetra, J.; García de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasper, P.; Martin, A.; Lang, S.; Demir, M.; Steffen, H.M. Hypertension in NAFLD: An uncontrolled burden. J. Hepatol. 2021, 74, 1258–1260. [Google Scholar] [CrossRef]
- Hoogwerf, B.J. Renin-angiotensin system blockade and cardiovascular and renal protection. Am. J. Cardiol. 2010, 105, 30A–35A. [Google Scholar] [CrossRef]
- Perdomo, C.; D’Ingianna, P.; Escalada, J.; Petta, S.; Romero Gómez, M.; Ampuero, J. Nonalcoholic fatty liver disease and the risk of metabolic comorbidities: How to manage in clinical practice. Pol. Arch. Intern. Med. 2020, 130, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Sancho-Bru, P.; Ginès, P.; Lora, J.M.; Al-Garawi, A.; Solé, M.; Colmenero, J.; Nicolás, J.M.; Jiménez, W.; Weich, N.; et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 2003, 125, 117–125. [Google Scholar] [CrossRef]
- Gillespie, E.L.; White, C.M.; Kardas, M.; Lindberg, M.; Coleman, C.I. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care 2005, 28, 2261–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuissa, H.; Jones, P.G.; Marso, S.P.; O’Keefe, J.H., Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: A meta-analysis of randomized clinical trials. J. Am. Coll. Cardiol. 2005, 46, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Fukui, H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 2001, 34, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Sturzeneker, M.C.S.; de Noronha, L.; Olandoski, M.; Wendling, L.U.; Precoma, D.B. Ramipril significantly attenuates the development of non-alcoholic steatohepatitis in hyperlipidaemic rabbits. Am. J. Cardiovasc. Dis. 2019, 9, 8–17. [Google Scholar]
- Enjoji, M.; Kotoh, K.; Kato, M.; Higuchi, N.; Kohjima, M.; Nakashima, M.; Nakamuta, M. Therapeutic effect of ARBs on insulin resistance and liver injury in patients with NAFLD and chronic hepatitis C: A pilot study. Int J. Mol. Med. 2008, 22, 521–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israili, Z.H. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J. Hum. Hypertens. 2000, 14, S73–S86. [Google Scholar] [CrossRef] [Green Version]
- Oparil, S. Newly emerging pharmacologic differences in angiotensin II receptor blockers. Am. J. Hypertens. 2000, 13, 18S–24S. [Google Scholar] [CrossRef] [Green Version]
- Georgescu, E.F.; Ionescu, R.; Niculescu, M.; Mogoanta, L.; Vancica, L. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J. Gastroenterol. 2009, 15, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Yokohama, S.; Tokusashi, Y.; Nakamura, K.; Tamaki, Y.; Okamoto, S.; Okada, M.; Aso, K.; Hasegawa, T.; Aoshima, M.; Miyokawa, N.; et al. Inhibitory effect of angiotensin II receptor antagonist on hepatic stellate cell activation in non-alcoholic steatohepatitis. World J. Gastroenterol. 2006, 12, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Yokohama, S.; Yoneda, M.; Haneda, M.; Okamoto, S.; Okada, M.; Aso, K.; Hasegawa, T.; Tokusashi, Y.; Miyokawa, N.; Nakamura, K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004, 40, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Cannon, C.P.; Murphy, S.A.; Mega, S.; Pasceri, V.; Briguori, C.; Colombo, A.; Yun, K.H.; Jeong, M.H.; Kim, J.S.; et al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: A collaborative patient-level meta-analysis of 13 randomized studies. Circulation 2011, 123, 1622–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Davis, A.M. Primary Prevention of Cardiovascular Disease. JAMA 2019, 322, 1817–1818. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Petta, S.; Mannisto, V.; Mancina, R.M.; Pipitone, R.; Karja, V.; Maggioni, M.; Kakela, P.; Wiklund, O.; Mozzi, E.; et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J. Hepatol. 2015, 63, 705–712. [Google Scholar] [CrossRef]
- Foster, T.; Budoff, M.J.; Saab, S.; Ahmadi, N.; Gordon, C.; Guerci, A.D. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: The St Francis Heart Study randomized clinical trial. Am. J. Gastroenterol. 2011, 106, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mitsiou, E.; Boutari, C.; Kotsis, V.; Georgianou, E.; Doumas, M.; Karagiannis, A.; Athyros, V.G. Effect of Low (5 mg) vs. High (20-40 mg) Rosuvastatin Dose on 24h Arterial Stiffness, Central Haemodynamics, and Non-Alcoholic Fatty Liver Disease in Patients with Optimally Controlled Arterial Hypertension. Curr. Vasc. Pharmacol. 2018, 16, 393–400. [Google Scholar] [CrossRef]
- Kim, R.G.; Loomba, R.; Prokop, L.J.; Singh, S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1521–1530.e8. [Google Scholar] [CrossRef] [Green Version]
- Onofrei, M.D.; Butler, K.L.; Fuke, D.C.; Miller, H.B. Safety of statin therapy in patients with preexisting liver disease. Pharmacotherapy 2008, 28, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D. Statins and hepatic steatosis: Perspectives from the Dallas Heart Study. Hepatology 2006, 44, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC Scientific Document Group 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Oza, N.; Takahashi, H.; Eguchi, Y.; Kitajima, Y.; Kuwashiro, T.; Ishibashi, E.; Nakashita, S.; Iwane, S.; Kawaguchi, Y.; Mizuta, T.; et al. Efficacy of ezetimibe for reducing serum low-density lipoprotein cholesterol levels resistant to lifestyle intervention in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2014, 44, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sirlin, C.B.; Ang, B.; Bettencourt, R.; Jain, R.; Salotti, J.; Soaft, L.; Hooker, J.; Kono, Y.; Bhatt, A.; et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: Assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015, 61, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, N.; Lenton, R.; Bhartia, M.; Abbas, A.; Raju, J.; Ramachandran, S. Effect of fibrate treatment on liver function tests in patients with the metabolic syndrome. Springerplus 2014, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbrini, E.; Mohammed, B.S.; Korenblat, K.M.; Magkos, F.; McCrea, J.; Patterson, B.W.; Klein, S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2010, 95, 2727–2735. [Google Scholar] [CrossRef]
- Argo, C.K.; Patrie, J.T.; Lackner, C.; Henry, T.D.; de Lange, E.E.; Weltman, A.L.; Shah, N.L.; Al-Osaimi, A.M.; Pramoonjago, P.; Jayakumar, S.; et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: A double-blind, randomized, placebo-controlled trial. J. Hepatol. 2015, 62, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Theocharidou, E.; Papademetriou, M.; Reklou, A.; Sachinidis, A.; Boutari, C.; Giouleme, O. The Role of PCSK9 in the Pathogenesis of Non-alcoholic Fatty Liver Disease and the Effect of PCSK9 Inhibitors. Curr. Pharm. Des. 2018, 24, 3654–3657. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Pattou, F.; Mathurin, P. Perspectives on Treatment for Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; D’Errico, V.; Fallucca, S.; Menini, S.; Pugliese, G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res. Rev. 2014, 30, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Blazina, I.; Selph, S. Diabetes drugs for nonalcoholic fatty liver disease: A systematic review. Syst. Rev. 2019, 8, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzotti, A.; Caletti, M.T.; Marchignoli, F.; Forlani, G.; Marchesini, G. Which treatment for type 2 diabetes associated with non-alcoholic fatty liver disease? Dig. Liver Dis. 2017, 49, 235–240. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef]
- Barb, D.; Portillo-Sanchez, P.; Cusi, K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1183–1195. [Google Scholar] [CrossRef] [Green Version]
- Górriz, J.L.; Soler, M.J.; Navarro-González, J.F.; García-Carro, C.; Puchades, M.J.; D’Marco, L.; Martínez Castelao, A.; Fernández-Fernández, B.; Ortiz, A.; Górriz-Zambrano, C.; et al. GLP-1 Receptor Agonists and Diabetic Kidney Disease: A Call of Attention to Nephrologists. J. Clin. Med. 2020, 9, 947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, M.J.; Houlihan, D.D.; Rowe, I.A.; Clausen, W.H.; Elbrønd, B.; Gough, S.C.; Tomlinson, J.W.; Newsome, P.N. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment. Pharmacol. Ther. 2013, 37, 234–242. [Google Scholar] [CrossRef]
- Shao, N.; Kuang, H.Y.; Hao, M.; Gao, X.Y.; Lin, W.J.; Zou, W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2014, 30, 521–529. [Google Scholar] [CrossRef]
- Liu, L.; Yan, H.; Xia, M.; Zhao, L.; Lv, M.; Zhao, N.; Rao, S.; Yao, X.; Wu, W.; Pan, B.; et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3292. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, M.P.; Pardo, V.; Ruiz, L.; Castro-Sánchez, L.; Lanzón, B.; Fernández-Millán, E.; García-Monzón, C.; Arroba, A.I.; González‐Rodríguez, Á.; Escrivá, F.; et al. A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology 2017, 65, 950–968. [Google Scholar] [CrossRef]
- Kannt, A.; Madsen, A.N.; Kammermeier, C.; Elvert, R.; Klöckener, T.; Bossart, M.; Haack, T.; Evers, A.; Lorenz, K.; Hennerici, W.; et al. Incretin combination therapy for the treatment of non-alcoholic steatohepatitis. Diabetes Obes. Metab. 2020, 22, 1328–1338. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athyros, V.G.; Polyzos, S.A.; Kountouras, J.; Katsiki, N.; Anagnostis, P.; Doumas, M.; Mantzoros, C.S. Non-Alcoholic Fatty Liver Disease Treatment in Patients with Type 2 Diabetes Mellitus; New Kids on the Block. Curr. Vasc. Pharmacol. 2020, 18, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Raj, H.; Durgia, H.; Palui, R.; Kamalanathan, S.; Selvarajan, S.; Kar, S.S.; Sahoo, J. SGLT-2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: A systematic review. World J. Diabetes 2019, 10, 114–132. [Google Scholar] [CrossRef]
- Xing, B.; Zhao, Y.; Dong, B.; Zhou, Y.; Lv, W.; Zhao, W. Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. J. Diabetes Investig. 2020, 11, 1238–1247. [Google Scholar] [CrossRef]
- Coelho, F.D.S.; Borges-Canha, M.; von Hafe, M.; Neves, J.S.; Vale, C.; Leite, A.R.; Carvalho, D.; Leite-Moreira, A. Effects of sodium-glucose co-transporter 2 inhibitors on liver parameters and steatosis: A meta-analysis of randomized clinical trials. Diabetes Metab. Res. Rev. 2020, e3413. [Google Scholar] [CrossRef] [PubMed]
- Chehrehgosha, H.; Sohrabi, M.R.; Ismail-Beigi, F.; Malek, M.; Reza Babaei, M.; Zamani, F.; Ajdarkosh, H.; Khoonsari, M.; Fallah, A.E.; Khamseh, M.E. Empagliflozin Improves Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Ther. 2021, 12, 843–861. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Shimizu, S.; Inoue, K.; Saito, D.; Yanagisawa, M.; Inukai, K.; Akiyama, Y.; Morimoto, Y.; Noda, M.; Shimada, A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care 2017, 40, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes. Metab. 2018, 20, 438–442. [Google Scholar] [CrossRef]
- Muthiah, M.D.; Sanyal, A.J. Current management of non-alcoholic steatohepatitis. Liver Int. 2020, 40, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo, C.M.; Garcia-Fernandez, N.; Escalada, J. Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate? J. Clin. Med. 2021, 10, 2040. https://doi.org/10.3390/jcm10092040
Perdomo CM, Garcia-Fernandez N, Escalada J. Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate? Journal of Clinical Medicine. 2021; 10(9):2040. https://doi.org/10.3390/jcm10092040
Chicago/Turabian StylePerdomo, Carolina M., Nuria Garcia-Fernandez, and Javier Escalada. 2021. "Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate?" Journal of Clinical Medicine 10, no. 9: 2040. https://doi.org/10.3390/jcm10092040
APA StylePerdomo, C. M., Garcia-Fernandez, N., & Escalada, J. (2021). Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate? Journal of Clinical Medicine, 10(9), 2040. https://doi.org/10.3390/jcm10092040





