The Uterocervical Angle Combined with Bishop Score as a Predictor for Successful Induction of Labor in Term Vaginal Delivery
Abstract
1. Introduction
2. Materials and Methods
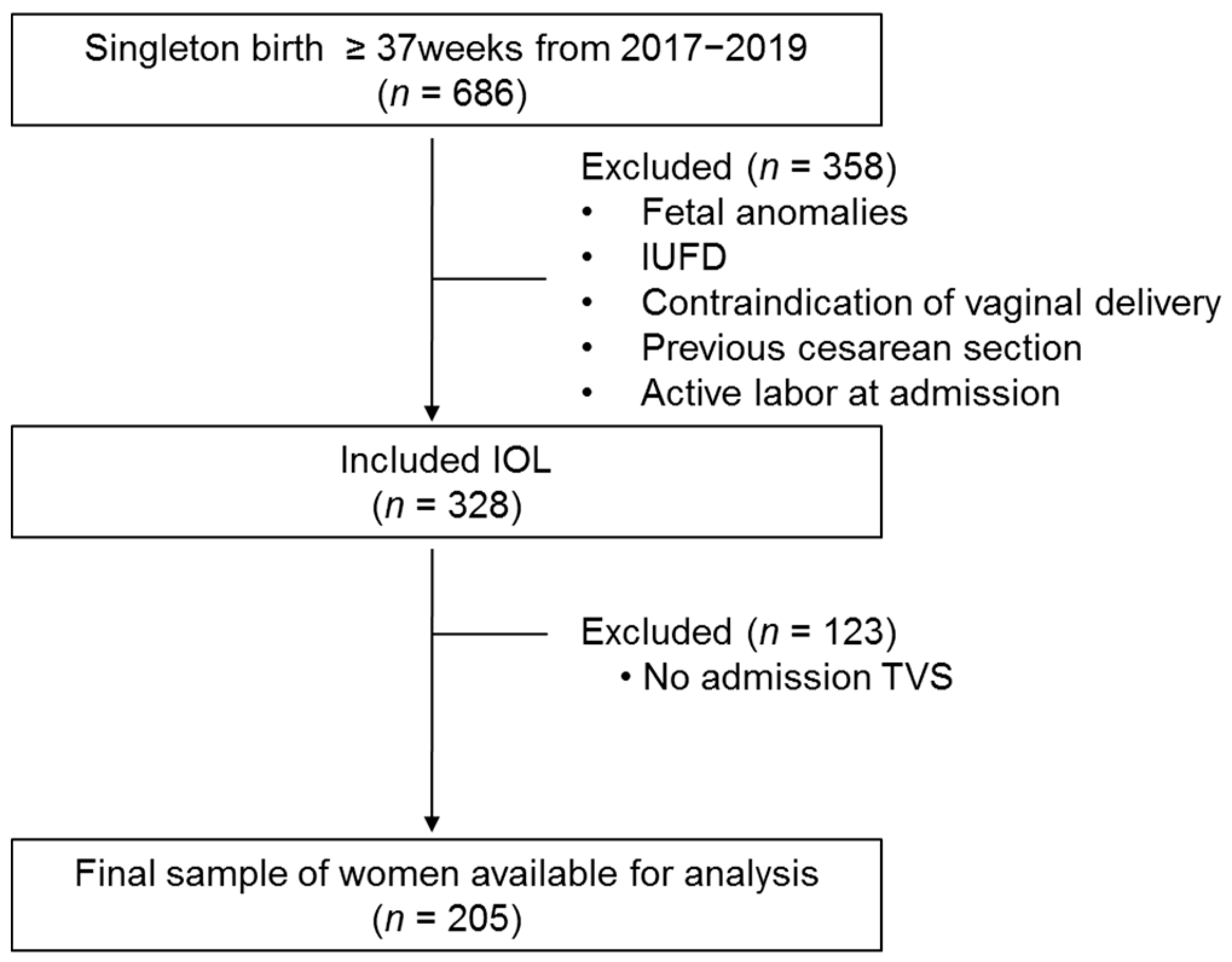
2.1. Patients
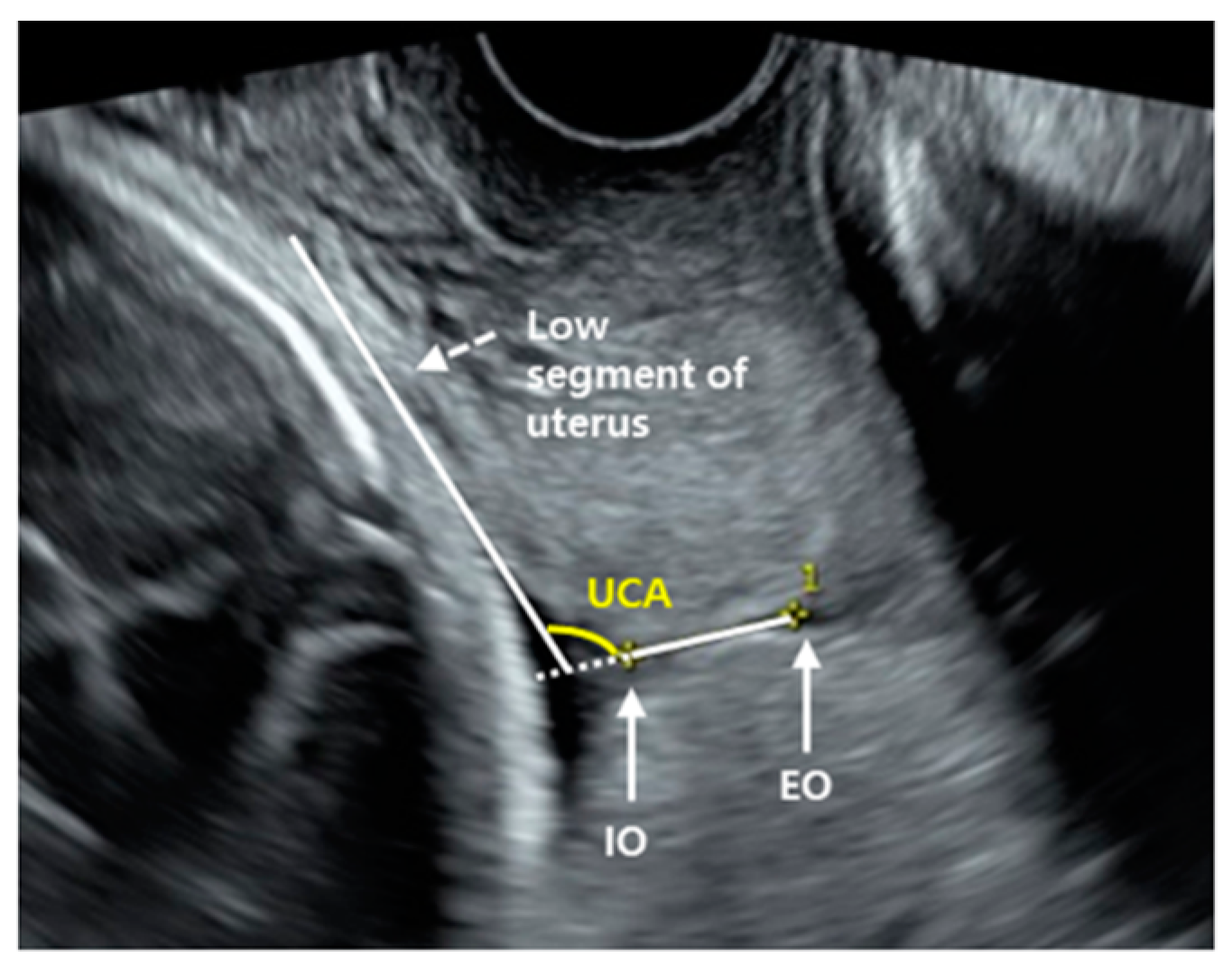
2.2. Uterocervical Angle Measurement
2.3. Labor Induction
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marconi, A.M. Recent advances in the induction of labor. F1000Research 2019, 8, 1829. [Google Scholar] [CrossRef] [PubMed]
- Getahun, D. Epidemiologic Considerations. Clin. Obstet. Gynecol. 2014, 57, 326–330. [Google Scholar] [CrossRef][Green Version]
- alaulikar, V.S.; Arulkumaran, S. Failed Induction of Labor: Strategies to Improve the Success Rates. Obstet. Gynecol. Surv. 2011, 66, 717–728. [Google Scholar] [CrossRef]
- Giugliano, E.; Cagnazzo, E.; Milillo, V.; Moscarini, M.; Vesce, F.; Caserta, D.; Marci, R. The Risk Factors for Failure of Labor Induction: A Cohort Study. J. Obstet. Gynecol. India 2013, 64, 111–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cammu, H.H.; Martens, G.; Ruyssinck, G.G.; Amy, J.-J. Outcome after elective labor induction in nulliparous women: A matched cohort study. Am. J. Obstet. Gynecol. 2002, 186, 240–244. [Google Scholar] [CrossRef]
- Baños, N.; Migliorelli, F.; Posadas, E.; Ferreri, J.; Palacio, M. Definition of Failed Induction of Labor and Its Predictive Factors: Two Unsolved Issues of an Everyday Clinical Situation. Fetal Diagn. Ther. 2015, 38, 161–169. [Google Scholar] [CrossRef]
- Dagdeviren, E.; Çetin, B.A.; Mathyk, B.A.; Koroglu, N.; Topcu, E.G.; Yuksel, M.A. Can uterocervical angles successfully predict induction of labor in nulliparous women? Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 87–91. [Google Scholar] [CrossRef]
- ACOG Committee on Practice Bulletins—Obstetrics ACOG Practice Bulletin No. 107: Induction of Labor. Obstet. Gynecol. 2009, 114, 386–397. [CrossRef]
- Parkes, I.; Kabiri, D.; Hants, Y.; Ezra, Y. The indication for induction of labor impacts the risk of cesarean delivery. J. Matern. Neonatal Med. 2014, 29, 224–228. [Google Scholar] [CrossRef]
- Crane, J.M.G. Factors Predicting Labor Induction Success: A Critical Analysis. Clin. Obstet. Gynecol. 2006, 49, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Pevzner, L.; Rayburn, W.F.; Rumney, P.; Wing, D.A. Factors Predicting Successful Labor Induction with Dinoprostone and Misoprostol Vaginal Inserts. Obstet. Gynecol. 2009, 114, 261–267. [Google Scholar] [CrossRef]
- Hwang, H.S.; Sohn, I.S.; Kwon, H.S. Imaging Analysis of Cervical Elastography for Prediction of Successful Induction of Labor at Term. J. Ultrasound Med. 2013, 32, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, D.G.E.; Brinkhorst, S.J.; Van Der Post, J.A.M.; Pajkrt, E.; Opmeer, B.C.; Mol, B.W.J.; Verhoeven, C.J.M. The Bishop Score as a Predictor of Labor Induction Success: A Systematic Review. Am. J. Perinatol. 2013, 30, 625–630. [Google Scholar] [CrossRef]
- Papillon-Smith, J.; Abenhaim, H.A. The role of sonographic cervical length in labor induction at term. J. Clin. Ultrasound 2014, 43, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Faltin-Traub, E.F.; Boulvain, M.; Faltin, D.L.; Extermann, P.; Irion, O. Reliability of the Bishop score before labour induction at term. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 178–181. [Google Scholar] [CrossRef]
- Paterson-Brown, S.; Fisk, N.; Edmonds, D.; Rodeck, C. Preinduction cervical assessment by Bishop’s score and transvaginal ultrasound. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 40, 17–23. [Google Scholar] [CrossRef]
- Dückelmann, A.M.; Bamberg, C.; Michaelis, S.A.M.; Lange, J.; Nonnenmacher, A.; Dudenhausen, J.W.; Kalache, K.D. Measurement of fetal head descent using the ‘angle of progression’ on transperineal ultrasound imaging is reliable regardless of fetal head station or ultrasound expertise. Ultrasound Obstet. Gynecol. 2010, 35, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gokturk, U.; Cavkaytar, S.; Danısman, N. Can measurement of cervical length, fetal head position and posterior cervical angle be an alternative method to Bishop score in the prediction of successful labor induction? J. Matern. Neonatal Med. 2015, 28, 1360–1365. [Google Scholar] [CrossRef]
- Dziadosz, M.; Bennett, T.-A.; Dolin, C.; Honart, A.W.; Pham, A.; Lee, S.S.; Pivo, S.; Roman, A.S. Uterocervical angle: A novel ultrasound screening tool to predict spontaneous preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 376.e1–376.e7. [Google Scholar] [CrossRef] [PubMed]
- Szlachetka, K.; Seligman, N.S.; Lynch, T.A. Ultrasonographic Change in Uterocervical Angle is not a Risk Factor for Preterm Birth in Women with a Short Cervix. Am. J. Perinatol. 2017, 34, 1058–1064. [Google Scholar] [CrossRef]
- Keepanasseril, A.; Suri, V.; Bagga, R.; Aggarwal, N. A new objective scoring system for the prediction of successful induction of labour. J. Obstet. Gynaecol. 2012, 32, 145–147. [Google Scholar] [CrossRef]
- Kehila, M.; Abouda, H.; Sahbi, K.; Cheour, H.; Chanoufi, M.B. Ultrasound cervical length measurement in prediction of labor induction outcome. J. Neonatal-Perinatal Med. 2016, 9, 127–131. [Google Scholar] [CrossRef]
- Eser, A.; Ozkaya, E. Uterocervical angle: An ultrasound screening tool to predict satisfactory response to labor induction. J. Matern. Neonatal Med. 2018, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pruksanusak, N.; Sawaddisan, R.; Kor-Anantakul, O.; Suntharasaj, T.; Suwanrath, C.; Geater, A. Comparison of reliability between uterocervical angle and cervical length measurements by various experienced operators using transvaginal ultrasound. J. Matern. Neonatal Med. 2018, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H. Transvaginal Ultrasonographic Cervical Measurement in Predicting Failed Labor Induction and Cesarean Delivery for Failure to Progress in Nulliparous Women. J. Korean Med. Sci. 2007, 22, 722–727. [Google Scholar] [CrossRef]
- Park, K.H.; Hong, J.-S.; Shin, D.M.; Kang, W.S. Prediction of failed labor induction in parous women at term: Role of previous obstetric history, digital examination and sonographic measurement of cervical length. J. Obstet. Gynaecol. Res. 2009, 35, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, A.S.; Sanchez-Ramos, L.; Kaunitz, A.M. Sonographic cervical assessment to predict the success of labor induction: A systematic review with metaanalysis. Am. J. Obstet. Gynecol. 2007, 197, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, G.; Thomakos, N.; Hatziioannou, L.; Mesogitis, S.; Papantoniou, N.; Antsaklis, A. Sonographic Cervical Length Measurement before Labor Induction in Term Nulliparous Women. Fetal Diagn. Ther. 2005, 21, 34–38. [Google Scholar] [CrossRef]
- Elghorori, M.R.M.; Hassan, I.; Dartey, W.; Abdel-Aziz, E. A way to lend objectivity to Bishop score. J. Obstet. Gynaecol. 2006, 26, 311–316. [Google Scholar] [CrossRef]
- Bueno, B.; San-Frutos, L.; Salazar, F.; Pérez-Medina, T.; Engels, V.; Archilla, B.; Izquierdo, F.; Bajo, J. Variables that predict the success of labor induction. Acta Obstet. Gynecol. Scand. 2005, 84, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.A.; Mathyk, B.A.; Tuten, A.; Bahat, P.Y.; Koroglu, N.; Topcu, E.G. The predictive nature of uterocervical angles in the termination of second trimester pregnancy. J. Matern. Neonatal Med. 2018, 32, 1952–1957. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 205) | Non-Success (n = 65) | Success (n = 140) | p | |
|---|---|---|---|---|---|
| Parity (n, %) | Nulliparous | 125 (61.0) | |||
| Multiparous | 80 (39.0) | ||||
| Maternal age (years) | 32 (24–42) | 33 (27–41) | 32 (24–42) | 0.278 | |
| BMI (kg/m2) | 26 (15–32) | 26 (15–31) | 25 (20–32) | 0.643 | |
| Gestational age at delivery (weeks) | 39 (36–41) | 39 (37–41) | 39 (36–41) | 0.834 | |
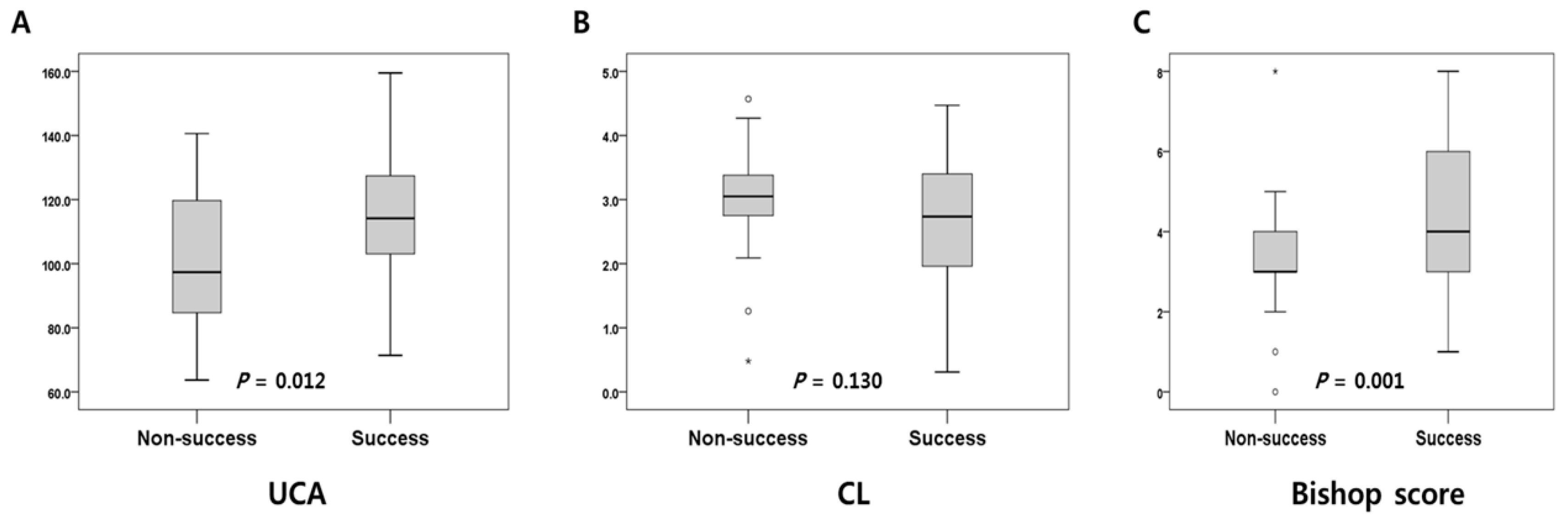
| Bishop score | 4 (0–8) | 3 (0–8) | 4 (1–8) | 0.001 | |
| Cervical length (cm) | 2.87 (0.31–4.57) | 3.05 (0.48–4.57) | 2.73 (0.31–4.47) | 0.130 | |
| UCA (degree) | 112.5 (63.7–159.5) | 97.3 (63.7–140.6) | 114.1 (71.4–159.5) | 0.012 | |
| Cesarean delivery (n, %) | 30 (14.6) | 20 (30.8) | 10 (7.1) | 0.001 | |
| Indication of induction | |||||
| At ≥39 weeks elective induction | 118 (52.6) | 30 (25.4) | 88 (74.6) | ||
| Pregnancy-induced hypertension | 18 (2.9) | 7 (38.9) | 11 (61.1) | ||
| Diabetes | 24 (6.8) | 11 (45.8) | 13 (54.2) | ||
| Fetal macrosomia | 9 (3.9) | 5 (55.6) | 4 (44.4) | ||
| Fetal growth restriction | 15 (8.7) | 5 (33.3) | 10 (66.7) | ||
| Oligohydramnios | 12 (5.8) | 5 (41.7) | 7 (58.3) | ||
| Others | 9 (4.3) | 2 (22.2) | 7 (77.8) | ||
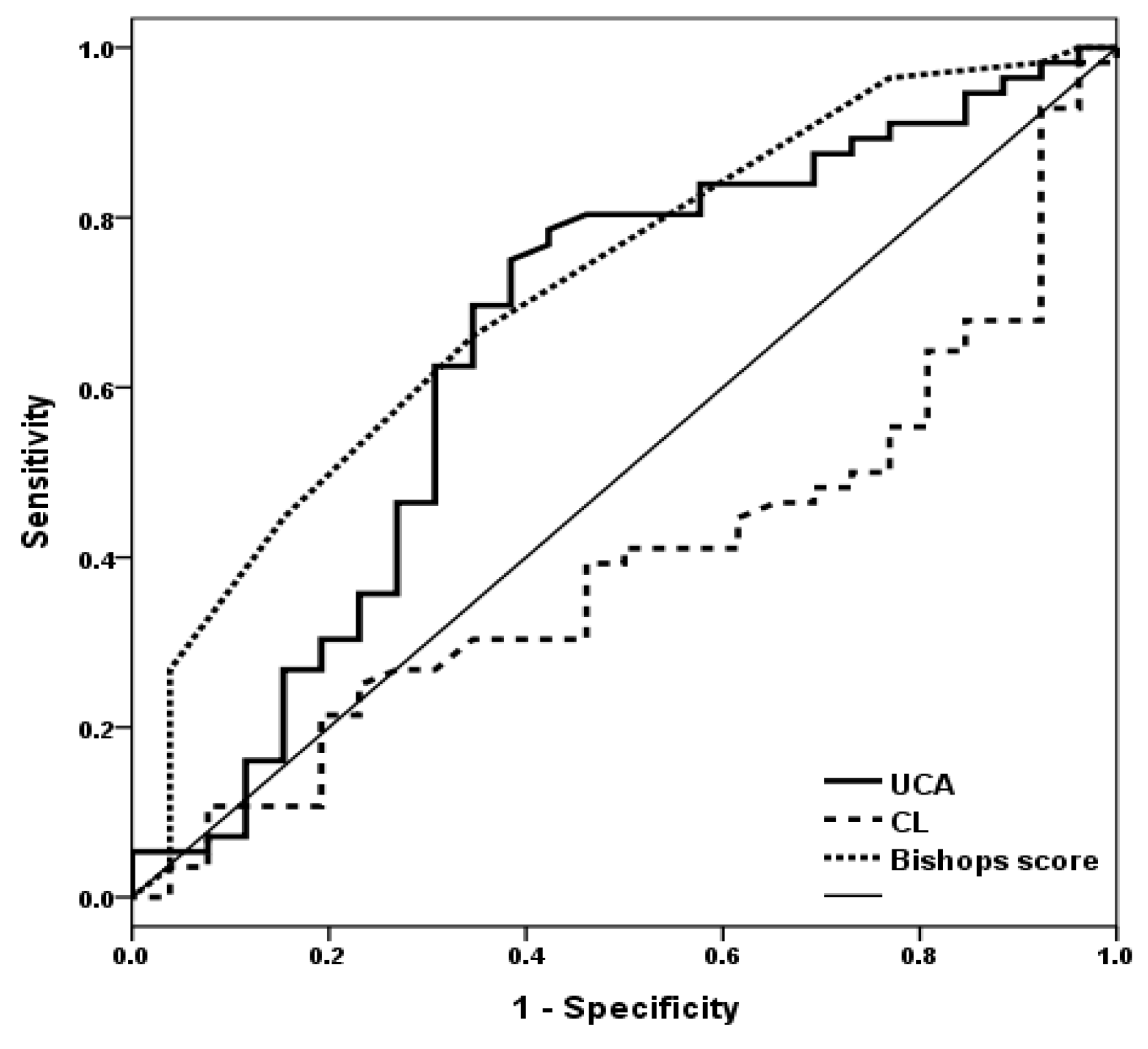
| UCA | Bishop Score | Combined (AND) | Combined (OR) | |
|---|---|---|---|---|
| Sensitivity (%) | 69.6 | 60.7 | 44.6 | 85.7 |
| Specificity (%) | 65.2 | 76.9 | 96.0 | 50.0 |
| PPV (%) | 81.4 | 85.0 | 96.2 | 78.7 |
| NPV (%) | 46.2 | 47.6 | 43.6 | 61.9 |
| OR (95% CI) | 4.333 (1.612–11.645) | 5.152 (1.788–14.843) | 10.667 (2.446–28.410) | 6.000 (2.052–17.544) |
| p-value | 0.004 | 0.002 | <0.001 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-W.; Kim, S.-Y.; Hwang, H.-S.; Kim, H.-S.; Sohn, I.-S.; Kwon, H.-S. The Uterocervical Angle Combined with Bishop Score as a Predictor for Successful Induction of Labor in Term Vaginal Delivery. J. Clin. Med. 2021, 10, 2033. https://doi.org/10.3390/jcm10092033
Yang S-W, Kim S-Y, Hwang H-S, Kim H-S, Sohn I-S, Kwon H-S. The Uterocervical Angle Combined with Bishop Score as a Predictor for Successful Induction of Labor in Term Vaginal Delivery. Journal of Clinical Medicine. 2021; 10(9):2033. https://doi.org/10.3390/jcm10092033
Chicago/Turabian StyleYang, Seung-Woo, Seo-Yeon Kim, Han-Sung Hwang, Hee-Sun Kim, In-Sook Sohn, and Han-Sung Kwon. 2021. "The Uterocervical Angle Combined with Bishop Score as a Predictor for Successful Induction of Labor in Term Vaginal Delivery" Journal of Clinical Medicine 10, no. 9: 2033. https://doi.org/10.3390/jcm10092033
APA StyleYang, S.-W., Kim, S.-Y., Hwang, H.-S., Kim, H.-S., Sohn, I.-S., & Kwon, H.-S. (2021). The Uterocervical Angle Combined with Bishop Score as a Predictor for Successful Induction of Labor in Term Vaginal Delivery. Journal of Clinical Medicine, 10(9), 2033. https://doi.org/10.3390/jcm10092033






