Fetal Growth Restriction and Subsequent Low Grade Fetal Inflammatory Response Are Associated with Early-Onset Neonatal Sepsis in the Context of Early Preterm Sterile Intrauterine Environment
Abstract
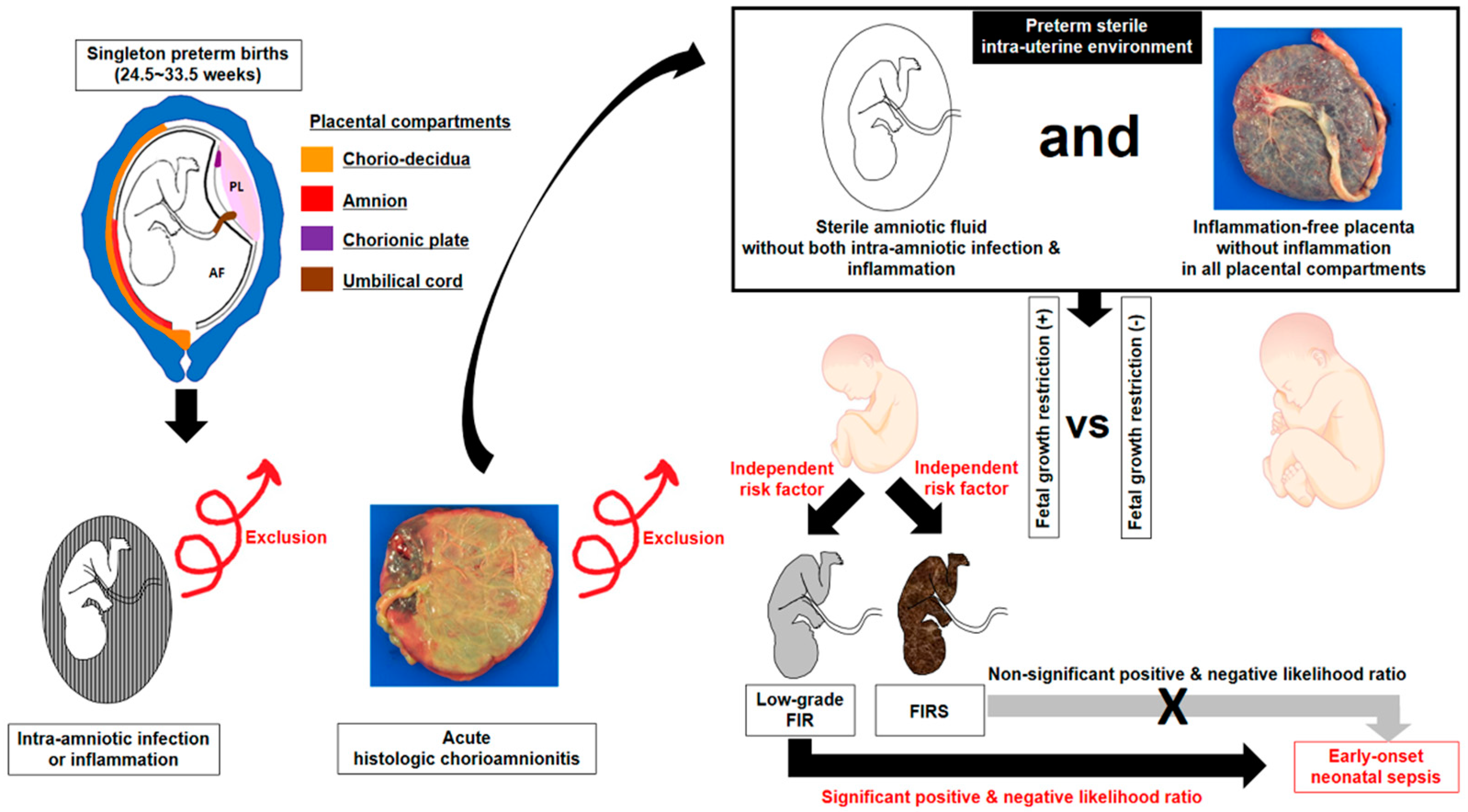
1. Introduction
2. Materials and Methods
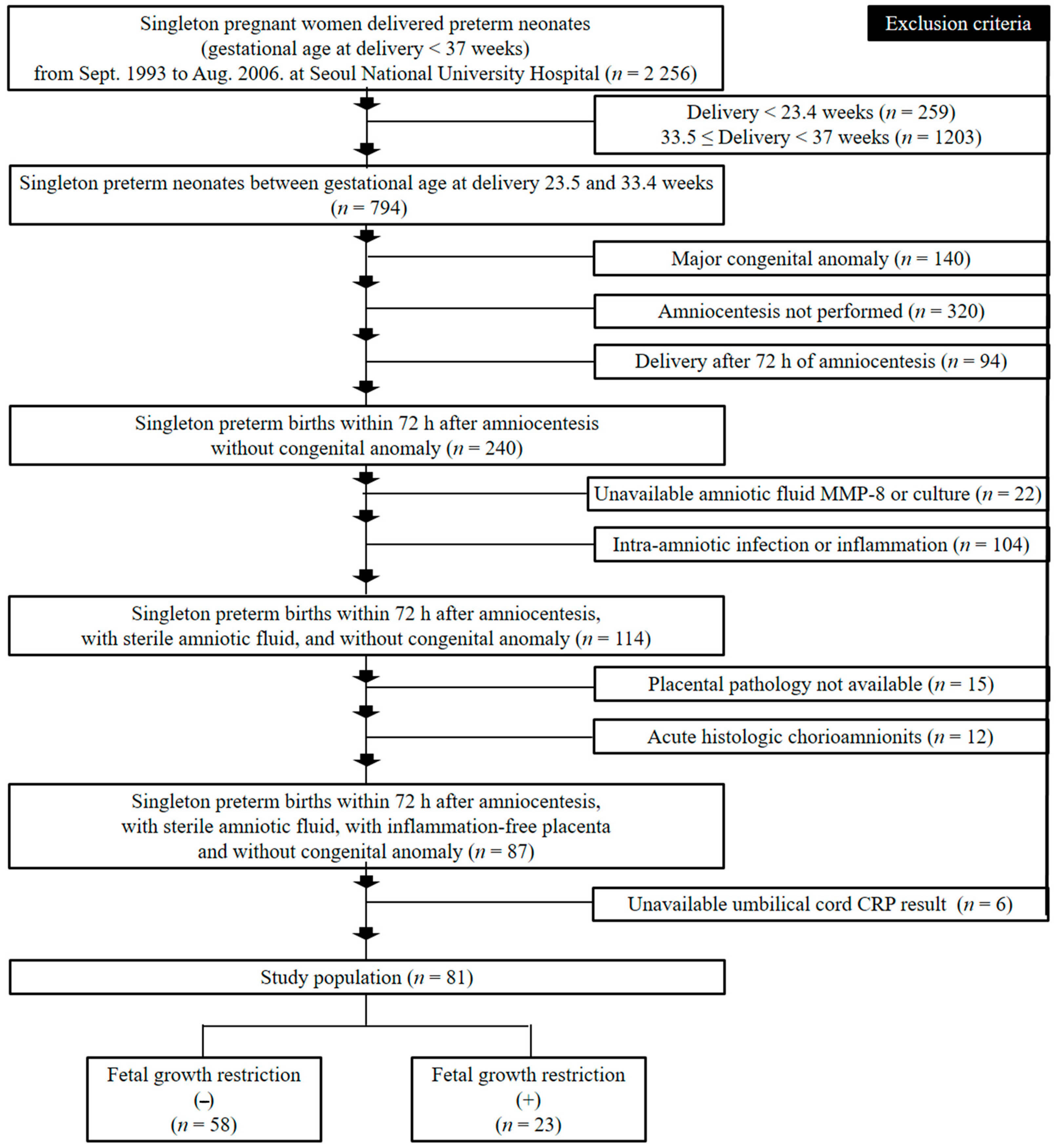
2.1. Study Design and Patient Population
2.2. Clinical Characteristics, Fetal Growth Restriction (FGR) and Early Onset Neonatal Sepsis (EONS)
2.3. Placental Pathologic Examination
2.4. Amniotic Fluid (AF) Infection and Inflammation
2.5. Fetal Inflammatory Response (FIR), Low-Grade FIR and Fetal Inflammatory Response Syndrome (FIRS)
2.6. Statistical Analysis
3. Results
3.1. Clinical and Pregnant Information According to the Presence or Absence of Fetal Growth Restriction (FGR) and Early Onset Neonatal Sepsis (EONS)
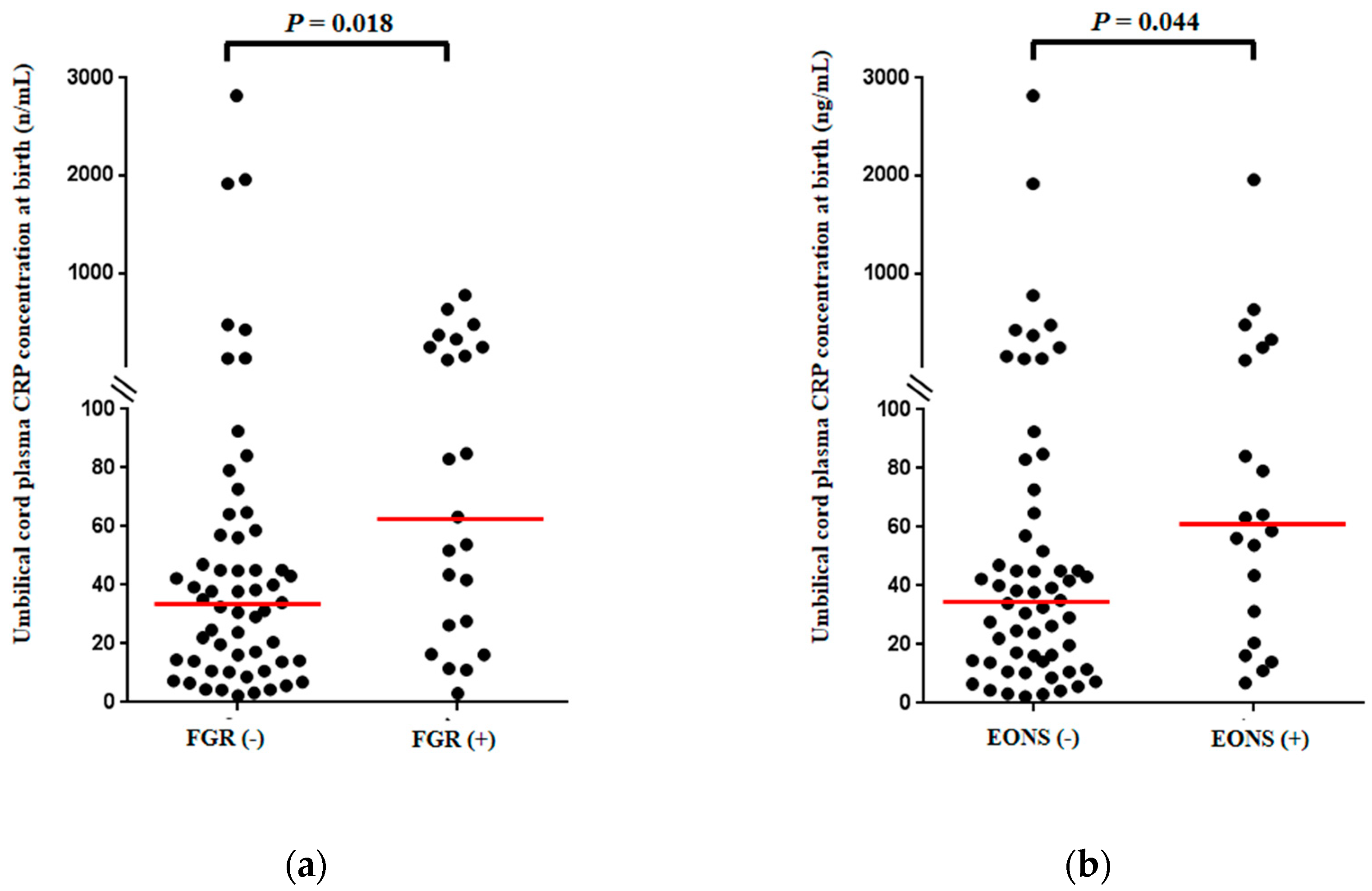
3.2. Umbilical Cord Plasma (UCP) CRP Concentrations at Birth According to the Presence or Absence of Fetal Growth Restriction (FGR) and Early Onset Neonatal Sepsis (EONS)
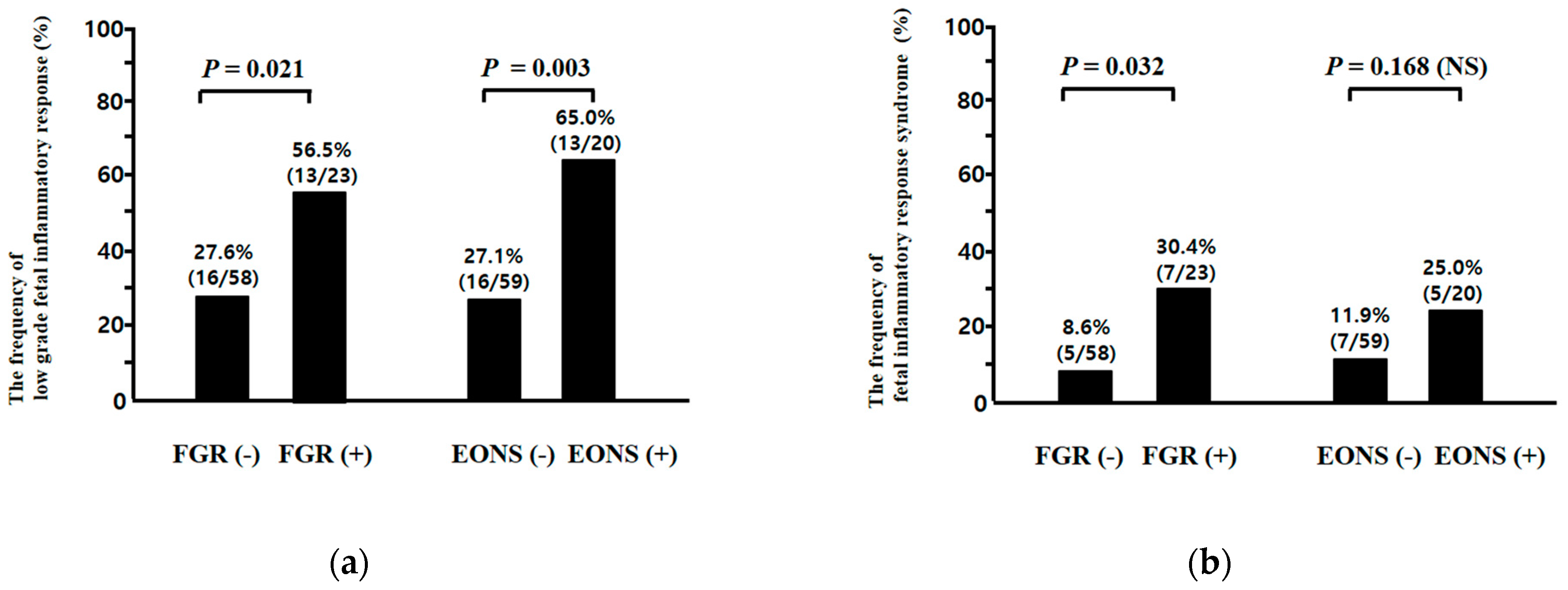
3.3. Low-Grade Fetal Inflammatory Response (FIR) and Fetal Inflammatory Response Syndrome (FIRS) According to the Presence or Absence of Fetal Growth Restriction (FGR) and Early-Onset Neonatal Sepsis (EONS)
3.4. Clinical and Pregnant Information According to the Presence or Absence of Low-Grade Fetal Inflammatory Response (FIR) and Fetal Inflammatory Response Syndrome (FIRS)
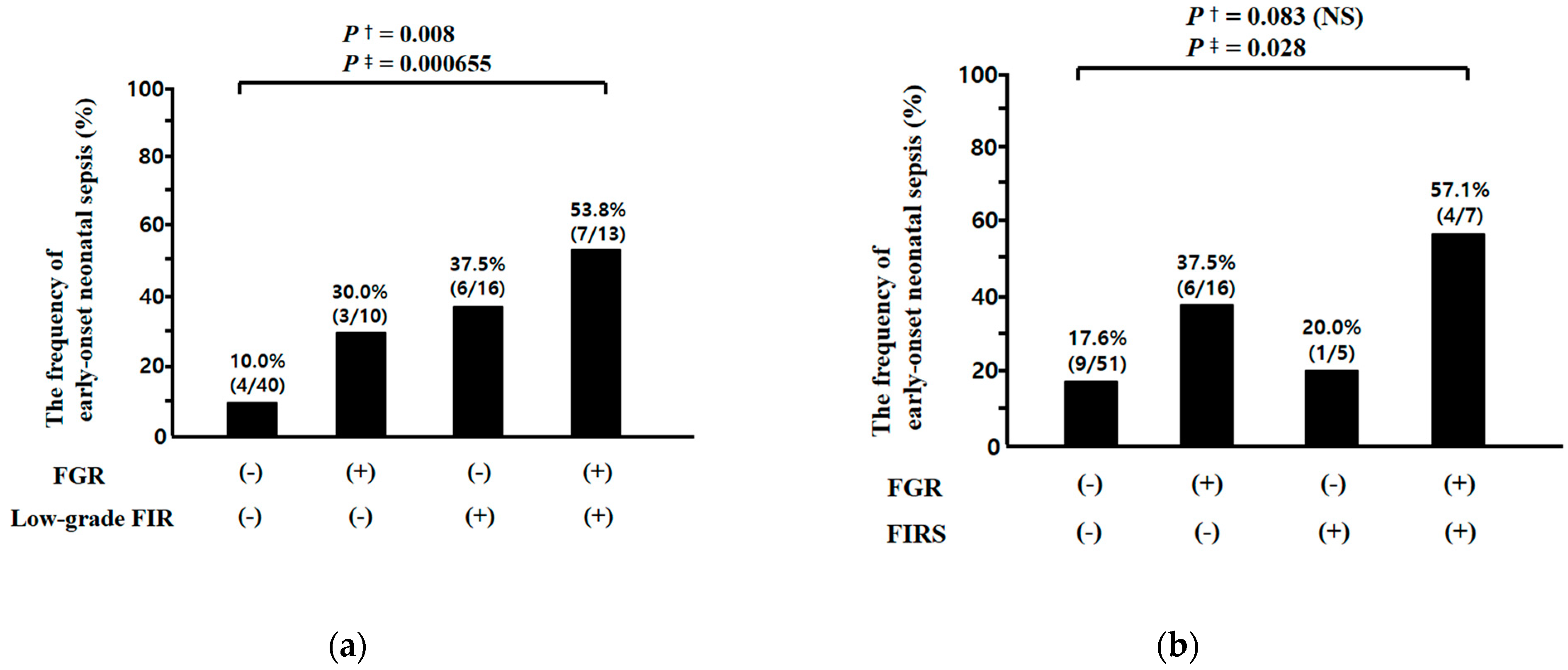
3.5. Relationships between Fetal Growth Restriction (FGR) and Fetal Inflammatory Response (FIR) (i.e., Low Grade FIR and Fetal Inflammatory Response Syndrome (FIRS)), and between FIR and Early-Onset Neonatal Sepsis (EONS) in Preterm Sterile Intrauterine Environment
3.6. Relationship between Umbilical Cord Plasma (UCP) CRP Concentrations at Birth and Birth Weight (BW) Percentile for Gestational Age (GA) at Delivery
4. Discussion
4.1. Principal Findings
4.2. The Pathophysiology of Fetal Inflammatory Response (FIR) Associated with Fetal Growth Restriction (FGR) in the Context of Preterm Sterile Intrauterine Environment
4.3. Relationship between Chronic Hypoxemia Absent Bacterial Infection and Fetal Inflammatory Response Syndrome (FIRS) in Animal Model
4.4. Low-Grade Fetal Inflammatory Response (FIR) and Fetal Growth Restriction (FGR) in the Context of Preterm Sterile Intrauterine Environment
4.5. Low-Grade Fetal Inflammatory Response (FIR) for the Identification of Early Onset Neonatal Sepsis (EONS) in the Context of Preterm Sterile Intrauterine Environment
4.6. The Value for the Diagnosis of Intra-Amniotic Infection and Amniotic Fluid (AF) Inflammation by Amniocentesis to Diagnose Either Fetal Infection/Fetal Inflammatory Response Syndrome (FIRS)
4.7. Major Strengths and Weakness of This Study
4.8. Clinical Implication
4.9. Further Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bamfo, J.E.; Odibo, A.O. Diagnosis and management of fetal growth restriction. J. Pregnancy 2011, 2011, 640715. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G.F.; Leveno, K.J.; Bloom, S.L.; Spong, C.Y.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Sheffield, J.S. Fetal-growth disorders. In Williams Obstetrics; Cunningham, G.F., Leveno, K.J., Bloom, S.L., Spong, C.Y., Dashe, J.S., Hoffman, B.L., Casey, B.M., Sheffield, J.S., Eds.; McGraw-Hill Education Inc.: New York, NY, USA, 2014; p. 883. [Google Scholar]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int. J. Pediatr. 2010, 2010, 401323. [Google Scholar] [CrossRef] [PubMed]
- Krishna, U.; Bhalerao, S. Placental insufficiency and fetal growth restriction. J. Obstet. Gynaecol. India 2011, 61, 505–511. [Google Scholar] [CrossRef]
- Baschat, A.A. Fetal responses to placental insufficiency: An update. BJOG 2004, 111, 1031–1041. [Google Scholar] [CrossRef]
- Dong, Y.; Hou, W.; Wei, J.; Weiner, C.P. Chronic hypoxemia absent bacterial infection is one cause of the fetal inflammatory response syndrome (FIRS). Reprod. Sci. 2009, 16, 650–656. [Google Scholar] [CrossRef]
- Guo, R.; Hou, W.; Dong, Y.; Yu, Z.; Stites, J.; Weiner, C.P. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod. Sci. 2010, 17, 540–548. [Google Scholar] [CrossRef]
- Tapanainen, P.J.; Bang, P.; Wilson, K.; Unterman, T.G.; Vreman, H.J.; Rosenfeld, R.G. Maternal hypoxia as a model for intrauterine growth retardation: Effects on insulin-like growth factors and their binding proteins. Pediatr. Res. 1994, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, T.; Zhang, X.; Li, W. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones 2010, 15, 335–342. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, Z.; Sun, Y.; Zhou, H.; Stites, J.; Katherine, N.; Weiner, C.P. Chronic fetal hypoxia produces selective brain injury associated with altered nitric oxide synthases. Am. J. Obstet. Gynecol. 2011, 204, e16–e28. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.C.; Liu, H.; Pinkas, G.A.; Thompson, L.P. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr. Res. 2012, 71, 25–31. [Google Scholar] [CrossRef]
- Li, W.; Zhong, X.; Zhang, L.; Wang, Y.; Wang, T. Heat shock protein 70 expression is increased in the liver of neonatal intrauterine growth retardation piglets. Asian Australas. J. Anim. Sci. 2012, 25, 1096–1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figueroa, H.; Cifuentes, J.; Lozano, M.; Alvarado, C.; Cabezas, C.; Eixarch, E.; Fernández, E.; Contreras, L.; Illanes, S.E.; Hernández-Andrade, E.; et al. Nitric oxide synthase and changes in oxidative stress levels in embryonic kidney observed in a rabbit model of intrauterine growth restriction. Prenat. Diagn. 2016, 36, 628–635. [Google Scholar] [CrossRef]
- Schiff, E.; Friedman, S.A.; Baumann, P.; Sibai, B.M.; Romero, R. Tumor necrosis factor-alpha in pregnancies associated with preeclampsia or small-for-gestational-age newborns. Am. J. Obstet. Gynecol. 1994, 170, 1224–1229. [Google Scholar] [CrossRef]
- Street, M.E.; Seghini, P.; Fieni, S.; Ziveri, M.A.; Volta, C.; Martorana, D.; Viani, I.; Gramellini, D.; Bernasconi, S. Changes in interleukin-6 and IGF system and their relationships in placenta and cord blood in newborns with fetal growth restriction compared with controls. Eur. J. Endocrinol. 2006, 155, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Trevisanuto, D.; Doglioni, N.; Altinier, S.; Zaninotto, M.; Plebani, M.; Zanardo, V. High-sensitivity C-reactive protein in umbilical cord of small-for-gestational-age neonates. Neonatology 2007, 91, 186–189. [Google Scholar] [CrossRef]
- Boutsikou, T.; Mastorakos, G.; Kyriakakou, M.; Margeli, A.; Hassiakos, D.; Papassotiriou, I.; Kanaka-Gantenbein, C.; Malamitsi-Puchner, A. Circulating levels of inflammatory markers in intrauterine growth restriction. Mediat. Inflamm. 2010, 2010, 790605. [Google Scholar] [CrossRef]
- Amarilyo, G.; Oren, A.; Mimouni, F.B.; Ochshorn, Y.; Deutsch, V.; Mandel, D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J. Perinatol. 2011, 31, 30–32. [Google Scholar] [CrossRef]
- Visentin, S.; Lapolla, A.; Londero, A.P.; Cosma, C.; Dalfrà, M.; Camerin, M.; Faggian, D.; Plebani, M.; Cosmi, E. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. BioMed Res. Int. 2014, 2014, 401595. [Google Scholar] [CrossRef]
- Gomez, R.; Romero, R.; Ghezzi, F.; Yoon, B.H.; Mazor, M.; Berry, S.M. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 1998, 179, 194–202. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Shim, J.Y.; Shim, S.S.; Kim, C.J.; Jun, J.K. C-reactive protein in umbilical cord blood: A simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J. Matern. Fetal Neonatal Med. 2003, 14, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, F.; Romero, R.; Kusanovic, J.P.; Mazaki-Tovi, S.; Pineles, B.L.; Erez, O.; Espinoza, J.; Hassan, S.S. The fetal inflammatory response syndrome. Clin. Obstet. Gynecol. 2007, 50, 652–683. [Google Scholar] [CrossRef]
- Savasan, Z.A.; Chaiworapongsa, T.; Romero, R.; Hussein, Y.; Kusanovic, J.P.; Xu, Y.; Dong, Z.; Kim, C.J.; Hassan, S.S. Interleukin-19 in fetal systemic inflammation. J. Matern. Fetal Neonatal Med. 2012, 25, 995–1005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.E.; Romero, R.; Jung, H.; Park, C.W.; Park, J.S.; Yoon, B.H. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am. J. Obstet. Gynecol. 2007, 197, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K.; Romero, R.; Yoon, B.H. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: Clinical implications. Placenta 2009, 30, 56–61. [Google Scholar] [CrossRef]
- Park, C.W.; Yoon, B.H.; Park, J.S.; Jun, J.K. A fetal and an intra-amniotic inflammatory response is more severe in preterm labor than in preterm prom in the context of funisitis: Unexpected observation in human gestations. PLoS ONE 2013, 8, e62521. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Yoon, B.H.; Park, J.S.; Jun, J.K. An elevated maternal serum C-reactive protein in the context of intra-amniotic inflammation is an indicator that the development of amnionitis, an intense fetal and af inflammatory response are likely in patients with preterm labor: Clinical implications. J. Matern. Fetal Neonatal Med. 2013, 26, 847–853. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, S.M.; Park, J.S.; Jun, J.K.; Yoon, B.H. Fetal, amniotic and maternal inflammatory responses in early stage of ascending intrauterine infection, inflammation restricted to chorio-decidua, in preterm gestation. J. Matern. Fetal Neonatal Med. 2014, 27, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Park, J.S.; Moon, K.C.; Jun, J.K.; Yoon, B.H. Preterm labor and preterm premature rupture of membranes have a different pattern in the involved compartments of acute histologoic chorioamnionitis and/or funisitis: Patho-physiologic implication related to different clinical manifestations. Pathol. Int. 2016, 66, 325–332. [Google Scholar] [CrossRef]
- Oh, J.W.; Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PLoS ONE 2019, 14, e0225328. [Google Scholar] [CrossRef]
- Oh, J.W.; Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K. Fetal inflammatory response is positively correlated with the progress of inflammation in chorionic plate. Placenta 2020, 97, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Hofer, N.; Kothari, R.; Morris, N.; Müller, W.; Resch, B. The fetal inflammatory response syndrome is a risk factor for morbidity in preterm neonates. Am. J. Obstet. Gynecol. 2013, 209, e1–e542. [Google Scholar] [CrossRef]
- Sursal, T.; Stearns-Kurosawa, D.J.; Itagaki, K.; Oh, S.; Sun, S.; Kurosawa, S.; Hauser, C.J. Plasma bacterial and mito-chondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify in-flammatory tissue injury in nonhuman primates. Shock 2013, 39, 55–62. [Google Scholar] [CrossRef]
- Kaplan, L.J. Systemic Inflammatory Response Syndrome. Medscape, eMedicine. 2019. Available online: http://www.emedicine.medscape.com/article/168943-overview (accessed on 13 May 2019).
- Horeczko, T.; Green, J.P.; Panacek, E.A. Epidemiology of the systemic inflammatory response syndrome (SIRS) in the emergency department. West. J. Emerg. Med. 2014, 15, 329–336. [Google Scholar] [CrossRef]
- Yoon, B.H.; Yang, S.H.; Jun, J.K.; Park, K.H.; Kim, C.J.; Romero, R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: A comparison with amniotic fluid white blood cell count. Obstet. Gynecol. 1996, 87, 231–237. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jun, J.K.; Park, K.H.; Syn, H.C.; Gomez, R.; Romero, R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet. Gynecol. 1996, 88, 1034–1340. [Google Scholar] [CrossRef]
- ACOG Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obste-tricians-gynecologists. Management of postterm pregnancy. Obstet. Gynecol. 2004, 104, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mikolajczyk, R.; Grewal, J.; Neta, G.; Klebanoff, M. Prenatal application of the individualized fetal growth reference. Am. J. Epidemiol. 2011, 173, 539–543. [Google Scholar] [CrossRef] [PubMed]
- McIntire, D.D.; Bloom, S.L.; Casey, B.M.; Leveno, K.J. Birth weight in relation to morbidity and mortality among new born infants. N. Engl. J. Med. 1999, 340, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Doubilet, P.M.; Benson, C.B.; Nadel, A.S.; Ringer, S.A. Improved birth weight table for neonates developed from ges-tations dated by early ultrasonography. J. Ultrasound Med. 1997, 16, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D. National institute of child health and human development neonatal research network. neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.H.; Syn, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Park, C.W.; Yoon, B.H.; Kim, S.M.; Park, J.S.; Jun, J.K. The frequency and clinical significance of intra-amniotic inflam-mation defined as an elevated amniotic fluid matrix metalloproteinase-8 in patients with preterm labor and low amniotic fluid white blood cell counts. Obstet. Gynecol. Sci. 2013, 56, 167–175. [Google Scholar] [CrossRef]
- Park, J.S.; Romero, R.; Yoon, B.H.; Moon, J.B.; Oh, S.Y.; Han, S.Y.; Ko, E.M. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am. J. Obstet. Gynecol. 2001, 185, 1156–1161. [Google Scholar] [CrossRef]
- Shim, S.S.; Romero, R.; Hong, J.S.; Park, C.W.; Jun, J.K.; Kim, B.I.; Yoon, B.H. Clinical significance of intra-amniotic in-flammation in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2004, 191, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Perrone, S.; Kenanidis, A.; Vezzosi, P.; Marzocchi, B.; Petraglia, F.; Centini, G.; Buonocore, G. Isoprostanes in amniotic fluid: A predictive marker for fetal growth restriction in pregnancy. Free Radic. Biol. Med. 2005, 38, 1537–1541. [Google Scholar] [CrossRef]
- Kamath, U.; Rao, G.; Kamath, S.U.; Rai, L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J. Clin. Biochem. 2006, 21, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hracsko, Z.; Safar, Z.; Orvos, H.; Novak, Z.; Pal, A.; Varga, I.S. Evaluation of oxidative stress markers after vaginal delivery or Caesarean section. In Vivo 2007, 21, 703–706. [Google Scholar]
- Mert, I.; Oruc, A.S.; Yuksel, S.; Cakar, E.S.; Buyukkagnici, U.; Karaer, A.; Danisman, N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012, 38, 658–664. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Karowicz-Bilinska, A.; Kedziora-Kornatowska, K.; Bartosz, G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic. Res. 2007, 41, 870–873. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Teles, A.; Zenclussen, A.C. Regulatory T cells and their role in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 445–459. [Google Scholar] [CrossRef]
- Petroff, M.G.; Perchellet, A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 506–519. [Google Scholar] [CrossRef]
- Lee, J.; Romero, R.; Chaiworapongsa, T.; Dong, Z.; Tarca, A.L.; Xu, Y.; Chiang, P.J.; Kusanovic, J.P.; Hassan, S.S.; Yeo, L.; et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: Evidence of a distinct and novel type of human fetal systemic inflammatory response. Am. J. Reprod. Immunol. 2013, 70, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Girardi, G.; Yarilin, D.; Thurman, J.M.; Holers, V.M.; Salmon, J.E. Complement activation induces dysregulation of an-giogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006, 203, 2165–2175. [Google Scholar] [CrossRef]
- Wood, C.E.; Keller-Wood, M. Current paradigms and new perspectives on fetal hypoxia: Implications for fetal brain development in late gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R1–R13. [Google Scholar] [CrossRef]
- Barker, D.J.P. Mothers, Babies, and Health in Later Life; Churchill Livingston: Edinburgh, UK, 1998. [Google Scholar]
- Davy, P.; Nagata, M.; Bullard, P.; Fogelson, N.S.; Allsopp, R. Fetal growth restriction is associated with accelerated te-lomere shortening and increased expression of cell senescence markers in the placenta. Placenta 2009, 30, 539–542. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated dis-eases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Sarkar, D.; Fisher, P.B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006, 236, 13–23. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Damodaram, M.; Story, L.; Kulinskaya, E.; Rutherford, M.; Kumar, S. Early adverse perinatal complications in preterm growth-restricted fetuses. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Engineer, N.; Kumar, S. Perinatal variables and neonatal outcomes in severely growth restricted preterm fetuses. Acta Obstet. Gynecol. Scand. 2010, 89, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Simchen, M.J.; Beiner, M.E.; Strauss-Liviathan, N.; Dulitzky, M.; Kuint, J.; Mashiach, S.; Schiff, E. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am. J. Perinatol. 2000, 17, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Romero, R.; Park, J.S.; Kim, M.; Oh, S.Y.; Kim, C.J.; Jun, J.K. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am. J. Obstet. Gynecol. 2000, 183, 1124–1129. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazor, M. Infection and preterm labor. Clin. Obstet. Gynecol. 1988, 31, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef]
- Pacora, P.; Chaiworapongsa, T.; Maymon, E.; Kim, Y.M.; Gomez, R.; Yoon, B.H.; Ghezzi, F.; Berry, S.M.; Qureshi, F.; Jacques, S.M.; et al. Funisitis and chorionic vasculitis: The histological counterpart of the fetal inflammatory response syndrome. J. Matern. Fetal Neonatal Med. 2002, 11, 18–25. [Google Scholar] [CrossRef]
| Primary Author (Year), [Reference Number] | Type of Animal | n | GA at Delivery (Weeks) | GA at Stimulation of Inducing FGR (Weeks) | Stimuli ofInducing FGR | Inflammatory Markers | Intensity of Inflammatory Markers: FGR vs. Control |
|---|---|---|---|---|---|---|---|
| Fetal blood | |||||||
| Dong Y (2009) [6] | Guinea pig | 24 | Near term | 71% of term | Hypoxia | IL-6 | FGR > Control |
| TNF-alpha | FGR > Control | ||||||
| Tapanainen PJ (1994) [8] | Rat | 12 | Term | 62% of term | Hypoxia | IGFBP-1 | FGR > Control |
| IGFBP-2 | FGR > Control | ||||||
| Fetal organs | |||||||
| Dong Y (2009) [6] | Guinea pig | 24 | Near term | 71% of term | Hypoxia | * Lung | |
| IL-6 (mRNA) | FGR > Control | ||||||
| * Heart | |||||||
| IL-6 (mRNA) | FGR > Control | ||||||
| * Brain | |||||||
| IL-6 (mRNA) | FGR > Control | ||||||
| * Liver | |||||||
| IL-6 (mRNA) | FGR = Control | ||||||
| Guo R (2010) [7] | Guinea pig | 12 | Near term | 71% of term | Hypoxia | * Brain (cDNA) | |
| TNF-alpha | FGR > Control | ||||||
| IL-1beta | FGR > Control | ||||||
| Zhong X (2010) [9] | Piglet | 10 | Term | N/A | N/A | * Intestine | |
| Hsp70 (protein) | FGR > Control | ||||||
| Hsp70 (mRNA) | FGR > Control | ||||||
| Dong Y (2011) [10] | Guinea pig | 18 | Near term | 79% of term | Hypoxia | * Brain (mRNA) | |
| iNOS | FGR > Control | ||||||
| Evans LC (2012) [11] | Guinea pig | 132 | Near term | 79% of term | Hypoxia | * Heart | |
| MDA (protein) | FGR > Control | ||||||
| 3-NT (protein) | FGR > Control | ||||||
| MMP-9 (protein) | FGR > Control | ||||||
| Li W (2012) [12] | Piglet | 10 | Term | N/A | N/A | * Liver | |
| T-SOD (protein) | FGR < Control | ||||||
| GPx (Protein) | FGR < Control | ||||||
| CAT (Protein) | FGR < Control | ||||||
| MDA (protein) | FGR > Control | ||||||
| Hsp70 (protein) | FGR > Control | ||||||
| Figueroa H (2016) [13] | Rabbit | 20 | Term | 81% of term | Uterine artery ligation | * Kidney (mRNA) | |
| iNOS | FGR > Control | ||||||
| HO-1 | FGR < Control | ||||||
| ROS | FGR > Control | ||||||
| Nitrotyrosine | FGR > Control | ||||||
| Primary Author (Year), [Reference Number] | n | GA at Delivery (Weeks) | Exclusion of Acute-HCA and AF Infection/Inflammation | Inflammatory Markers | Intensity of Inflammatory Markers: FGR vs. Control |
|---|---|---|---|---|---|
| Fetal blood | |||||
| Schiff E (1994) [14] | 85 | FGR: N/A Control: N/A | N/A | TNF-alpha | FGR < Control |
| Street ME (2006) [15] | 36 | FGR: 35.3 Control: 36.6 | N/A | IL-6 | FGR = Control |
| Trevisanuto D (2007) [16] | 104 | FGR: 34.6 Control: 34.2 | N/A | hs-CRP | FGR > Control |
| Boutsikou T (2014) [17] | 40 | FGR: 37.9 Control: 38.9 | N/A | hs-CRP | FGR = Control |
| PAI-1 | FGR = Control | ||||
| S100B | FGR = Control | ||||
| Amarilyo G (2011) [18] | 40 | FGR: 38.2 Control: 39.1 | N/A | IL-6 | FGR > Control |
| TNF-alpha | FGR = Control | ||||
| TPO | FGR > Control | ||||
| CRP | FGR > Control | ||||
| Visentin S (2014) [19] | 140 | FGR: 36.75 Control: 38.57 | N/A | IL-6 | FGR > Control |
| TNF-alpha | FGR > Control | ||||
| CRP | FGR = Control | ||||
| FGR (−) (n = 58) 71.6% (58/81) | FGR (+) (n = 23) 28.4% (23/81) | p Value | |
|---|---|---|---|
| Mean maternal age, y (±SD) | 32.1 ± 4.8 | 30.5 ± 4.0 | 0.227 |
| Parity ≥1 | 56.9% (33/58) | 30.4% (7/23) | 0.048 |
| Median GA at amniocentesis, week (range) | 31.1 (25.9–33.4) | 30.6 (25.3–33.4) | 0.529 |
| Antenatal corticosteroids use | 63.8% (37/58) | 73.9% (17/23) | 0.443 |
| Antibiotics use | 20.7% (12/58) | 17.4% (4/23) | 1.000 |
| Cesarean delivery | 89.7% (52/58) | 100% (23/23) | 0.176 |
| Male newborn | 60.3% (35/58) | 43.5% (10/23) | 0.217 |
| Causes of preterm delivery | 0.024 | ||
| PTL | 25.9%% (15/58) | 0% (0/23) | |
| Preterm-PROM | 5.2% (3/58) | 0% (0/23) | |
| Preeclampsia | 56.9% (33/58) | 87.0% (20/23) | |
| Other maternal fetal indication | 12.1% (7/58) | 13.0% (3/23) | |
| Median GA at delivery, week (range) | 31.1 (25.9–33.4) | 30.6 (25.3–33.4) | 0.457 |
| Birth weight, g (±SD) | 1389 ± 395 | 860 ± 284 | 0.000 |
| 1-min Apgar score of <7 | 67.2% (39/58) | 87.0% (20/23) | 0.098 |
| 5-min Apgar score of <7 | 41.4% (24/58) | 65.2% (15/23) | 0.083 |
| Umbilical arterial pH at birth ≤7.15 a | 15.8% (9/57) | 27.8% (5/18) | 0.303 |
| EONS (−) (n = 59) 74.7% (59/79) | EONS (+) (n = 20) 25.3% (20/79) | p Value | |
|---|---|---|---|
| Mean maternal age, y (±SD) | 31.9 ± 4.6 | 30.9 ± 4.7 | 0.415 |
| Parity ≥1 | 54.2% (32/59) | 30.0% (6/20) | 0.074 |
| Median GA at amniocentesis, week (range) | 31.4 (26.9–33.4) | 29.8 (25.3–32.7) | 0.002 |
| Antenatal corticosteroids use | 69.5% (41/59) | 60.0% (12/20) | 0.583 |
| Antibiotics use | 22.0% (13/59) | 15.0% (3/20) | 0.749 |
| Cesarean delivery | 89.8% (53/59) | 100% (20/20) | 0.329 |
| Male newborn | 54.2% (32/59) | 55.0% (11/20) | 1.000 |
| Causes of preterm delivery | 0.410 | ||
| PTL | 20.3% (12/59) | 10.0% (2/20) | |
| Preterm-PROM | 5.1% (3/59) | 0% (0/20) | |
| Preeclampsia | 61.0% (36/59) | 80.0% (16/20) | |
| Other maternal fetal indication | 13.6% (8/59) | 10.0% (2/20) | |
| Median GA at delivery, week (range) | 31.4 (26.9–33.4) | 29.8 (25.3–33.4) | 0.002 |
| Birth weight, g (±SD) | 1356 ± 394 | 909 ± 408 | 0.000 |
| 1-min Apgar score of <7 | 64.4% (38/59) | 95.0% (19/20) | 0.009 |
| 5-min Apgar score of <7 | 37.3% (22/59) | 75.0% (15/20) | 0.004 |
| Umbilical arterial pH at birth ≤7.15 b | 12.7% (7/55) | 33.3% (6/18) | 0.073 |
| FGR | 22.0% (13/59) | 50.0% (10/20) | 0.024 |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| FGR | 3.003 | 1.024–8.812 | 0.045 |
| GA at delivery | 0.746 | 0.572–0.972 | 0.030 |
| Antenatal corticosteroids use | 0.747 | 0.248–2.248 | 0.603 |
| Antibiotics use | 0.294 | 0.064–1.345 | 0.115 |
| Vaginal delivery | 0.000 | 0.000– | 0.999 |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| FGR | 4.184 | 1.101–15.902 | 0.036 |
| GA at delivery | 0.877 | 0.625–1.232 | 0.449 |
| Antenatal corticosteroids use | 1.718 | 0.387–7.638 | 0.477 |
| Antibiotics use | 0.000 | 0.000– | 0.998 |
| Vaginal delivery | 0.000 | 0.000– | 0.999 |
| Sensitivity | Specificity | Positive PV | Negative PV | Positive LR (95% CI) | Negative LR (95% CI) | |
|---|---|---|---|---|---|---|
| Low-grade FIR | 65.0% (13/20) | 72.9% (43/59) | 44.8% (13/29) | 86.0% (43/50) | 2.3969 [1.4141–4.0625] | 0.4802 [0.2591–0.8902] |
| FIRS | 25.0% (5/20) | 88.1% (52/59) | 41.7% (5/12) | 77.6% (52/67) | 2.1071 [0.7526–5.8993] | 0.8510 [0.6497–1.1145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.C.; Park, C.-W.; Park, J.S.; Jun, J.K. Fetal Growth Restriction and Subsequent Low Grade Fetal Inflammatory Response Are Associated with Early-Onset Neonatal Sepsis in the Context of Early Preterm Sterile Intrauterine Environment. J. Clin. Med. 2021, 10, 2018. https://doi.org/10.3390/jcm10092018
Moon KC, Park C-W, Park JS, Jun JK. Fetal Growth Restriction and Subsequent Low Grade Fetal Inflammatory Response Are Associated with Early-Onset Neonatal Sepsis in the Context of Early Preterm Sterile Intrauterine Environment. Journal of Clinical Medicine. 2021; 10(9):2018. https://doi.org/10.3390/jcm10092018
Chicago/Turabian StyleMoon, Kyung Chul, Chan-Wook Park, Joong Shin Park, and Jong Kwan Jun. 2021. "Fetal Growth Restriction and Subsequent Low Grade Fetal Inflammatory Response Are Associated with Early-Onset Neonatal Sepsis in the Context of Early Preterm Sterile Intrauterine Environment" Journal of Clinical Medicine 10, no. 9: 2018. https://doi.org/10.3390/jcm10092018
APA StyleMoon, K. C., Park, C.-W., Park, J. S., & Jun, J. K. (2021). Fetal Growth Restriction and Subsequent Low Grade Fetal Inflammatory Response Are Associated with Early-Onset Neonatal Sepsis in the Context of Early Preterm Sterile Intrauterine Environment. Journal of Clinical Medicine, 10(9), 2018. https://doi.org/10.3390/jcm10092018







