Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Randomization, Immunosuppression Protocol and Interventions
2.3. Protocol Biopsies and Histological Assessment
2.4. Testing for dnDSA
2.5. Cardiovascular and Renal Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Clinical and Histological Data
3.2. Acute Rejection during Follow-Up
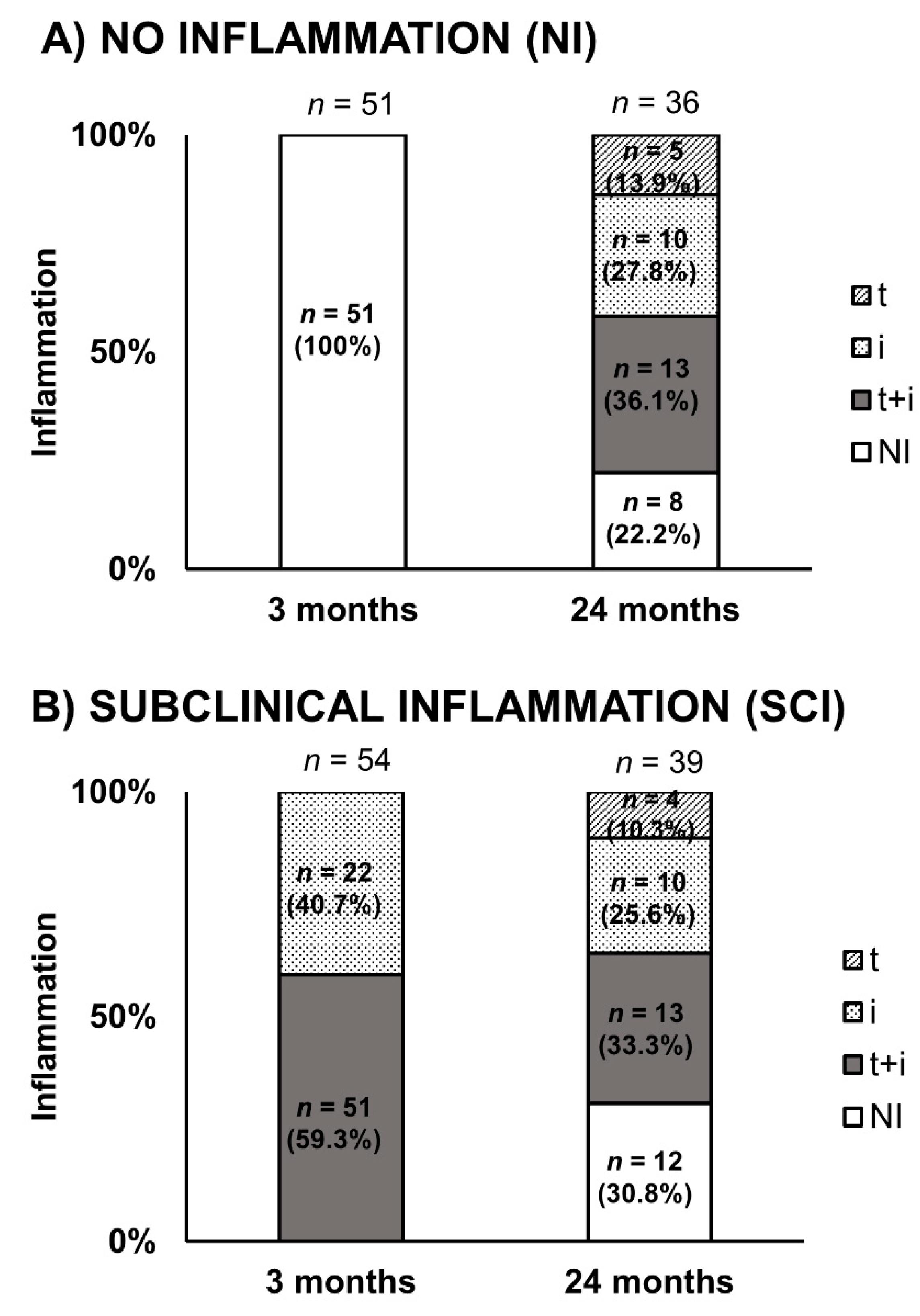
3.3. Histological Data Evolution
3.4. De Novo DSA
3.5. Clinical and Biochemical Data
3.6. Safety and Graft and Patient Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascual, J. Steroid avoidance or withdrawal in kidney transplantation. Curr. Opin. Organ Transplant. 2011, 16, 600–605. [Google Scholar] [CrossRef]
- Srinivas, T.R.; Meier-Kriesche, H.-U. Minimizing Immunosuppression, an Alternative Approach to Reducing Side Effects: Objectives and Interim Result. Clin. J. Am. Soc. Nephrol. 2008, 3, S101–S116. [Google Scholar] [CrossRef] [PubMed]
- Serrano, O.K.; Kandaswamy, R.; Gillingham, K.; Chinnakotla, S.; Dunn, T.B.; Finger, E.; Payne, W.; Ibrahim, H.; Kukla, A.; Spong, R.; et al. Rapid Discontinuation of Prednisone in Kidney Transplant Recipients. Transplantation 2017, 101, 2590–2598. [Google Scholar] [CrossRef]
- Haller, M.C.; Royuela, A.; Nagler, E.V.; Pascual, J.; Webster, A.C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Heilman, R.L.; Devarapalli, Y.; Chakkera, H.A.; Mekeel, K.L.; Moss, A.A.; Mulligan, D.C.; Mazur, M.J.; Hamawi, K.; Williams, J.W.; Reddy, K.S. Impact of Subclinical Inflammation on the Development of Interstitial Fibrosis and Tubular Atrophy in Kidney Transplant Recipients. Arab. Archaeol. Epigr. 2010, 10, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Bhusal, S.; Randhawa, P.; Sood, P.; Cherukuri, A.; Wu, C.; Puttarajappa, C.; Hoffman, W.; Shah, N.; Mangiola, M.; et al. Short-term adverse effects of early subclinical allograft inflammation in kidney transplant recipients with a rapid steroid withdrawal protocol. Am. J. Transpl. 2018, 18, 1710–1717. [Google Scholar] [CrossRef]
- Mehta, R.; Sood, P.; Hariharan, S. Subclinical Rejection in Renal Transplantation. Transplantation 2016, 100, 1610–1618. [Google Scholar] [CrossRef]
- Rush, D.; Arlen, D.; Boucher, A.; Busque, S.; Cockfield, S.M.; Girardin, C.; Knoll, G.; Lachance, J.-G.; Landsberg, D.; Shapiro, J.; et al. Lack of Benefit of Early Protocol Biopsies in Renal Transplant Patients Receiving TAC and MMF: A Randomized Study. Arab. Archaeol. Epigr. 2007, 7, 2538–2545. [Google Scholar] [CrossRef]
- Rush, D.N.; Gibson, I.W. Subclinical Inflammation in Renal Transplantation. Transplantation 2019, 103, e139–e145. [Google Scholar] [CrossRef]
- Kumar, M.S.A.; Khan, S.; Ranganna, K.; Malat, G.; Sustento-Reodica, N.; Meyers, W.C. Long-Term Outcome of Early Steroid Withdrawal After Kidney Transplantation in African American Recipients Monitored by Surveillance Biopsy. Arab. Archaeol. Epigr. 2008, 8, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Rostaing, L.; Hertig, A.; Albano, L.; Anglicheau, D.; Durrbach, A.; Vuiblet, V.; Moulin, B.; Merville, P.; Hazzan, M.; Lang, P.; et al. Fibrosis Progression According to Epithelial-Mesenchymal Transition Profile: A Randomized Trial of Everolimus Versus CsA. Arab. Archaeol. Epigr. 2015, 15, 1303–1312. [Google Scholar] [CrossRef]
- Gatault, P.; Kamar, N.; Büchler, M.; Colosio, C.; Bertrand, D.; Durrbach, A.; Albano, L.; Rivalan, J.; Le Meur, Y.; Essig, M.; et al. Reduction of Extended-Release Tacrolimus Dose in Low-Immunological-Risk Kidney Transplant Recipients Increases Risk of Rejection and Appearance of Donor-Specific Antibodies: A Randomized Study. Am. J. Transpl. 2016, 17, 1370–1379. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes-2010. Diabetes Care 2010, 33, S11–S61. [Google Scholar] [CrossRef]
- Cupps, T.R.; Edgar, L.C.; Thomas, C.A.; Fauci, A.S. Multiple mechanisms of B cell immunoregulation in man after administration of in vivo corticosteroids. J. Immunol. 1984, 132, 170–175. [Google Scholar] [PubMed]
- Pascual, J.; Galeano, C.; Royuela, A.; Zamora, J. A Systematic Review on Steroid Withdrawal Between 3 and 6 Months After Kidney Transplantation. Transplantation 2010, 90, 343–349. [Google Scholar] [CrossRef]
- van Sandwijk, M.S.; de Vries, A.P.; Bakker, S.J.; Berge, I.J.T.; Berger, S.P.; Bouatou, Y.R.; de Fijter, J.W.; Florquin, S.; van der Heide, J.J.H.; Idu, M.M.; et al. Early Steroid Withdrawal Compared With Standard Immunosuppression in Kidney Transplantation: Interim Analysis of the Amsterdam-Leiden-Groningen Randomized Controlled Trial. Transplant. Direct 2018, 4, e354. [Google Scholar] [CrossRef]
- Suszynski, T.M.; Gillingham, K.J.; Rizzari, M.D.; Dunn, T.B.; Payne, W.D.; Chinnakotla, S.; Finger, E.B.; Sutherland, D.E.; Najarian, J.S.; Pruett, T.L.; et al. Prospective Randomized Trial of Maintenance Immunosuppression With Rapid Discontinuation of Prednisone in Adult Kidney Transplantation. Arab. Archaeol. Epigr. 2013, 13, 961–970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vanrenterghem, Y.; van Hooff, J.P.; Squifflet, J.P.; Salmela, K.; Rigotti, P.; Jindal, R.M.; Pascual, J.; Ekberg, H.; Sicilia, L.S.; Boletis, J.N.; et al. Minimization of immunosuppressive therapy after renal transplantation: Results of a randomized controlled trial. Am. J. Transplant. 2005, 5, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Gelpi, R.; Helanterä, I.; Melilli, E.; Honkanen, E.; Bestard, O.; Grinyo, J.M.; Cruzado, J.M. Decreased Kidney Graft Survival in Low Immunological Risk Patients Showing Inflammation in Normal Protocol Biopsies. PLoS ONE 2016, 11, e0159717. [Google Scholar] [CrossRef]
- Wehmeier, C.; Amico, P.; Hirt-Minkowski, P.; Georgalis, A.; Höenger, G.; Menter, T.; Mihatsch, M.; Burkhalter, F.; Steiger, J.; Dickenmann, M.; et al. Acute Rejection Phenotypes in the Current Era of Immunosuppression: A Single-Center Analysis. Transplant. Direct 2017, 3, e136. [Google Scholar] [CrossRef]
- García-Carro, C.; Dörje, C.; Åsberg, A.; Midtvedt, K.; Scott, H.; Reinholt, F.P.; Holdaas, H.; Seron, D.; Reisæter, A.V. Inflammation in Early Kidney Allograft Surveillance Biopsies with and without Associated Tubulointerstitial Chronic Damage as a Predictor of Fibrosis Progression and Development of De Novo Donor Specific Antibodies. Transplantation 2017, 101, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Lerut, E.; Damme, B.V.; Vanrenterghem, Y.; Kuypers, D.R.J. Tacrolimus Exposure and Evolution of Renal Allograft Histology in the First Year After Transplantation. Arab. Archaeol. Epigr. 2007, 7, 2114–2123. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Agrawal, N.; Sharma, A.; Taverniti, A.; P’Ng, C.H.; Shingde, M.; Wong, G.; Chapman, J.R. The clinical and pathological significance of borderline T cell–mediated rejection. Arab. Archaeol. Epigr. 2018, 19, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Randhawa, P.; Jordan, M.L.; Scantlebury, V.P.; Vivas, C.; Jain, A.; Corry, R.J.; McCauley, J.; Johnston, J.; Donaldson, J.; et al. An Analysis of Early Renal Transplant Protocol Biopsies - the High Incidence of Subclinical Tubulitis. Arab. Archaeol. Epigr. 2001, 1, 47–50. [Google Scholar] [CrossRef]
- Rush, D.N.; Jeffery, J.R.; Gough, J. Sequential protocol biopsies in renal transplant patients. Clinico-pathological correlations using the Banff schema. Transplantation 1995, 59, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Moreso, F.; Ibernon, M.; Gomà, M.; Carrera, M.; Fulladosa, X.; Hueso, M.; Gil-Vernet, S.; Cruzado, J.M.; Torras, J.; Grinyó, J.M.; et al. Subclinical Rejection Associated with Chronic Allograft Nephropathy in Protocol Biopsies as a Risk Factor for Late Graft Loss. Arab. Archaeol. Epigr. 2006, 6, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Cosio, F.G.; Grande, J.P.; Wadei, H.; Larson, T.S.; Griffin, M.D.; Stegall, M.D. Predicting Subsequent Decline in Kidney Allograft Function from Early Surveillance Biopsies. Arab. Archaeol. Epigr. 2005, 5, 2464–2472. [Google Scholar] [CrossRef]
- Ortiz-Fernandez, L.; Carmona, F.D.; Montes-Cano, M.A.; Garcia-Lozano, J.R.; Conde-Jaldón, M.; Ortego-Centeno, N.; Castillo, M.J.; Espinosa, G.; Graña-Gil, G.; Sánchez-Bursón, J.; et al. Genetic Analysis with the Immunochip Platform in Behçet Disease. Identification of Residues Associated in the HLA Class I Region and New Susceptibility Loci. PLoS ONE 2016, 11, e0161305. [Google Scholar] [CrossRef]
- Hoffman, W.; Mehta, R.; Jorgensen, D.R.; Sood, P.; Randhawa, P.; Wu, C.M.; Puttarajappa, C.; Shah, N.A.; Tevar, A.D.; Hariharan, S. The Impact of Early Clinical and Subclinical T Cell–mediated Rejection After Kidney Transplantation. Transplantation 2019, 103, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Gago, M.; Cornell, L.D.; Kremers, W.K.; Stegall, M.D.; Cosio, F.G. Kidney Allograft Inflammation and Fibrosis, Causes and Consequences. Arab. Archaeol. Epigr. 2012, 12, 1199–1207. [Google Scholar] [CrossRef]
- Thierry, A.; Thervet, E.; Vuiblet, V.; Goujon, J.-M.; Machet, M.-C.; Noël, L.-H.; Rioux-Leclercq, N.; Comoz, F.; Cordonnier, C.; François, A.; et al. Long-term Impact of Subclinical Inflammation Diagnosed by Protocol Biopsy One Year After Renal Transplantation. Arab. Archaeol. Epigr. 2011, 11, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.; Bouchard-Boivin, F.; Béland, S.; Noël, R.; Côté, I.; Lapointe, I.; Lesage, J.; Latulippe, E.; Riopel, J.; Santoriello, D.; et al. Impact of the Current Versus the Previous Diagnostic Threshold on the Outcome of Patients with Borderline Changes Suspicious for T Cell–mediated Rejection Diagnosed on Indication Biopsies. Transplantation 2018, 102, 2120–2125. [Google Scholar] [CrossRef]
- Nankivell, B.J.; P’Ng, C.H.; Chapman, J.R. Does tubulitis without interstitial inflammation represent borderline acute T cell mediated rejection? Arab. Archaeol. Epigr. 2019, 19, 132–144. [Google Scholar] [CrossRef]
- Wu, K.; Budde, K.; Lu, H.; Schmidt, D.; Liefeldt, L.; Glander, P.; Neumayer, H.H.; Rudolph, B. The Severity of Acute Cellular Rejection Defined by Banff Classification Is Associated with Kidney Allograft Outcomes. Transplantation 2014, 97, 1146–1154. [Google Scholar] [CrossRef]
- Loupy, A.; Vernerey, D.; Tinel, C.; Aubert, O.; Van Huyen, J.P.D.; Rabant, M.; Verine, J.; Nochy, D.; Empana, J.P.; Martinez, F.; et al. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allografts. J. Am. Soc. Nephrol. 2015, 26, 1721–1731. [Google Scholar] [CrossRef]
- Torres, I.B.; Reisaeter, A.V.; Moreso, F.; Âsberg, A.; Vidal, M.; Garcia-Carro, C.; Midtvedt, K.; Reinholt, F.P.; Scott, H.; Castellà, E.; et al. Tacrolimus and mycophenolate regimen and subclinical tubulo-interstitial inflammation in low immunological risk renal transplants. Transpl. Int. 2017, 30, 1119–1131. [Google Scholar] [CrossRef]
- Stegall, M.D.; Park, W.D.; Larson, T.S.; Gloor, J.M.; Cornell, L.D.; Sethi, S.; Dean, P.G.; Prieto, M.; Amer, H.; Textor, S.; et al. The Histology of Solitary Renal Allografts at 1 and 5 Years After Transplantation. Arab. Archaeol. Epigr. 2010, 11, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.B.; Tandukar, S.; Jorgensen, D.; Randhawa, P.; Sood, P.; Puttarajappa, C.; Zeevi, A.; Tevar, A.D.; Hariharan, S. Early subclinical tubulitis and interstitial inflammation in kidney transplantation have adverse clinical implications. Kidney Int. 2020, 98, 436–447. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, D.G.; Sellarés, J.; Mengel, M.; Chang, J.; Hidalgo, L.G.; Famulski, K.S.; Sis, B.; Einecke, G.; Halloran, P.F. The Nature of Biopsies with “Borderline Rejection” and Prospects for Eliminating This Category. Arab. Archaeol. Epigr. 2011, 12, 191–201. [Google Scholar] [CrossRef]
- Serón, D.; Moreso, F.; Bover, J.; Condom, E.; Gil-Vernet, S.; Cañas, C.; Fulladosa, X.; Torras, J.; Carrera, M.; Grinyó, J.M.; et al. Early protocol renal allograft biopsies and graft outcome. Kidney Int. 1997, 51, 310–316. [Google Scholar] [CrossRef]
- Espinoza, E.; Gonzalez-Parra, C.; Macías-Díaz, D.; Uribe-Uribe, N.; Alberu, J.; Morales-Buenrostro, L. Risk Factors for Banff Borderline Acute Rejection in Protocol Biopsies and Effect on Renal Graft Function. Transplant. Proc. 2010, 42, 2376–2378. [Google Scholar] [CrossRef]
- Laftavi, M.R.; Stephan, R.; Stefanick, B.; Kohli, R.; Dagher, F.; Applegate, M.; O’Keefe, J.; Pierce, D.; Rubino, A.; Guzowski, H.; et al. Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery 2005, 137, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Shin, M.J.; Shin, S.J.; Kim, Y.S.; Choi, Y.J.; Moon, I.S.; Kim, S.Y.; Koh, Y.B.; Bang, B.K.; Yang, C.W. Clinical Significance of an Early Protocol Biopsy in Living-Donor Renal Transplantation: Ten-Year Experience at a Single Center. Arab. Archaeol. Epigr. 2005, 5, 1354–1360. [Google Scholar] [CrossRef]
- Leeaphorn, N.; Pena, J.R.A.; Thamcharoen, N.; Khankin, E.V.; Pavlakis, M.; Cardarelli, F. HLA-DQ Mismatching and Kidney Transplant Outcomes. Clin. J. Am. Soc. Nephrol. 2018, 13, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Mannon, R.B.; Matas, A.J.; Grande, J.; LeDuc, R.; Connett, J.; Kasiske, B.; Cecka, J.M.; Gaston, R.S.; Cosio, F.; Gourishankar, S.; et al. Inflammation in Areas of Tubular Atrophy in Kidney Allograft Biopsies: A Potent Predictor of Allograft Failure. Am. J. Transplant. 2010, 10, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Mengel, M.; Chapman, J.R.; Cosio, F.G.; Cavaille-Coll, M.W.; Haller, H.; Halloran, P.F.; Kirk, A.D.; Mihatsch, M.J.; Nan-kivell, B.J.; Racusen, L.C.; et al. Protocol biopsies in renal transplantation: Insights into patient management and pathogenesis. Am. J. Transplant. 2007, 7, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Heilman, R.L.; Nijim, S.; Chakkera, H.A.; Devarapalli, Y.; Moss, A.A.; Mulligan, D.C.; Mazur, M.J.; Hamawi, K.; Williams, J.W.; Reddy, K.S. Impact of Acute Rejection on Kidney Allograft Outcomes in Recipients on Rapid Steroid Withdrawal. J. Transplant. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Lefaucheur, C.; Gosset, C.; Rabant, M.; Viglietti, D.; Verine, J.; Aubert, O.; Louis, K.; Glotz, D.; Legendre, C.; Van Huyen, J.D.; et al. T cell–mediated rejection is a major determinant of inflammation in scarred areas in kidney allografts. Am. J. Transpl. 2018, 18, 377–390. [Google Scholar] [CrossRef]
- Delgado, J.C.; Fuller, A.; Ozawa, M.; Smith, L.; Terasaki, P.I.; Shihab, F.S.; Eckels, D.D. No Occurrence of De Novo HLA Antibodies in Patients with Early Corticosteroid Withdrawal in a 5-year Prospective Randomized Study. Transplantation 2009, 87, 546–548. [Google Scholar] [CrossRef]
- Li, L.; Chaudhuri, A.; Chen, A.; Zhao, X.; Bezchinsky, M.; Concepcion, W.; Salvatierra, O.; Sarwal, M.M. Efficacy and Safety of Thymoglobulin Induction as an Alternative Approach for Steroid-Free Maintenance Immunosuppression in Pediatric Renal Transplantation. Transplantation 2010, 90, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, N.; Terasaki, P.I.; Schonemann, C. Donor-specific HLA antibodies in chronic renal allograft rejection: A prospective trial with a four-year follow-up. Available online: https://pubmed.ncbi.nlm.nih.gov/18365377/ (accessed on 5 May 2021).
- Everly, M.J.; Rebellato, L.M.; Haisch, C.E.; Ozawa, M.; Parker, K.; Briley, K.P.; Catrou, P.G.; Bolin, P.; Kendrick, W.T.; Kendrick, S.A.; et al. Incidence and Impact of De Novo Donor-Specific Alloantibody in Primary Renal Allografts. Transplantation 2013, 95, 410–417. [Google Scholar] [CrossRef]
- Wiebe, C.; Gibson, I.W.; Blydt-Hansen, T.D.; Karpinski, M.; Ho, J.; Storsley, L.J.; Goldberg, A.; Birk, P.E.; Rush, D.N.; Nickerson, P.W. Evolution and Clinical Pathologic Correlations of De Novo Donor-Specific HLA Antibody Post Kidney Transplant. Arab. Archaeol. Epigr. 2012, 12, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Wang, J.M.G.; Massie, A.B.; Jackson, K.R.; McAdams-DeMarco, M.A.; Brennan, D.C.; Lentine, K.L.; Coresh, J.; Segev, D.L. Early Steroid Withdrawal in Deceased-Donor Kidney Transplant Recipients with Delayed Graft Function. J. Am. Soc. Nephrol. 2020, 31, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Dohler, B.; Laux, G. Collaborative Transplant Study Long-Term Prospective Study of Steroid Withdrawal in Kidney and Heart Transplant Recipients. Arab. Archaeol. Epigr. 2005, 5, 720–728. [Google Scholar] [CrossRef]
- Arnol, M.; de Mattos, A.M.; Chung, J.S.; Prather, J.C.; Mittalhenkle, A.; Norman, D.J. Late steroid withdrawal and cardiovascular events in kidney transplant recipients. Transplantation 2008, 86, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Vanrenterghem, Y.; Lebranchu, Y.; Hené, R.; Oppenheimer, F.; Ekberg, H. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 2000, 70, 1352–1359. [Google Scholar] [CrossRef]
- Knight, S.R.; Morris, P.J. Steroid Avoidance or Withdrawal After Renal Transplantation Increases the Risk of Acute Rejection but Decreases Cardiovascular Risk. A Meta-Analysis. Transplantation 2010, 89, 1–14. [Google Scholar] [CrossRef]
- Kumar, M.S.A.; Heifets, M.; Moritz, M.J.; Saeed, M.I.; Khan, S.M.; Fyfe, B.; Sustento-Riodeca, N.; Daniel, J.N.; Kumar, A. Safety and Efficacy of Steroid Withdrawal Two Days after Kidney Transplantation: Analysis of Results at Three Years. Transplantation 2006, 81, 832–839. [Google Scholar] [CrossRef]
- Pascual, J.; Zamora, J.; Galeano, C.; Royuela, A.; Quereda, C. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Thomusch, O.; Wiesener, M.; Opgenoorth, M.; Pascher, A.; Woitas, R.P.; Witzke, O.; Jaenigen, B.; Rentsch, M.; Wolters, H.; Rath, T.; et al. Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): An open-label, multicentre, randomised controlled trial. Lancet 2016, 388, 3006–3016. [Google Scholar] [CrossRef]
- Pirsch, J.D.; Henning, A.K.; First, M.R.; Fitzsimmons, W.; Gaber, A.O.; Reisfield, R.; Shihab, F.; Woodle, E.S. New-Onset Diabetes After Transplantation: Results from a Double-Blind Early Corticosteroid Withdrawal Trial. Arab. Archaeol. Epigr. 2015, 15, 1982–1990. [Google Scholar] [CrossRef]
- Torres, A.; Hernández, D.; Moreso, F.; Serón, D.; Burgos, M.D.; Pallardó, L.M.; Kanter, J.; Díaz Corte, C.; Rodríguez, M.; Diaz, J.M.; et al. Randomized Controlled Trial Assessing the Impact of Tacrolimus Versus Cyclosporine on the Incidence of Posttransplant Diabetes Mellitus. Kidney Int. Rep. 2018, 3, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Woodle, E.S.; First, M.R.; Pirsch, J.; Shihab, F.; Gaber, A.O.; Van Veldhuisen, P.; Corticosteroid Withdrawal Study Group. A Prospective, Randomized, Double-Blind, Placebo-Controlled Multicenter Trial Comparing Early (7 Day) Corticosteroid Cessation Versus Long-Term, Low-Dose Corticosteroid Therapy. Ann. Surg. 2008, 248, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; De Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings From an International Consensus Meeting on Posttransplantation Diabetes Mellitus: Recommendations and Future Directions. Arab. Archaeol. Epigr. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed]


| CSC (n = 52) | CSW (n = 53) | p | |
|---|---|---|---|
| Donor age (years) | 52.7 ± 13 | 54.7 ± 12 | 0.403 |
| ECD (%) | 36.5 | 47 | 0.270 |
| Living donor (%) | 13.5 | 15.7 | 0.787 |
| Recipient weight (kg) | 78.3 ± 13 | 74.7 ± 17 | 0.338 |
| Recipient BMI (kg/m2) | 27 ± 4.1 | 25.7 ± 4.2 | 0.211 |
| Male (%) | 72 | 75 | 0.826 |
| Prior CVD (%) | 27 | 11.3 | 0.050 |
| Hemodialysis (%) | 71 | 65.4 | 0.664 |
| Cause of ESRD (%) | 0.060 | ||
| Glomerulonephritis | 23 | 21 | |
| Diabetes | 21 | 11 | |
| APKD | 31 | 15 | |
| Interstitial nephropathy | 2 | 19 | |
| Nephrosclerosis | 9.6 | 11 | |
| Unknown | 11.5 | 13 | |
| Other | 2 | 5.7 | |
| Induction therapy (%) | 0.096 | ||
| Basiliximab | 51 | 67.3 | |
| Thymoglobulin | 49 | 32.7 | |
| Cold ischemia time (h) | 10.4 ± 6 | 11 ± 6.4 | 0.640 |
| Pretransplant PRA (%) | 1.7 ± 6.6 | 1.2 ± 5.1 | 0.653 |
| Glycemia (mg/dL) | 119.9 ± 66 | 102.7 ± 23 | 0.088 |
| HbA1c % | 6.3 ± 1.5 | 5.7 ± 0.8 | 0.070 |
| Total cholesterol (mg/dL) | 171.3 ± 30.5 | 175.2 ± 40 | 0.609 |
| HDL-cholesterol (mg/dL) | 48.2 ± 13.3 | 47.2 ± 10 | 0.698 |
| LDL-cholesterol (mg/dL) | 95.6 ± 26 | 99.4 ± 29.4 | 0.532 |
| Triglycerides (mg/dL) | 139.3 ± 57 | 152 ± 10.4 | 0.478 |
| Hypertension (%) | 90.2 | 90.4 | 1.000 |
| SBP (mmHg) | 131 ± 17 | 130.5 ± 16 | 0.861 |
| DBP (mmHg) | 72.6 ± 9.7 | 75.5 ± 8 | 0.133 |
| Tacrolimus levels (ng/mL) | 9.8 ± 2.7 | 8.9 ± 2.2 | 0.081 |
| MMF dose (mg) | 1029 ± 314 | 1102 ± 297 | 0.253 |
| Total HLA mismatches * (n) | 6.2 ± 2.2 | 6.1 ± 2.3 | 0.763 |
| Proteinuria (mg/dL) | 286.3 ± 214 | 286.1 ± 238 | 0.998 |
| MDRD-4 (mL/min) | 57.6 ± 22 | 50.4 ± 16.4 | 0.064 |
| CSC (n = 52) | CSW (n = 53) | p | |
|---|---|---|---|
| g (0–3) | 0.04 ± 0.2 | 0.07 ± 0.3 | 0.444 |
| ptc (0–3) | 0.06 ± 0.2 | 0.16 ± 0.4 | 0.158 |
| t (0–3) | 0.3 ± 0.46 | 0.36 ± 0.48 | 0.533 |
| i (0–3) | 0.48 ± 0.5 | 0.53 ± 0.5 | 0.628 |
| v (0–3) | 0 | 0.02 ± 0.14 | 0.322 |
| ci (0–3) | 0.36 ± 0.48 | 0.46 ± 0.5 | 0.302 |
| ct (0–3) | 0.28 ± 0.46 | 0.41 ± 0.5 | 0.175 |
| cg (0–3) | 0 | 0 | |
| cv (0–3) | 0.4 ± 0.6 | 0.36 ± 0.5 | 0.716 |
| ah (0–3) | 0.36 ± 0.6 | 0.34 ± 0.6 | 0.865 |
| ct + ci | 0.63 ± 0.9 | 0.86 ± 0.92 | 0.206 |
| IFTA ≥ 2 (%) | 32.6 | 39 | 0.535 |
| ct + ci + cg + cv | 1.04 ± 1.24 | 1.22 ± 1.11 | 0.451 |
| Rejection | CSC (n = 52) | CSW (n = 53) |
|---|---|---|
| Number of total rejections (%) | 11 (21.2) | 13 (24.5) |
| Subclinical | 10 | 10 |
| Type | ||
| Borderline | 8 | 7 |
| TCMR IA | 0 | 2 |
| TCMRIB | 1 | 1 |
| ABMR | 1 | 0 |
| Clinically suspected | 1 | 3 |
| Type | ||
| Borderline | 0 | 3 |
| TCMRIB | 1 | 0 |
| NI (n = 51) | SCI (n = 54) | p | |
|---|---|---|---|
| g (0–3) | 0.02 ± 0.15 | 0.09 ± 0.29 | 0.122 |
| ptc (0–3) | 0 | 0.21 ± 0.46 | 0.002 |
| t (0–3) | 0 | 0.63 ± 0.49 | 0.000 |
| i (0–3) | 0 | 0.96 ± 0.19 | 0.000 |
| v (0–3) | 0 | 0.02 ± 0.14 | 0.357 |
| ci (0–3) | 0.29 ± 0.46 | 0.58 ± 0.49 | 0.004 |
| ct (0–3) | 0.25 ± 0.44 | 0.51 ± 0.51 | 0.009 |
| cg (0–3) | 0 | 0 | |
| cv (0–3) | 0.27 ± 0.45 | 0.53 ± 0.62 | 0.023 |
| ah (0–3) | 0.29 ± 0.58 | 0.41 ± 0.63 | 0.339 |
| ct + ci | 0.52 ± 0.88 | 1.06 ± 0.93 | 0.005 |
| IFTA ≥2 (%) | 25 | 45.8 | 0.037 |
| ct + ci + cg + cv | 0.76 ± 1.03 | 1.60 ± 1.20 | 0.001 |
| ECD (%) | 36 | 49 | 0.181 |
| DGF (%) | 24 | 27 | 0.735 |
| Creatinine (mg/dL) | 1.4 ± 0.5 | 1.6 ± 0.4 | 0.018 |
| Proteinuria (mg/dL) | 297 ± 229 | 279 ± 225 | 0.763 |
| MDRD (mL/min) | 60.0 ± 23.4 | 48.5 ± 13.6 | 0.003 |
| Total HLA mismatches * (n) | 5.4 ± 2.4 | 6.9 ± 2.0 | 0.002 |
| Tacrolimus levels (ng/mL) | 9.7 ± 2.9 | 9.2 ± 2.1 | 0.286 |
| CSC (n = 39) | CSW (n = 36) | p | |
|---|---|---|---|
| g (0–3) | 0.17 ± 0.38 | 0.08 ± 0.28 | 0.311 |
| ptc (0–3) | 0.24 ± 0.58 | 0.20 ± 0.50 | 0.781 |
| t (0–3) | 0.50 ± 0.69 | 0.56 ± 0.58 | 0.736 |
| i (0–3) | 0.83 ± 0.71 | 0.68 ± 0.69 | 0.444 |
| v (0–3) | 0 | 0 | |
| ci (0–3) | 0.61 ± 0.63 | 0.88 ± 0.78 | 0.165 |
| ct (0–3) | 0.61 ± 0.57 | 0.88 ± 0.78 | 0.149 |
| cg (0–3) | 0.15 ± 0.46 | 0 | 0.103 |
| cv (0–3) | 0.54 ± 0.69 | 0.70 ± 0.82 | 0.454 |
| ah (0–3) | 0.59 ± 0.87 | 0.64 ± 0.70 | 0.805 |
| ct + ci | 1.21 ± 1.13 | 1.76 ± 1.54 | 0.144 |
| IFTA ≥ 2 (%) | 50 | 64 | 0.305 |
| ct + ci + cg + cv | 1.96 ± 1.51 | 2.61 ± 1.47 | 0.136 |
| NI | SCI | |||||
|---|---|---|---|---|---|---|
| Month 3 | Month 24 | p | Month 3 | Month 24 | p | |
| g (0–3) | 0.04 ± 0.21 | 0.26 ± 0.45 | 0.057 | 0.09 ± 0.30 | 0.03 ± 0.18 | 0.325 |
| ptc (0–3) | 0 | 0.43 ± 0.73 | 0.009 | 0.19 ± 0.40 | 0.06 ± 0.25 | 0.103 |
| t (0–3) | 0 | 0.55 ± 0.59 | 0.000 | 0.56 ± 0.50 | 0.50 ± 0.67 | 0.645 |
| i (0–3) | 0 | 0.74 ± 0.62 | 0.000 | 0.97 ± 0.18 | 0.75 ± 0.76 | 0.109 |
| v (0–3) | 0 | 0 | 0 | 0 | ||
| ci (0–3) | 0.25 ± 0.44 | 0.80 ± 0.83 | 0.017 | 0.55 ± 0.51 | 0.79 ± 0.62 | 0.070 |
| ct (0–3) | 0.20 ± 0.41 | 0.80 ± 0.77 | 0.010 | 0.48 ± 0.51 | 0.79 ± 0.61 | 0.036 |
| cg (0–3) | 0 | 0.20 ± 0.52 | 0.104 | 0 | 0 | |
| cv (0–3) | 0.17 ± 0.38 | 0.56 ± 0.71 | 0.049 | 0.61 ± 0.63 | 0.61 ± 0.79 | 1 |
| ah (0–3) | 0.23 ± 0.53 | 0.68 ± 0.89 | 0.038 | 0.25 ± 0.44 | 0.50 ± 0.67 | 0.073 |
| ct + ci | 0.45 ± 0.83 | 1.6 ± 1.54 | 0.011 | 1.03 ± 0.94 | 1.59 ± 1.21 | 0.036 |
| IFTA ≥ 2 (%) | 25 | 54.5 | 0.625 | 45.8 | 59.4 | 0.244 |
| ct + ci + cg + cv | 0.67 ± 0.97 | 2.50 ± 1.72 | 0.001 | 1.68 ± 1.12 | 2.25 ± 1.38 | 0.088 |
| CSC n = 52 | CSW n = 53 | p Value | |
|---|---|---|---|
| Weight (kg) | |||
| 12 months | 81.5 ± 13.4 | 78.0 ± 16.6 | 0.323 |
| 24 months | 83.9 ± 14.3 | 81.4 ± 17.4 | 0.531 |
| BMI (kg/m2) | |||
| 12 months | 28.6 ± 4.1 | 27.2 ± 4.7 | 0.181 |
| 24 months | 29.9 ± 4.6 | 28.1 ± 4.8 | 0.162 |
| HbA1c (%) | |||
| 12 months | 6.5 ± 1.5 | 5.7 ± 0.8 | 0.017 |
| 24 months | 6.4 ± 1.2 | 5.7 ± 0.3 | 0.013 |
| Glucose (mg/dL) | |||
| 12 months | 110.6 ± 38.1 | 106.3 ± 20.9 | 0.494 |
| 24 months | 107.8 ± 26.5 | 112.3 ± 32.9 | 0.494 |
| Total cholesterol (mg/dL) | |||
| 12 months | 163.0 ± 30.4 | 149.2 ± 20.9 | 0.017 |
| 24 months | 162.7 ± 26.7 | 165.2 ± 30.3 | 0.704 |
| HDL-cholesterol (mg/dL) | |||
| 12 months | 49.8 ± 15.2 | 42.0 ± 13.6 | 0.024 |
| 24 months | 49.7 ± 14.0 | 44.6 ± 12.2 | 0.117 |
| LDL-cholesterol (mg/dL) | |||
| 12 months | 85.8 ± 23.9 | 80.4 ± 19.0 | 0.286 |
| 24 months | 86.3 ± 19.7 | 95.1 ± 21.5 | 0.087 |
| Triglycerides (mg/dL) | |||
| 12 months | 137.2 ± 49.5 | 132.6 ± 63.9 | 0.717 |
| 24 months | 137.5 ± 56.1 | 131.2 ± 73.1 | 0.681 |
| SBP (mmHg) | |||
| 12 months | 133.4 ± 18.9 | 129.3 ± 14.8 | 0.278 |
| 24 months | 134.2 ± 14.9 | 125.7 ± 15.3 | 0.016 |
| DBP (mmHg) | |||
| 12 months | 75.2 ± 9.3 | 74.3 ± 10 | 0.709 |
| 24 months | 74.5 ± 10.7 | 75.4 ± 8.8 | 0.699 |
| Tacrolimus levels (ng/mL) | |||
| 12 months | 8.6 ± 2.8 | 7.9 ± 1.8 | 0.158 |
| 24 months | 7.5 ± 2.5 | 7.3 ± 1.6 | 0.582 |
| MMF Doses (mg) | |||
| 12 months | 937 ± 162 | 1005 ± 174 | 0.208 |
| 24 months | 935 ± 156 | 909 ± 240 | 0.570 |
| Creatinine (mg/dL) | |||
| 12 months | 1.4 ± 1.0 | 1.5 ± 0.5 | 0.714 |
| 24 months | 1.3 ± 0.4 | 1.5 ± 0.4 | 0.133 |
| Proteinuria (mg/24 h) | |||
| 12 months | 284.6 ± 288.0 | 172.2 ± 144.5 | 0.075 |
| 24 months | 512.5 ± 1306.5 | 160.4 ± 110.5 | 0.284 |
| MDRD (mL/min) | |||
| 12 months | 59.1 ± 16.6 | 54.3 ± 18.0 | 0.171 |
| 24 months | 60.1 ± 18.2 | 55.4 ± 19.5 | 0.235 |
| CSC n = 52 | CSW n = 53 | p Value | |
|---|---|---|---|
| Urinary sepsis, n (%) | 1 (1.9) | 2 (3.8) | 0.569 |
| CMV infection, n (%) | 3 (5.7) | 8 (15) | 0.191 |
| BK virus infection, n (%) | 1 (1.9) | 6 (11.3) | 0.108 |
| Patients with any serious infection * (%) | 5 (9.6) | 13 (24.5) | 0.070 |
| Cardiovascular disease, n (%) | 3 (5.7) | 2 (3.8) | 0.677 |
| Neoplasia, n (%) | 2 (3.8) | 1 (1.9) | 0.618 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, D.; Alonso-Titos, J.; Vázquez, T.; León, M.; Caballero, A.; Cobo, M.A.; Sola, E.; López, V.; Ruiz-Esteban, P.; Cruzado, J.M.; et al. Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients. J. Clin. Med. 2021, 10, 2005. https://doi.org/10.3390/jcm10092005
Hernández D, Alonso-Titos J, Vázquez T, León M, Caballero A, Cobo MA, Sola E, López V, Ruiz-Esteban P, Cruzado JM, et al. Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients. Journal of Clinical Medicine. 2021; 10(9):2005. https://doi.org/10.3390/jcm10092005
Chicago/Turabian StyleHernández, Domingo, Juana Alonso-Titos, Teresa Vázquez, Myriam León, Abelardo Caballero, María Angeles Cobo, Eugenia Sola, Verónica López, Pedro Ruiz-Esteban, Josep María Cruzado, and et al. 2021. "Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients" Journal of Clinical Medicine 10, no. 9: 2005. https://doi.org/10.3390/jcm10092005
APA StyleHernández, D., Alonso-Titos, J., Vázquez, T., León, M., Caballero, A., Cobo, M. A., Sola, E., López, V., Ruiz-Esteban, P., Cruzado, J. M., Sellarés, J., Moreso, F., Manonelles, A., Torío, A., Cabello, M., Delgado-Burgos, J., Casas, C., Gutiérrez, E., Jironda, C., ... Torres, A. (2021). Clinical Relevance of Corticosteroid Withdrawal on Graft Histological Lesions in Low-Immunological-Risk Kidney Transplant Patients. Journal of Clinical Medicine, 10(9), 2005. https://doi.org/10.3390/jcm10092005






