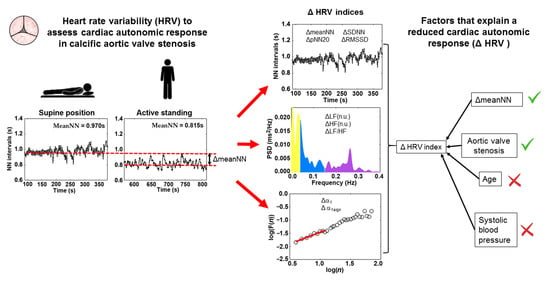
Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Protocol
2.2. Echocardiographic Assessment and Study Groups
2.3. Electrocardiogram Recording and HRV Indices
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindman, B.R.; Marie-Annick, C.; Patrick, M.; Bernard, I.; Patrizio, L.; Otto, C.M.; Philippe, P. Calcific aortic stenosis. Nat. Rev. Dis. Prim. 2016, 2, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Sathyamurthy, I.; Alex, S. Calcific aortic valve disease: Is it another face of atherosclerosis? Indian Heart J. 2015, 67, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.J.; Nkomo, V.T.; Enriquez-Sarano, M. The Global Burden of Aortic Stenosis. Prog. Cardiovasc. Dis. 2014, 56, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, N.; Otto, C.M. Aortic stenosis: Changing disease concepts. J. Cardiovasc. Ultrasound 2015, 23, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rassa, A.; Zahr, F. Hypertension and Aortic Stenosis: A Review. Curr. Hypertens. Rev. 2018, 14, 6–14. [Google Scholar] [CrossRef]
- Lindman, B.R.; Jay, P. Multimorbidity in Older Adults with Aortic Stenosis. Clin. Geriatr. Med. 2016, 32, 305–314. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 117, pp. 379–393. [Google Scholar]
- Papaioannou, V.; Pneumatikos, I.; Maglaveras, N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Front. Physiol. 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1–19. [Google Scholar] [CrossRef]
- Vukasovic, J.L.; Florenzano, F.; Adriazola, P.; Escobar, E. Heart rate variability in severe aortic stenosis. J. Heart Valve Dis. 1999, 8, 143–148. [Google Scholar]
- Arslan, U.; Ozdemir, M.; Kocaman, S.A.; Balcioglu, S.; Cemri, M.; Cengel, A. Heart rate variability and heart rate turbulence in mild-to-moderate aortic stenosis. Europace 2008, 10, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.; Piorecka-Makula, A.; Bobkowski, W. Heart rate variability in children with aortic valve stenosis—A pilot study. Arch. Med. Sci. 2013, 9, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Zuern, C.S.; Eick, C.; Rizas, K.D.; Stoleriu, C.; Barthel, P.; Scherer, C.; Müller, K.A.L.; Gawaz, M.; Bauer, A. Severe autonomic failure in moderate to severe aortic stenosis: Prevalence and association with hemodynamics and biomarkers. Clin. Res. Cardiol. 2012, 101, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Zuern, C.S.; Rizas, K.D.; Eick, C.; Vogtt, M.I.; Bigalke, B.; Gawaz, M.; Bauer, A. Severe autonomic failure as a predictor of mortality in aortic valve stenosis. Int. J. Cardiol. 2014, 176, 782–787. [Google Scholar] [CrossRef]
- Rodriguez, J.; Blaber, A.P.; Kneihsl, M.; Trozic, I.; Ruedl, R.; Green, D.A.; Broadbent, J.; Xu, D.; Rössler, A.; Hinghofer-Szalkay, H.; et al. Poststroke alterations in heart rate variability during orthostatic challenge. Medicine 2017, 96, 4–8. [Google Scholar] [CrossRef]
- Nygaard, S.; Christensen, A.H.; Rolid, K.; Nytrøen, K.; Gullestad, L.; Fiane, A.; Thaulow, E.; Døhlen, G.; Godang, K.; Saul, J.P.; et al. Autonomic cardiovascular control changes in recent heart transplant recipients lead to physiological limitations in response to orthostatic challenge and isometric exercise. Eur. J. Appl. Physiol. 2019, 119, 2225–2236. [Google Scholar] [CrossRef]
- Dunn, C.E.; Monroe, D.C.; Crouzet, C.; Hicks, J.W.; Choi, B. Speckleplethysmographic (SPG) Estimation of Heart Rate Variability During an Orthostatic Challenge. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Barantke, M.; Krauss, T.; Ortak, J.; Lieb, W.; Reppel, M.; Burgdorf, C.; Pramstaller, P.P.; Schunkert, H.; Bonnemeier, H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J. Cardiovasc. Electrophysiol. 2008, 19, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Reulecke, S.; Charleston-Villalobos, S.; Voss, A.; González-Camarena, R.; González-Hermosillo, J.; Gaitán-González, M.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Dynamics of the cardiovascular autonomic regulation during orthostatic challenge is more relaxed in women. Biomed. Tech. 2018, 63, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Reulecke, S.; Charleston-Villalobos, S.; Voss, A.; González-Camarena, R.; González-Hermosillo, J.; Gaitán-González, M.J.; Hernández-Pacheco, G.; Schroeder, R.; Aljama-Corrales, T. Orthostatic stress causes immediately increased blood pressure variability in women with vasovagal syncope. Comput. Methods Programs Biomed. 2016, 127, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, E.; Konttinen, N.; Kinnunen, U.; Kyröläinen, H.; Rusko, H. The incidence of stress symptoms and heart rate variability during sleep and orthostatic test. Eur. J. Appl. Physiol. 2011, 111, 733–741. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Hughson, R.L.; Mäkikallio, T.H.; Airaksinen, K.E.J.; Seppänen, T.; Huikuri, H.V. Effects of exercise and passive head-up tilt on fractal and complexity properties of heart rate dynamics. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Lerma, C.; Echeverría, J.C.; Infante, O.; Pérez-Grovas, H.; González-Gómez, H. Sign and magnitude scaling properties of heart rate variability in patients with end-stage renal failure: Are these properties useful to identify pathophysiological adaptations? Chaos 2017, 27, 093906. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.C.; Infante, O.; Pérez-Grovas, H.; González, H.; José, M.V.; Lerma, C. Effects of Orthostatism and Hemodialysis on Mean Heart Period and Fractal Heart Rate Properties of Chronic Renal Failure Patients. Artif. Organs 2017, 41, 1026–1034. [Google Scholar] [CrossRef]
- Peng, C.-K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, Y.; Ivanov, P.C.; Havlin, S.; Peng, C.-K.; Goldberger, A.L.; Stanley, H.E. Magnitude and Sign Correlations in Heartbeat Fluctuations. Phys. Rev. Lett. 2001, 86, 1900–1903. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Torres-Arellano, J.M.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío, J.; Fajardo, E.; Lerma, C. Inflammation and Reduced Parasympathetic Cardiac Modulation in Aortic-Valve Sclerosis. Appl. Sci. 2019, 9, 4020. [Google Scholar] [CrossRef]
- Chandra, H.R.; Goldstein, J.A.; Choudhary, N.; O’Neill, C.S.; George, P.B.; Gangasani, S.R.; Cronin, L.; Marcovitz, P.A.; Hauser, A.M.; O’Neill, W.W. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J. Am. Coll. Cardiol. 2004, 43, 169–175. [Google Scholar] [CrossRef]
- Otto, C.M.; Lind, B.K.; Kitzman, D.W.; Gersh, B.J.; Siscovick, D.S. Association of Aortic-Valve Sclerosis with Cardiovascular Mortality and Morbidity in the Elderly. N. Engl. J. Med. 1999, 341, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.K. Aortic sclerosis: Not an innocent murmur but a marker of increased cardiovascular risk. Heart 2005, 91, 1389–1393. [Google Scholar] [CrossRef]
- Figuerola, S.; Quintanar, E.; Lerma, C. Development and validation of a graphical user interface for assessment of cardiorespiratory coupling. Congr. Int. Ing. Electrón. Mem. ELECTRO 2019, 41, 148–153. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Peña, M.A.; Echeverría, J.C.; García, M.T.; González-Camarena, R. Applying fractal analysis to short sets of heart rate variability data. Med. Biol. Eng. Comput. 2009, 47, 709–717. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Perkiömäki, J.S.; Maestri, R.; Pinna, G.D. Clinical impact of evaluation of cardiovascular control by novel methods of Heart rate dynamics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schmidt, G.; Yamamoto, Y. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- Poggio, P.; Cavallotti, L.; Myasoedova, V.A.; Bonomi, A.; Songia, P.; Gripari, P.; Valerio, V.; Amato, M.; Barbieri, S.; Faggiano, P.; et al. Aortic Valve Sclerosis Adds to Prediction of Short-Term Mortality in Patients with Documented Coronary Atherosclerosis. J. Clin. Med. 2019, 8, 1172. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. Circulation 2014, 129, 521–644. [Google Scholar] [CrossRef]
- Aurigemma, G.P.; Colleen, M.H. Left Ventricular Systolic Function and Outcome in Aortic Stenosis. JACC Cardiovasc. Imaging 2020, 13, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Lucini, D. Autonomic dysregulation in essential hypertension: Insight from heart rate and arterial pressure variability. Auton. Neurosci. Basic Clin. 2001, 90, 76–82. [Google Scholar] [CrossRef]
- Pavithran, P.; Madanmohan, T.; Nandeesha, H. Sex differences in short-term heart rate variability in patients with newly diagnosed essential hypertension. J. Clin. Hypertens. 2008, 10, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Küçükosmanoğlu, O.; Ozbarlas, N.; Birand, A.; Kudaiberdieva, G.Z. Power spectral analysis of heart rate variability in children with aortic stenosis. Turk. J. Pediatr. 2002, 44, 109–115. [Google Scholar]
- Ivanov, P.; Nunes, L.; Goldberger, A.; Havlin, S.; Rosenblum, M.; Struzik, Z.; Stanley, E. Multifractality inhuman heartbeat dynamics. Nature 1999, 399, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Karasik, R.; Sapir, N.; Ashkenazy, Y.; Ivanov, P.C.; Dvir, I.; Lavie, P.; Havlin, S. Correlation differences in heartbeat fluctuations during rest and exercise. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2002, 66, 4. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Huang, J.W.; Lin, L.Y.; Chang, C.H.; Chu, F.Y.; Lin, Y.H.; Wu, C.K.; Lee, J.K.; Hwang, J.J.; Lin, J.L.; et al. Detrended Fluctuation Analysis of Heart Rate Dynamics Is an Important Prognostic Factor in Patients with End-Stage Renal Disease Receiving Peritoneal Dialysis. PLoS ONE 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Fang, S.C.; Wu, Y.L.; Tsai, P.S. Heart Rate Variability and Risk of All-Cause Death and Cardiovascular Events in Patients With Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Biol. Res. Nurs. 2020, 22, 45–56. [Google Scholar] [CrossRef]
- Kontogeorgos, S.; Thunström, E.; Basic, C.; Hansson, P.O.; Zhong, Y.; Ergatoudes, C.; Morales, D.; Mandalenakis, Z.; Rosengren, A.; Caidahl, K.; et al. Prevalence and risk factors of aortic stenosis and aortic sclerosis: A 21-year follow-up of middle-aged men. Scand. Cardiovasc. J. 2020, 54, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Ferreira-González, I.; Pinar-Sopena, J.; Ribera, A.; Marsal, J.R.; Cascant, P.; González-Alujas, T.; Evangelista, A.; Brotons, C.; Moral, I.; Permanyer-Miralda, G.; et al. Prevalence of calcific aortic valve disease in the elderly and associated risk factors: A population-based study in a Mediterranean area. Eur. J. Prev. Cardiol. 2013, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Engert, J.; Thanassoulis, G. Risk Factors for Valvular Calcification. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 96–102. [Google Scholar] [CrossRef]
| Variable | NAV (n = 22) | AVS (n = 25) | p Value |
|---|---|---|---|
| Age (years) | 41 ± 8 | 63 ± 7 | <0.001 |
| Female Male | 10 (45%) 12 (55%) | 8 (32%) 17 (68%) | 0.259 |
| Body mass index (kg/m2) | 27.35 ± 3.69 | 28.34 ± 3.56 | 0.354 |
| Heart rate (bpm) | 60.8 ± 9.7 | 62.2 ± 11.3 | 0.654 |
| SBP (mmHg) | 112 ± 11 | 136 ± 20 | <0.001 |
| DBP (mmHg) | 78 (70–80) | 80 (76–90) | 0.083 |
| Hypertension | 2 (9%) | 11 (44%) | 0.008 |
| Dyslipidemia | 0 (%) | 7 (28%) | 0.008 |
| Alcoholism | 10 (46%) | 13 (52%) | 0.438 |
| Smoking | 6 (32%) | 8 (32%) | 0.488 |
| Statins | 0 (0%) | 5 (20%) | 0.035 |
| Aspirin | 0 (0%) | 10 (40%) | 0.001 |
| Variable | (NAV) (n = 22) | (AVS) (n = 25) | p Value |
|---|---|---|---|
| AVA (cm2) | 4.20 (4.03–4.20) | 0.60 (0.41–1.21) | <0.001 |
| AVAi (cm2/m2) | 2.17 (2.06–2.38) | 0.36 (0.25–0.71) | <0.001 |
| Vmax (m/s) | 1.20 (1.02–1.37) | 4.30 (3.18–5.37) | <0.001 |
| AVGmean (mmHg) | 3 (2–3) | 43 (23–70) | <0.001 |
| AVGmax (mmHg) | 5 (4–7) | 74 (38–115) | <0.001 |
| LVEF (%) | 62 ± 6 | 54 ± 9 | <0.001 |
| Variable | NAV (n = 22) | AVS (n = 25) | p Value |
|---|---|---|---|
| Serum glucose (mg/dL) | 87.7 ± 12.1 | 97.6 ± 11.3 | <0.008 |
| Albumin (mg/dL) | 4.39 ± 0.22 | 4.48 ± 0.32 | 0.413 |
| Total cholesterol (mg/dL) | 191.76 ± 34.25 | 182.08 ± 37.37 | 0.359 |
| High density lipids (mg/dL) | 41.59 ± 10.54 | 42.82 ± 11.69 | 0.706 |
| Low density lipids (mg/dL) | 125 ± 32 | 107 ± 36 | 0.065 |
| Triglycerides (mg/dL) | 139 (113–163) | 151 (106–189) | 0.579 |
| Atherogenic index | 3.21 ± 1.19 | 2.72 ± 1.35 | 0.193 |
| C-reactive protein (mg/dL) | 2.60 (1.30–3.4) | 2.00 (0.89–4.17) | 0.880 |
| Hemoglobin (mg/dL) | 15.0 ± 1.7 | 14.7 ± 1.5 | 0.586 |
| Hematocrit (%) | 45.1 ± 4.3 | 43.6 ± 4.6 | 0.271 |
| Variable | NAV (n = 22) | AVS (n = 25) | p Value |
|---|---|---|---|
| Supine position | |||
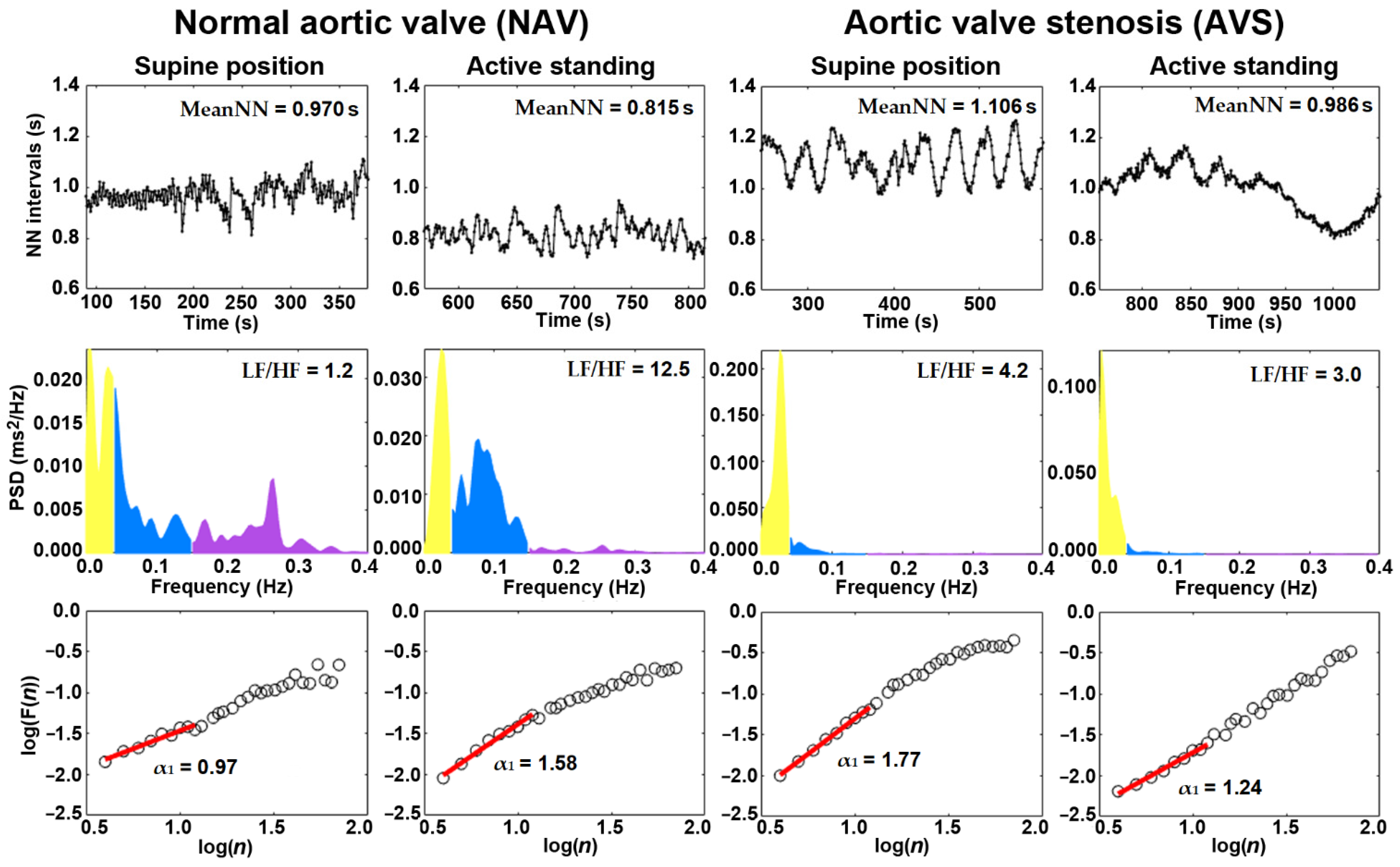
| MeanNN (s) | 0.994 ± 0.180 ** | 0.987 ± 0.167 ** | 0.890 |
| SDNN (ms) | 54.3 ± 23.5 | 50.3 ± 26.3 * | 0.591 |
| pNN20 (%) | 59.0 ± 31.2 ** | 41.2 ± 34.5 ** | 0.058 |
| RMSSD (ms) | 38.7 ± 16.9 ** | 26.3 ± 13.3 ** | 0.007 |
| LF (nu) | 56.8 (44.0–68.5) ** | 76.7 (54.2–84.7) * | 0.004 |
| HF (nu) | 43.2 (31.5–56.0) ** | 23.3 (15.3–45.8) * | 0.004 |
| LF/HF | 1.31 (0.78–2.17) ** | 3.29 (1.18–5.53) | 0.004 |
| α1 | 0.993 ± 0.21 ** | 1.16 ± 0.42 | 0.083 |
| α1sign | 0.171 ± 0.14 ** | 0.33 ± 0.23 | 0.006 |
| Active standing | |||
| MeanNN (s) | 0.825 ± 0.15 | 0.888 ± 0.12 | 0.132 |
| SDNN (ms) | 53.3 ± 30.6 | 41.8 ± 18.4 | 0.138 |
| pNN20 (%) | 40.5 ± 26.9 | 28.9 ± 20.4 | 0.108 |
| RMSSD (ms) | 26.3 ± 13.9 | 19.1 ± 9.3 | 0.048 |
| LF (nu) | 85.4 (73.2–88.6) | 82.2 (70.8–87.4) | 0.277 |
| HF (nu) | 14.6 (11.4–26.8) | 17.8 (12.6–29.2) | 0.277 |
| LF/HF | 5.83 (2.73–7.74) | 4.60 (2.42–6.92) | 0.277 |
| α1 | 1.40 ± 0.21 | 1.24 ± 0.32 | 0.038 |
| α1sign | 0.466 ± 0.14 | 0.366 ± 0.13 | 0.019 |
| Variable | NAV (n = 22) | AVS (n = 25) | p Value |
|---|---|---|---|
| ΔmeanNN (s) | 0.170 ± 0.070 | 0.100 ± 0.100 | 0.010 |
| ΔSDNN (ms) | 1 ± 18 | 8 ± 23 | 0.221 |
| ΔpNN20 (%) | 18.53 ± 12.55 | 12.28 ± 23.17 | 0.250 |
| ΔRMSSD (ms) | 12.48 ± 10.06 | 7.24 ± 11.47 | 0.102 |
| ΔLF (nu) | −26.48 ± 18.03 | −7.03 ± 15.92 | <0.001 |
| ΔHF (nu) | 26.52 ± 18.06 | 7.05 ± 15.91 | <0.001 |
| Δ(LF/HF) | −5.20 ± 4.52 | −0.937 ± 3.77 | <0.001 |
| Δα1 | −0.42 ± 0.23 | −0.07 ± 0.32 | <0.001 |
| Δα1sign | −0.29 ± 0.20 | −0.03 ± 0.22 | <0.001 |
| Variables | Standardized β | β (C.I.95%) | p | R2 |
|---|---|---|---|---|
| Predicted HRV index: ΔpNN20 | 0.409 | |||
| ΔmeanNN | 0.650 | 126.67 (79.98–173.37) | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Predicted HRV index: ΔRMSSD | 0.249 | |||
| ΔmeanNN | 0.516 | 57.02 (27.19–86.85) | <0.001 | |
| AVS condition | Excluded variable | |||
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Predicted HRV index: ΔLF | 0.365 | |||
| ΔmeanNN | −0.353 | −65.53 (−114.50–−16.55) | 0.010 | |
| AVS condition | 0.415 | 7.54 (2.74–12.33) | 0.003 | |
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Predicted HRV index: ΔHF | 0.367 | |||
| ΔmeanNN | 0.355 | 66.12 (17.11–115.1) | 0.009 | |
| AVS condition | −0.414 | −7.54 (−12.34–−2.74) | 0.003 | |
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Predicted HRV index: ΔLF/HF | 0.202 | |||
| ΔmeanNN | Excluded variable | |||
| AVS condition | 0.471 | 2.16 (0.88–3.44) | 0.001 | |
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Predicted HRV index: Δα1 | 0.437 | |||
| ΔmeanNN | −0.447 | −1.51 (−2.35–−0.67) | 0.001 | |
| AVS condition | 0.385 | 0.12 (0.04–0.21) | 0.003 | |
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
| Predicted HRV index: Δα1sign | 0.331 | |||
| ΔmeanNN | Excluded variable | |||
| AVS condition | 0.589 | 0.141 (0.08–0.20) | <0.001 | |
| Age | Excluded variable | |||
| SBP | Excluded variable | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Arellano, J.M.; Echeverría, J.C.; Ávila-Vanzzini, N.; Springall, R.; Toledo, A.; Infante, O.; Bojalil, R.; Cossío-Aranda, J.E.; Fajardo, E.; Lerma, C. Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis. J. Clin. Med. 2021, 10, 2004. https://doi.org/10.3390/jcm10092004
Torres-Arellano JM, Echeverría JC, Ávila-Vanzzini N, Springall R, Toledo A, Infante O, Bojalil R, Cossío-Aranda JE, Fajardo E, Lerma C. Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis. Journal of Clinical Medicine. 2021; 10(9):2004. https://doi.org/10.3390/jcm10092004
Chicago/Turabian StyleTorres-Arellano, José M., Juan C. Echeverría, Nydia Ávila-Vanzzini, Rashidi Springall, Andrea Toledo, Oscar Infante, Rafael Bojalil, Jorge E. Cossío-Aranda, Erika Fajardo, and Claudia Lerma. 2021. "Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis" Journal of Clinical Medicine 10, no. 9: 2004. https://doi.org/10.3390/jcm10092004
APA StyleTorres-Arellano, J. M., Echeverría, J. C., Ávila-Vanzzini, N., Springall, R., Toledo, A., Infante, O., Bojalil, R., Cossío-Aranda, J. E., Fajardo, E., & Lerma, C. (2021). Cardiac Autonomic Response to Active Standing in Calcific Aortic Valve Stenosis. Journal of Clinical Medicine, 10(9), 2004. https://doi.org/10.3390/jcm10092004