Cervical Human Papillomavirus Infection (HPV) and High Oncogenic Risk Genotypes among Women Living with HIV in Asia: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
- -
- Study settings: study identification (first author, publication year, journal of publication); location (country, region, or city) where the study was performed.
- -
- Sociodemographic data of WLHIV: number of WLHIV enrolled; mean age of WLHIV; and percentage of WLHIV who: use condoms consistently (always or most of the time), have at least a primary education, smoke, are sex workers, and have had at least one pregnancy.
- -
- Data related to HPV infection: number of WLHIV tested for HPV infection; HPV test used; number of HPV genotypes tested; percentage of WLHIV with HPV infection and multiple HPV infection, and mean number of genotypes for those with multiple infection; percentage of WLHIV with any HR-HPV infection; percentage of WLHIV positive for each of the 13 HR-HPV genotypes, according to WHO’s classification [3].
- -
- Data related to HIV infection: mean CD4 cell-count nadir and when tested for HPV; mean HIV-RNA viral load and percentage of WLHIV with detectable viral load when tested for HPV; percentage of WLHIV receiving antiretroviral therapy, and mean duration since treatment initiation.
2.3. Statistical Analyses
3. Results
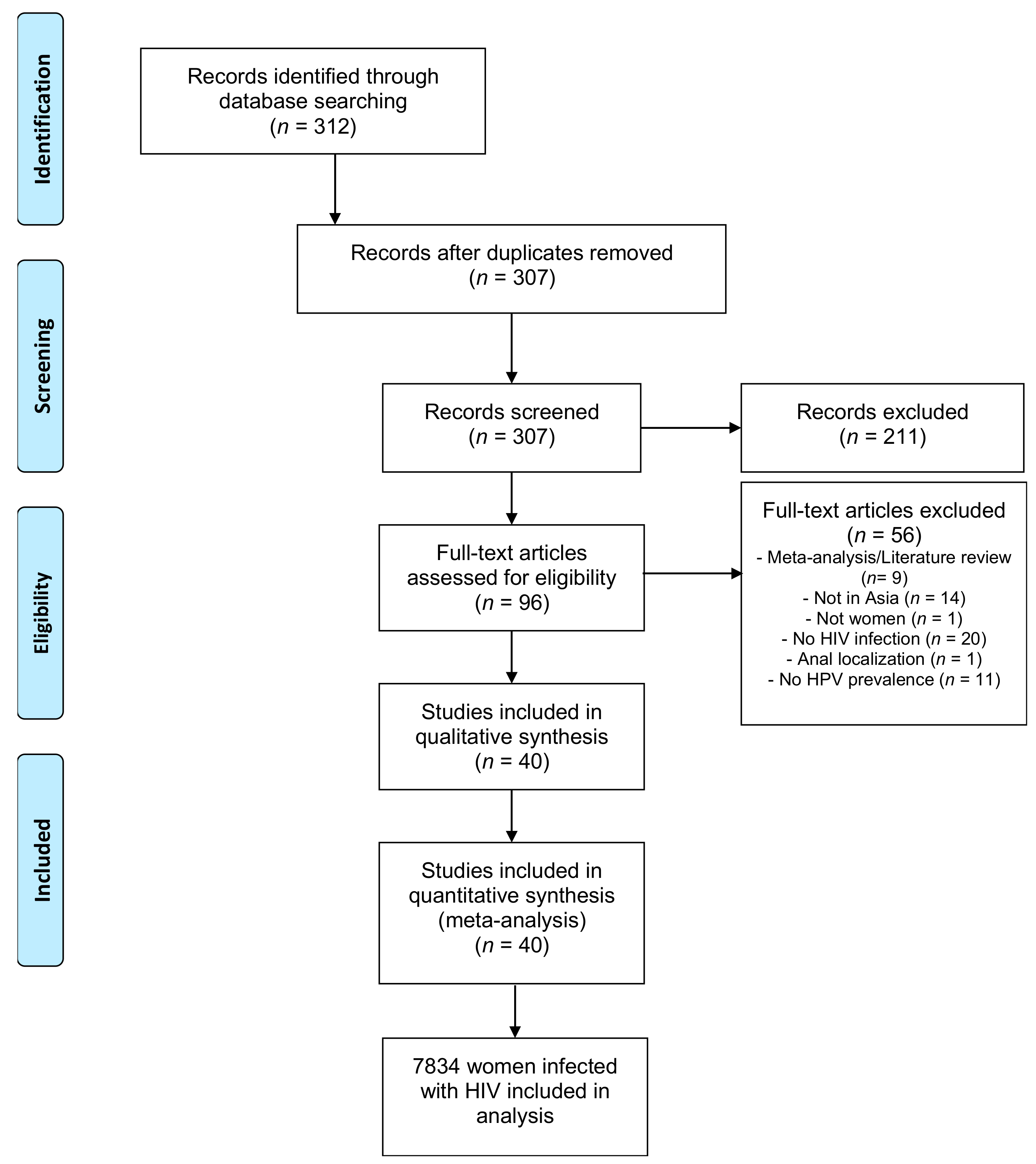
3.1. Studies Included
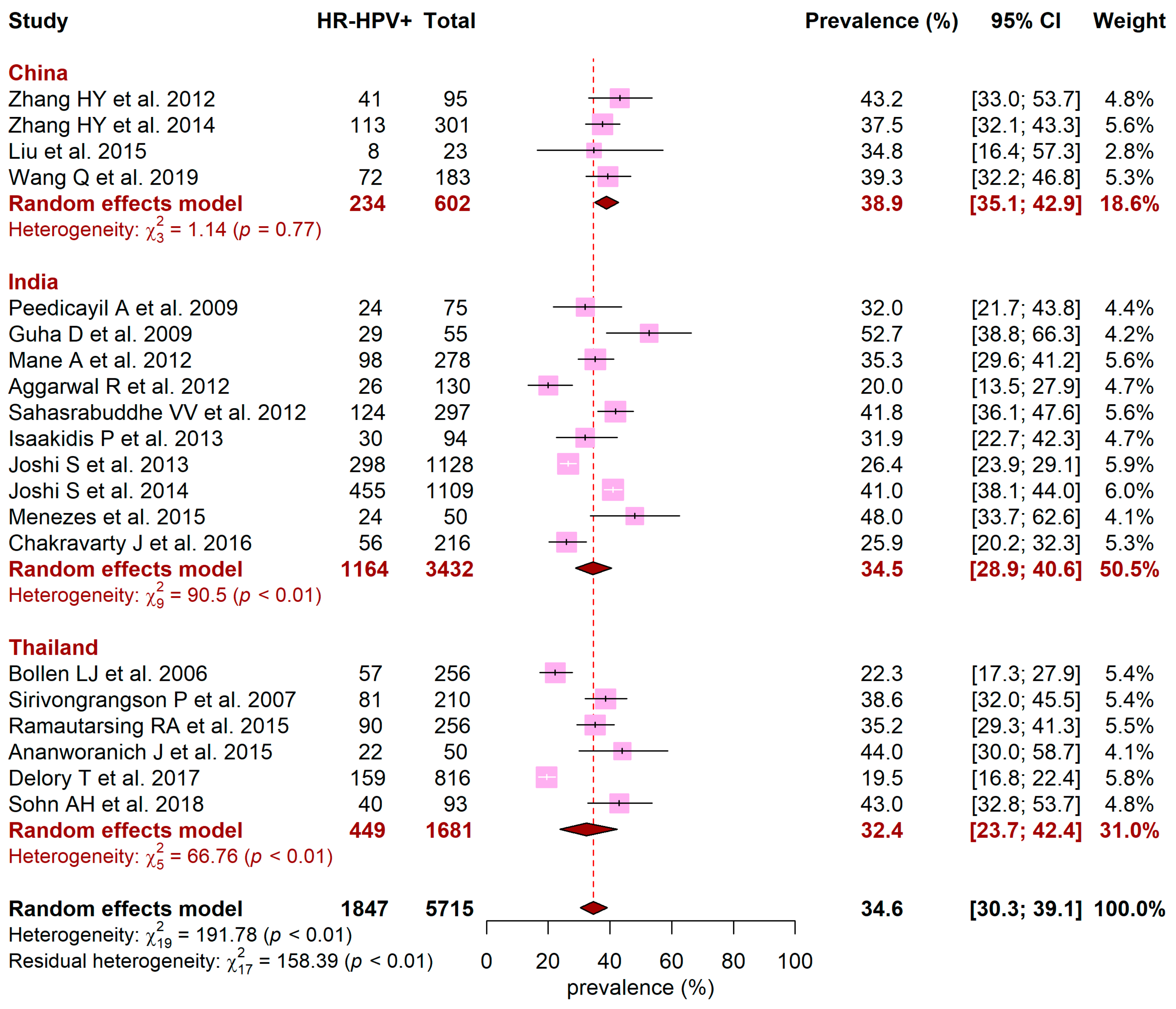
3.2. HPV Prevalence
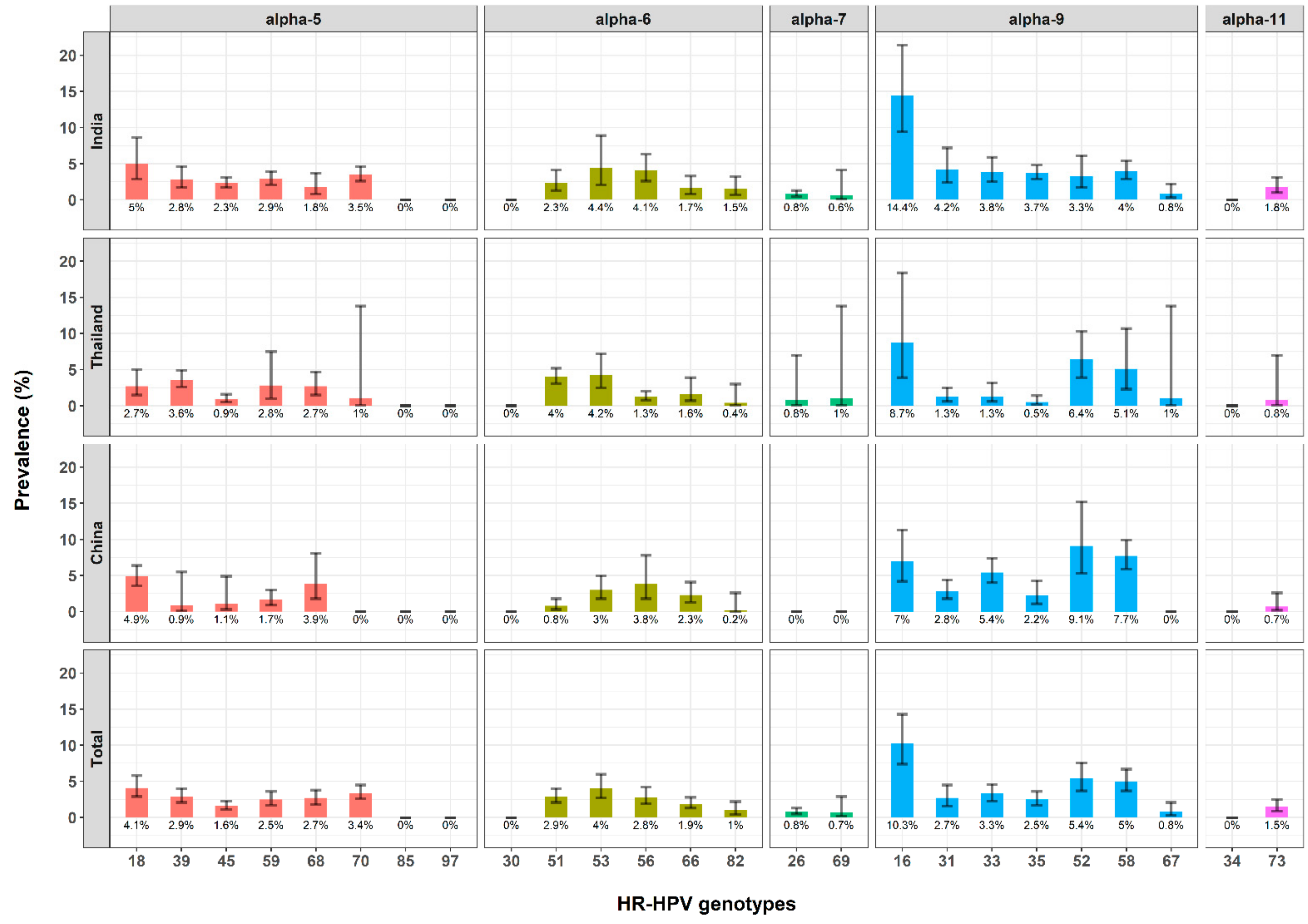
3.3. HR-HPV Prevalence and Distribution of HR-HPV Genotypes
3.4. Sources of Heterogeneity for HR-HPV Prevalence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- De Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- WHO/IARC. IARC Mongraphs on the Evaluation of Carcinogenic Risks to Humans; WHO: Geneva, Switzerland, 2012; p. 100B. [Google Scholar]
- Clifford, G.M.; Gonçalves, M.A.G.; Franceschi, S.; HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: A meta-analysis. AIDS 2006, 20, 2337–2344. [Google Scholar] [CrossRef]
- Tounkara, F.K.; Téguété, I.; Guédou, F.A.; Goma-Matsétsé, E.; Koné, A.; Béhanzin, L.; Traoré, S.; Aza-Gnandji, M.; Keita, B.; Guenoun, J.; et al. Human papillomavirus genotype distribution and factors associated among female sex workers in West Africa. PLoS ONE. 2020, 15, e0242711. [Google Scholar] [CrossRef]
- Luo, L.-P.; He, P.; Liu, Q.-T.; Jiang, Y.-H.; Zhang, Y.-N.; Li, Q.-Z.; Li, Q.; Li, S.T.; Yang, F.; Ling, H.; et al. Prevalence and genotype distribution of HPV infection among 214, 715 women from Southern China, 2012-2018: Baseline measures prior to mass HPV vaccination. BMC Infect. Dis. 2021, 21, 328. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Kulaga, S.; Robitaille, J.; Ferreira, S.; Santos, M.; Miyamura, R.A.; Duarte-Franco, E.; Rohan, T.E.; Ferenczy, A.; Villa, L.L.; et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001, 286, 3106–3114. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef]
- WHO/IARC. Cervix Uteri, Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf (accessed on 3 March 2021).
- Bonjour, M.; Charvat, H.; Franco, E.L.; Piñeros, M.; Clifford, G.M.; Bray, F.; Baussano, I. Global estimates of expected and preventable cervical cancers among girls born between 2005 and 2014: A birth cohort analysis. Lancet Public Health 2021. [Google Scholar] [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil, A.I.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2020. [Google Scholar] [CrossRef]
- Massad, L.S.; Xie, X.; Burk, R.; Keller, M.J.; Minkoff, H.; D’Souza, G.; Watts, D.H.; Palefsky, J.; Young, M.; Levine, A.M.; et al. Long-term cumulative detection of human papillomavirus among HIV seropositive women. AIDS 2014, 28, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Rowhani-Rahbar, A.; Hawes, S.E.; Sow, P.S.; Toure, P.; Feng, Q.; Dem, A.; Dembele, B.; Critchlow, C.W.; N’Doye, I.; Kiviat, N.B. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J. Infect. Dis. 2007, 196, 887–894. [Google Scholar] [CrossRef]
- Vermund, S.H.; Wilson, C.M.; Rogers, A.S.; Partlow, C.; Moscicki, A.B. Sexually transmitted infections among HIV infected and HIV uninfected high-risk youth in the REACH study. Reaching for Excellence in Adolescent Care and Health. J. Adolesc. Health 2001, 29 (Suppl. 3), 49–56. [Google Scholar] [CrossRef]
- Abraham, A.G.; D’Souza, G.; Jing, Y.; Gange, S.J.; Sterling, T.R.; Silverberg, M.J.; Saag, M.; Rourke, S.; Rachlis, A.; Napravnik, S.; et al. Invasive cervical cancer risk among HIV-infected women: A North American multicohort collaboration prospective study. J. Acquir. Immune Defic. Syndr. 1999 2013, 62, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Denny, L.A.; Franceschi, S.; de Sanjosé, S.; Heard, I.; Moscicki, A.B.; Palefsky, J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 2012, 30 (Suppl. 5), F168–F174. [Google Scholar] [CrossRef]
- Hawes, S.E.; Critchlow, C.W.; Faye Niang, M.A.; Diouf, M.B.; Diop, A.; Touré, P.; Aziz Kasse, A.; Dembele, B.; Salif Sow, P.; Coll-Seck, A.M.; et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J. Infect. Dis. 2003, 188, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, M.; Kronborg, G.; Larsen, C.S.; Pedersen, G.; Pedersen, C.; Gerstoft, J.; Obel, N. Causes of death among Danish HIV patients compared with population controls in the period 1995-2008. Infection 2012, 40, 627–634. [Google Scholar] [CrossRef]
- Mor, Z.; Sheffer, R.; Chemtob, D. Causes of death and mortality trends of all individuals reported with HIV/AIDS in Israel, 1985–2010. J. Public Health 2018, 40, 56–64. [Google Scholar] [CrossRef]
- Gheibi, Z.; Shayan, Z.; Joulaei, H.; Fararouei, M.; Beheshti, S.; Shokoohi, M. Determinants of AIDS and non-AIDS related mortality among people living with HIV in Shiraz, southern Iran: A 20-year retrospective follow-up study. BMC Infect. Dis. 2019, 19, 1094. [Google Scholar] [CrossRef]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Pendse, R.; Gupta, S.; Yu, D.; Sarkar, S. HIV/AIDS in the South-East Asia region: Progress and challenges. J. Virus Erad. 2016, 2 (Suppl. 4), 1–6. [Google Scholar]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of Human Papillomavirus (HPV) Types in HIV-Positive Women: A Meta-Analysis From HPV Infection to Cervical Cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjosé, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115, 789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Clifford, G.M.; Gallus, S.; Herrero, R.; Muñoz, N.; Snijders, P.J.F.; Vaccarella, S.; Anh, P.T.H.; Ferreccio, C.; Hieu, N.T.; Matos, E.; et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet 2005, 366, 991–998. [Google Scholar] [CrossRef]
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.-L.; Jin, C.; Wang, S.; Song, Y.; Xu, Z.-Y.; Yan, X.-J.; Li, L.M.; Ning, Y.; Wang, H.J. Prevalence of cervicovaginal human papillomavirus infection and genotypes in the pre-vaccine era in China: A nationwide population-based study. J. Infect. 2021, 82, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, J.-J.; Li, N.; Wang, Y.-W. Distribution of human papillomavirus genotypes in western China and their association with cervical cancer and precancerous lesions. Arch. Virol. 2021, 166, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Delory, T.; Ngo-Giang-Huong, N.; Rangdaeng, S.; Chotivanich, N.; Limtrakul, A.; Putiyanun, C.; Suriyachai, P.; Matanasarawut, W.; Jarupanich, T.; Liampongsabuddhi, P.; et al. Human Papillomavirus infection and cervical lesions in HIV infected women on antiretroviral treatment in Thailand. J. Infect. 2017, 74, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Higgins, J.P.T. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barba, M.; Venuti, A.; Pizzuti, L.; Cappuzzo, F.; Landi, L.; Carpano, S.; Marchetti, P.; Villa, A.; Vizza, E.; et al. Circulating HPV DNA in the Management of Oropharyngeal and Cervical Cancers: Current Knowledge and Future Perspectives. J. Clin. Med. 2021, 10, 1525. [Google Scholar] [CrossRef]
- Wijayanti, K.E.; Schütze, H.; MacPhail, C.; Braunack-Mayer, A. Parents’ knowledge, beliefs, acceptance and uptake of the HPV vaccine in members of The Association of Southeast Asian Nations (ASEAN): A systematic review of quantitative and qualitative studies. Vaccine 2021. [Google Scholar] [CrossRef] [PubMed]
- Ver, A.T.; Notarte, K.I.; Velasco, J.V.; Buac, K.M.; Nazareno, J.; Lozañes, J.A.; Antonio, D.; Bacorro, W. A systematic review of the barriers to implementing human papillomavirus vaccination programs in low- and middle-income countries in the Asia-Pacific. Asia-Pac. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Huber, B.; Wang, J.W.; Roden, R.B.S.; Kirnbauer, R. RG1-VLP and Other L2-Based, Broad-Spectrum HPV Vaccine Candidates. J. Clin. Med. 2021, 10, 1044. [Google Scholar] [CrossRef]

| Characteristics | Number of Studies | Total Number of WLHIV | Number of WLHIV with the Characteristic | Pooled Mean or Proportion | 95CI |
|---|---|---|---|---|---|
| Age (years) | 27 | 5683 | - | 32 years | (29 to 35) |
| Under ART (%) | 31 | 7383 | 4574 | 54.6% | (44.2% to 64.5%) |
| Undetectable viral load (%) | 9 | 1989 | 1248 | 52.6% | (31.7% to 72.7%) |
| CD4 when tested for HPV (cell/mm3) | 14 | 3053 | - | 422 cells/mm3 | (361.7 to 482.3) |
| Smokers (%) | 15 | 4443 | 850 | 24.1% | (17.9% to 31.6%) |
| Sex workers (%) | 13 | 1630 | 254 | 17.1% | (10.1% to 27.5%) |
| Consistent condom use (%) | 12 | 2406 | 1290 | 54.3% | (40.6% to 67.3%) |
| At least one pregnancy (%) | 14 | 4686 | 4012 | 83.9% | (73.5% to 90.7%) |
| At least primary education (%) | 17 | 5664 | 4142 | 72.9% | (60.7% to 82.4%) |
| Characteristics | Number of Studies | Total WLHIV in the Studies | Number of WLHIV with HR-HPV Results | Pooled Mean or Proportion (95CI) | Univariate Analysis | ||
|---|---|---|---|---|---|---|---|
| Exp(β) | 95CI | p Value | |||||
| Age (years) per year increment | 16 | 4363 | 32 (28–36) | 0.99 | [0.99–1.00] | 0.052 | |
| Under ART | 19 | 5756 | 3958 | 66.3% (56.2–75%) | 0.93 | [0.81–1.07] | 0.349 |
| Undetectable viral load | 6 | 1667 | 1163 | 62.6% (36.5–83%) | 1.06 | [0.77–1.45] | 0.736 |
| CD4 when tested for HPV (cells/mm3) | 10 | 2353 | 448.6 (388.4–508.8) | 1.00 | [0.99–1.00] | 0.731 | |
| Smokers | 9 | 3449 | 610 | 21.5% (15.8–28.6%) | 1.12 | [0.92–1.34] | 0.332 |
| Sex workers | 4 | 772 | 76 | 8.9% (5–15.5%) | 0.99 | [0.10–9.43] | 0.993 |
| Consistent condom use | 8 | 1892 | 1130 | 61.3% (48.5–72.7%) | 1.26 | [0.89–1.77] | 0.243 |
| At least one pregnancy | 9 | 3942 | 3375 | 82.5% (66.3–91.9%) | 0.95 | [0.73–1.25] | 0.741 |
| At least primary education | 12 | 4785 | 3716 | 78.2% (68.1–85.7%) | 1,17 | [0.87–1.59] | 0.326 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verrier, F.; Le Coeur, S.; Delory, T. Cervical Human Papillomavirus Infection (HPV) and High Oncogenic Risk Genotypes among Women Living with HIV in Asia: A Meta-Analysis. J. Clin. Med. 2021, 10, 1911. https://doi.org/10.3390/jcm10091911
Verrier F, Le Coeur S, Delory T. Cervical Human Papillomavirus Infection (HPV) and High Oncogenic Risk Genotypes among Women Living with HIV in Asia: A Meta-Analysis. Journal of Clinical Medicine. 2021; 10(9):1911. https://doi.org/10.3390/jcm10091911
Chicago/Turabian StyleVerrier, Florian, Sophie Le Coeur, and Tristan Delory. 2021. "Cervical Human Papillomavirus Infection (HPV) and High Oncogenic Risk Genotypes among Women Living with HIV in Asia: A Meta-Analysis" Journal of Clinical Medicine 10, no. 9: 1911. https://doi.org/10.3390/jcm10091911
APA StyleVerrier, F., Le Coeur, S., & Delory, T. (2021). Cervical Human Papillomavirus Infection (HPV) and High Oncogenic Risk Genotypes among Women Living with HIV in Asia: A Meta-Analysis. Journal of Clinical Medicine, 10(9), 1911. https://doi.org/10.3390/jcm10091911







