Facial Affect Recognition by Patients with Schizophrenia Using Human Avatars
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- Schizophrenia group:
- (a)
- fulfilling diagnostic criteria DSM-5 for schizophrenia
- (b)
- staying clinically stabilized during the 3 months prior to passing the semi-structured interview, according to criteria already used by our group [27]
- (c)
- being an outpatient
- (d)
- being aged between 18 and 65 years
- (e)
- speaking fluent Spanish
- (f)
- signing the informed consent form
- (a)
- other axis I major mental disorders of DSM-5
- (b)
- suffering mental retardation (intelligence quotient < 70)
- (c)
- suffering somatic pathology that might interfere with facial affect recognition (for example, a significant visual impairment)
- Healthy controls:
2.3. Data Collection Procedure
- Schizophrenia group:
- The Functioning Assessment Short Test, FAST [30]. The 24 items of the FAST scale are divided among six specific areas of functioning: autonomy, occupational functioning, cognitive functioning, financial issues, interpersonal relationships and leisure time. A recent study noted that the FAST showed strong psychometric properties and was a valid instrument for use in clinical practice, clinical trials, and research settings in subjects diagnosed with schizophrenia [31].
- Spanish version [32] of the WHOQOL-BREF [33] assessment tool. WHOQOL-BREF is a generic questionnaire developed by the Study Group on Quality of Life of the World Health Organization which introduces a total of 26 questions to measure the quality of life. The scale is divided into four domains: physical, psychological, social relationships and environmental health.
- Healthy controls:
2.4. Experimental Procedure
2.5. Statistical Analysis
3. Results
3.1. Comparison of Recognition Scores and Reaction Times of Healthy Controls and Patients with Schizophrenia
3.1.1. Recognition Scores and Reaction Times in the Schizophrenia Group
3.1.2. Recognition Scores
3.1.3. Reaction Times
3.2. Influence of Dynamism and Presentation Angle of the DVFs on Emotion Recognition
3.2.1. Dynamism of the DVFs
3.2.2. Presentation Angle of the DVFs
3.3. Influence of Sociodemographic Data on Emotion Recognition for the Schizophrenia Group
3.3.1. Influence of Gender
3.3.2. Influence of Age
3.3.3. Influence of Education Level
3.3.4. Influence of Being Active
3.4. Influence of the Number of Hospitalizations and the Dosage of Antipsychotic Drugs on Emotion Recognition for the Schizophrenia Group
3.5. Influence of the Results of the Schizophrenia Group’s Psychometric Scales on Emotion Recognition
3.5.1. Positive and Negative Syndrome Scale (PANSS)
3.5.2. Functional Assessment Short Test (FAST)
3.5.3. World Health Organization Quality of Life Field Trial Version (WHOQOL-BREF)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomotake, M. Quality of life and its predictors in people with schizophrenia. J. Med Investig. 2011, 58, 167–174. [Google Scholar] [CrossRef]
- Van, O.J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Green, M.F.; Kern, R.S.; Braff, D.L.; Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 2000, 26, 119–136. [Google Scholar] [CrossRef]
- Bowie, C.R.; Reichenberg, A.; Patterson, T.L.; Heaton, R.K.; Harvey, P.D. Determinants of real-world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. Am. J. Psychiatry 2006, 163, 418–425. [Google Scholar] [CrossRef]
- Pinkham, A.E.; Penn, D.L.; Green, M.F.; Buck, B.; Healey, K.; Harvey, P.D. The social cognition psychometric evaluation study: Results of the expert survey and RAND panel. Schizophr. Bull. 2014, 40, 813–823. [Google Scholar] [CrossRef]
- Fernández-Sotos, P.; Torio, I.; Fernández-Caballero, A.; Navarro, E.; González, P.; Dompablo, M.; Rodriguez-Jimenez, R. Social cognition remediation interventions: A systematic mapping review. PLoS ONE 2019, 14, e0218720. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.D.; Salovey, P.; Caruso, D.R.; Sitarenios, G. Emotional intelligence as a standard intelligence. Emotion 2001, 1, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Bortolon, C.; Capdevielle, D.; Raffard, S. Face recognition in schizophrenia disorder: A comprehensive review of behavioral, neuroimaging and neurophysiological studies. Neurosci. Biobehav. Rev. 2015, 53, 79–107. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, J.Y.; Martin, F.; Tiberghien, G.; Verlut, I.; Franck, N. Selective attention to facial emotion and identity in schizophrenia. Neuropsychologia 2002, 40, 503–511. [Google Scholar] [CrossRef]
- Kohler, C.G.; Walker, J.B.; Martin, E.A.; Healey, K.M.; Moberg, P.J. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophr. Bull. 2010, 36, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Wölwer, W.; Streit, M.; Gaebel, W.; Polzer, U. Facial affect recognition in the course of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 1996, 246, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.M.; Barker, J.D.; Barr, S.; Bittner, J.L.; Bromfield, W.D.; Chu, N.; Goode, R.A.; Lee, D.; Simmons, M.; Srinath, A. The efficiency of dynamic and static facial expression recognition. J. Vis. 2013, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pablos, S.; González-Pablos, E.; Martín-Lorenzo, C.; Flores, L.A.; Gómez-García-Bermejo, J.; Zalama, E. Virtual avatar for emotion recognition in patients with schizophrenia: A pilot study. Front. Hum. Neurosci. 2016, 10, 421. [Google Scholar] [CrossRef]
- García, A.S.; Fernández-Sotos, P.; Vicente-Querol, M.A.; Lahera, G.; Rodriguez-Jimenez, R.; Fernández-Caballero, A. Design of reliable virtual human facial expressions and validation by healthy people. Integr. Comput.-Aided Eng. 2020, 27, 287–299. [Google Scholar] [CrossRef]
- Edwards, J.; Jackson, H.J.; Pattison, P.E. Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clin. Psychol. Rev. 2002, 22, 789–832. [Google Scholar] [CrossRef]
- Dyck, M.; Winbeck, M.; Leiberg, S.; Chen, Y.; Gur, R.C.; Mathiak, K. Recognition profile of emotions in natural and virtual faces. PLoS ONE 2008, 3, e3628. [Google Scholar] [CrossRef]
- Dellazizzo, L.; Potvin, S.; Phraxayavong, K.; Dumais, A. Exploring the benefits of virtual reality-assisted therapy following cognitive-behavioral therapy for auditory hallucinations in patients with treatment-resistant schizophrenia: A proof of concept. J. Clin. Med. 2020, 9, 3169. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Fernández-Sotos, P.; Fernández-Caballero, A.; Navarro, E.; Latorre, J.M.; Rodriguez-Jimenez, R.; González, P. Acceptance and use of a multi-modal avatar-based tool for remediation of social cognition deficits. J. Ambient Intell. Humaniz. Comput. 2020, 11, 4513–4524. [Google Scholar] [CrossRef]
- Riva, G.; Serino, S. Virtual reality in the assessment, understanding and treatment of mental health disorders. J. Clin. Med. 2020, 9, 3434. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sotos, P.; Fernández-Caballero, A.; Rodriguez-Jimenez, R. Virtual reality for psychosocial remediation in schizophrenia: A systematic review. Eur. J. Psychiatry 2020, 34, 1–10. [Google Scholar] [CrossRef]
- García, A.S.; Navarro, E.; Fernández-Caballero, A.; González, P. Towards the design of avatar-based therapies for enhancing facial affect recognition. In International Symposium on Ambient Intelligence; Spring: Cham, Switzerland, 2019; pp. 306–313. [Google Scholar]
- Fernández-Caballero, A.; Fernández-Sotos, P.; Navarro, E.; González, P.; Ricarte, J.J.; Ros, L.; Latorre, J.M.; Rodriguez-Jimenez, R. Human-avatar symbiosis in cognitive cybertherapies: Proof of concept for auditory verbal hallucinations. In Ubiquitous Computing and Ambient Intelligence; Ochoa, S.F., Singh, P., Bravo, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 742–753. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W. Facial Action Coding System; Consulting Psychologists Press: Palo Alto, CA, USA, 1978. [Google Scholar]
- Fernández-Sotos, P.; García, A.S.; Vicente-Querol, M.A.; Lahera, G.; Rodriguez-Jimenez, R.; Fernández-Caballero, A. Validation of dynamic virtual faces for facial affect recognition. PLoS ONE 2021, 16, e0246001. [Google Scholar] [CrossRef]
- Rodriguez-Jimenez, R.; Dompablo, M.; Bagney, A.; Santabárbara, J.; Aparicio, A.; Torio, I.; Moreno-Ortega, M.; Lopez-Anton, R.; Lobo, A.; Kern, R.; et al. The MCCB impairment profile in a Spanish sample of patients with schizophrenia: Effects of diagnosis, age, and gender on cognitive functioning. Schizophr. Res. 2015, 169, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, W.; Lenhard, A. Calculation of Effect Sizes; Psychometrica: Dettelbach, Germany, 2016. [Google Scholar] [CrossRef]
- Sánchez-Morla, E.M.; Barabash, A.; Martínez-Vizcaíno, V.; Tabarés-Seisdedos, R.; Balanzá-Martínez, V.; Cabranes-Díaz, J.A.; Baca-Baldomero, E.; Sánchez Gómez, J.L. Comparative study of neurocognitive function in euthymic bipolar patients and stabilized schizophrenic patients. Psychiatry Res. 2009, 169, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.V.; Cuesta, Z.M. Validation of positive and negative symptom scale (PANSS) in a sample of Spanish schizophrenic patients. Actas Luso-Esp. De Neurol. Psiquiatr. Y Cienc. Afines 1994, 22, 171–177. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Rosa, A.R.; Sánchez-Moreno, J.; Martínez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Ayuso-Mateos, J.L.; et al. Validity and reliability of the functioning assessment short test (FAST) in bipolar disorder. Clin. Pract. Epidemiol. Ment. Health 2007, 3, 5. [Google Scholar] [CrossRef]
- González-Ortega, I.; Rosa, A.; Alberich, S.; Barbeito, S.; Vega, P.; Echeburúa, E.; Vieta, E.; González-Pinto, A. Validation and use of the functioning assessment short test in first psychotic episodes. J. Nerv. Ment. Dis. 2010, 198, 836–840. [Google Scholar] [CrossRef]
- Espinoza, I.; Osorio, P.; Torrejón, M.J.; Lucas-Carrasco, R.; Bunout, D. Validation of the WHOQOL-BREF quality of life questionnaire among Chilean older people. Rev. Médica De Chile 2011, 139, 579–586. [Google Scholar] [CrossRef]
- The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Lahera, G.; Ruiz, A.; Brañas, A.; Vicens, M.; Orozco, A. Reaction time, processing speed and sustained attention in schizophrenia: Impact on social functioning. Rev. De Psiquiatr. Y Salud Ment. 2017, 10, 197–205. [Google Scholar] [CrossRef]
- Ruffman, T.; Henry, J.D.; Livingstone, V.; Phillips, L.H. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neurosci. Biobehav. Rev. 2008, 32, 863–881. [Google Scholar] [CrossRef]
- Calder, A.J.; Keane, J.; Manly, T.; Sprengelmeyer, R.; Scott, S.; Nimmo-Smith, I.; Young, A.W. Facial expression recognition across the adult life span. Neuropsychologia 2003, 41, 195–202. [Google Scholar] [CrossRef]
- Gutiérrez-Maldonado, J.; Rus-Calafell, M.; Márquez-Rejón, S.; Ribas-Sabaté, J. Associations between facial emotion recognition, cognition and alexithymia in patients with schizophrenia: Comparison of photographic and virtual reality presentations. In Annual Review of Cybertherapy and Telemedicine 2012; IOS Press: Amsterdam, The Netherlands, 2012; Volume 181, pp. 88–92. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.J.; Kim, J.; Park, D.E.; Jang, H.J.; Ku, J.; Kim, C.H.; Kim, I.Y.; Kim, S.I. Characteristics of social perception assessed in schizophrenia using virtual reality. CyberPsychology Behav. 2007, 10, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Dyck, M.; Winbeck, M.; Leiberg, S.; Chen, Y.; Mathiak, K. Virtual faces as a tool to study emotion recognition deficits in schizophrenia. Psychiatry Res. 2010, 179, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.; Nichols, H.S.; Ichinose, M.; Bian, D.; Bekele, E.; Snodgress, M.; Amat, A.Z.; Granholm, E.; Park, S.; Sarkar, N. Extraction of emotional information via visual scanning patterns: A feasibility study of participants with schizophrenia and neurotypical individuals. ACM Trans. Access. Comput. 2018, 11, 23. [Google Scholar] [CrossRef]
- Fabri, M.; Moore, D.; Hobbs, D. Mediating the expression of emotion in educational collaborative virtual environments: An experimental study. Virtual Real. 2004, 7, 66–81. [Google Scholar] [CrossRef]
- Krumhuber, E.G.; Tamarit, L.; Roesch, E.B.; Scherer, K.R. FACSGen 2.0 animation software: Generating three-dimensional FACS-valid facial expressions for emotion research. Emotion 2012, 12, 351–363. [Google Scholar] [CrossRef]
- Gutiérrez-Maldonado, J.; Rus-Calafell, M.; González-Conde, J. Creation of a new set of dynamic virtual reality faces for the assessment and training of facial emotion recognition ability. Virtual Real. 2018, 18, 61–71. [Google Scholar] [CrossRef]
- Joyal, C.C.; Jacob, L.; Cigna, M.H.; Guay, J.P.; Renaud, P. Virtual faces expressing emotions: An initial concomitant and construct validity study. Front. Hum. Neurosci. 2014, 8, 787. [Google Scholar] [CrossRef]
- Savla, G.N.; Vella, L.; Armstrong, C.C.; Penn, D.L.; Twamley, E.W. Deficits in domains of social cognition in schizophrenia: A meta-analysis of the empirical evidence. Schizophr. Bull. 2012, 39, 979–992. [Google Scholar] [CrossRef]
- Barkl, S.J.; Lah, S.; Harris, A.W.; Williams, L.M. Facial emotion identification in early-onset and first-episode psychosis: A systematic review with meta-analysis. Schizophr. Res. 2014, 159, 62–69. [Google Scholar] [CrossRef]
- Kohler, C.G.; Turner, T.; Stolar, N.M.; Bilker, W.B.; Brensinger, C.M.; Gur, R.E.; Gur, R.C. Differences in facial expressions of four universal emotions. Psychiatry Res. 2004, 128, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Behere, R.V.; Venkatasubramanian, G.; Arasappa, R.; Reddy, N.; Gangadhar, B.N. Effect of risperidone on emotion recognition deficits in antipsychotic-naïve schizophrenia: A short-term follow-up study. Schizophr. Res. 2009, 113, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Jáni, M.; Kašpárek, T. Emotion recognition and theory of mind in schizophrenia: A meta-analysis of neuroimaging studies. World J. Biol. Psychiatry 2018, 19, S86–S96. [Google Scholar] [CrossRef]
- Rodriguez-Jimenez, R.; Fernandez-Garcimartín, H.; Bagney, A.; Dompablo, M.; Torio, I.; Rodríguez, C.; Horcajadas, F.A.; Rodríguez-Torrejano, J. Cognition and schizophrenia: From neurocognition to social cognition. Psilogos 2013, 11, 10–24. [Google Scholar] [CrossRef]
- Beer, A.L. Chapter 27-Nicotine and Cognition: Effects of Nicotine on Attention and Memory Systems in Humans. In Neuropathology of Drug Addictions and Substance Misuse; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 282–290. [Google Scholar] [CrossRef]
- Campos, M.W.; Serebrisky, D.; Castaldelli-Maia, J.M. Smoking and cognition. Curr. Drug Abus. Rev. 2016, 9, 76. [Google Scholar] [CrossRef] [PubMed]


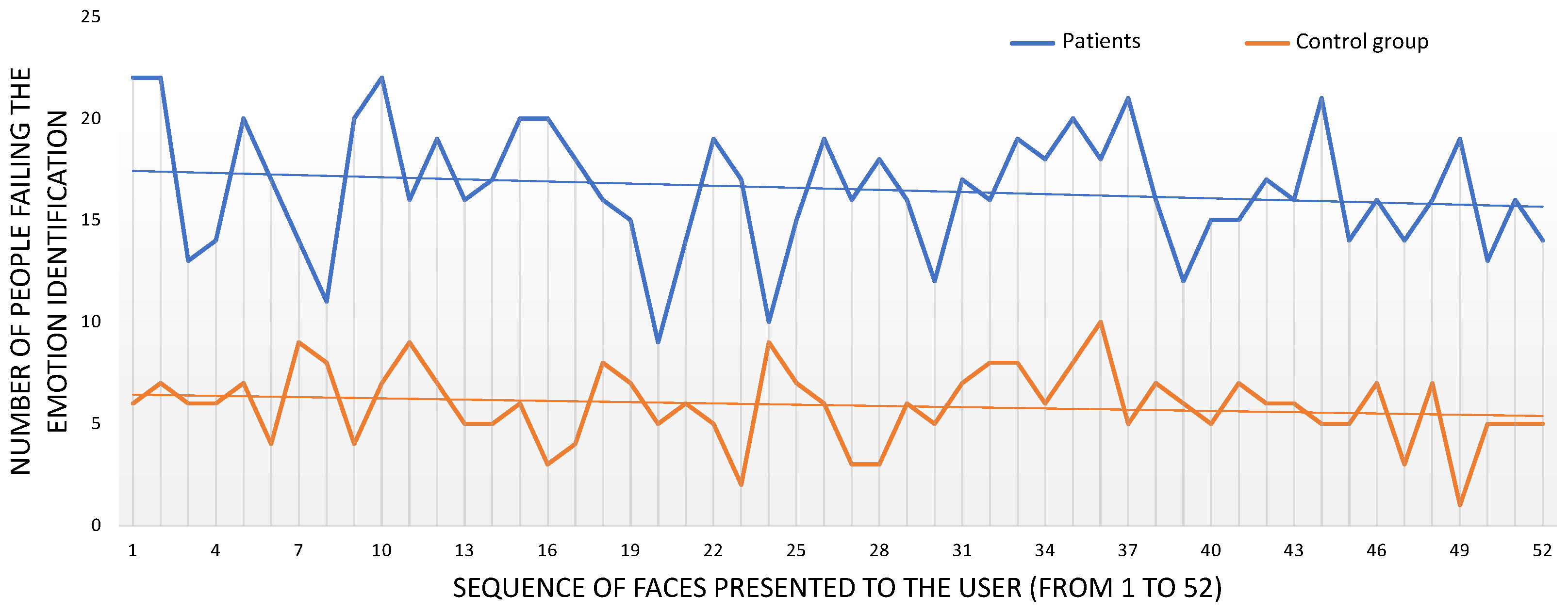
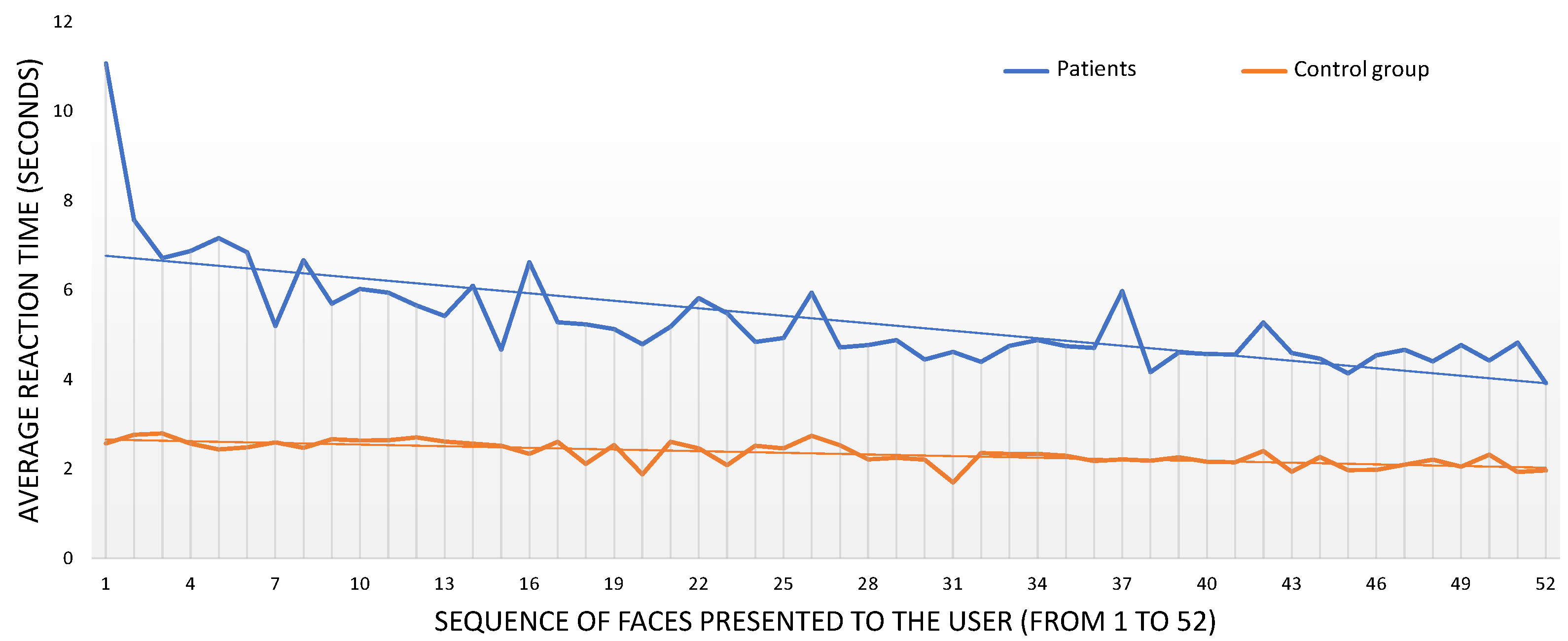
| Schizophrenia Group () | Control Group () | |
|---|---|---|
| Age | 38.0 ± 9.4 | 36.9 ± 11.1 |
| Gender | Men: 35.7% | Men: 35.7% |
| Women: 64.3% | Women: 64.3% | |
| Education level | Basic: 21.4% | Basic: 21.4% |
| Medium: 53.6% | Medium: 53.6% | |
| High: 25.0% | High: 25.0% |
| Patients treated with atypical antipsychotics only | 55 (98.2%) |
| Patients treated with typical antipsychotics only | 0 (0.0%) |
| Patients treated with atypical and typical antipsychotics | 1 (1.8%) |
| Chlorpromazine equivalents | 380 ± 53.6 mg/day |
| Age of onset of the disorder | 23.71 ± 6.05 years |
| Years of disorder evolution | 13.93 ± 9.66 years |
| Schizophrenia Group () | Control Group () | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Surprise | Fear | Anger | Disgust | Joy | Sadness | Neutral | Surprise | Fear | Anger | Disgust | Joy | Sadness | |
| Neutral | 86.2 | 0.9 | 2.2 | 1.8 | 1.8 | 3.6 | 3.6 | 97.3 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 1.8 |
| Surprise | 2.0 | 87.7 | 5.1 | 0.4 | 1.1 | 2.2 | 1.3 | 0.4 | 91.3 | 8.0 | 0.0 | 0.2 | 0.0 | 0.0 |
| Fear | 5.6 | 36.2 | 45.5 | 1.1 | 3.1 | 0.7 | 7.8 | 0.7 | 14.5 | 76.6 | 0.2 | 0.7 | 0.0 | 7.4 |
| Anger | 3.3 | 1.6 | 3.1 | 83.3 | 5.1 | 0.7 | 2.9 | 0.4 | 0.7 | 1.3 | 94.2 | 2.5 | 0.0 | 0.9 |
| Disgust | 4.5 | 2.2 | 3.8 | 23.9 | 61.4 | 0.4 | 3.8 | 0.4 | 1.1 | 1.6 | 8.5 | 88.4 | 0.0 | 0.0 |
| Joy | 10.3 | 4.7 | 1.1 | 2.5 | 3.3 | 76.8 | 1.3 | 2.0 | 0.9 | 0.0 | 0.0 | 0.0 | 97.1 | 0.0 |
| Sadness | 11.6 | 6.7 | 8.3 | 5.1 | 6.7 | 1.3 | 60.3 | 3.1 | 2.7 | 4.5 | 0.4 | 3.6 | 0.4 | 85.3 |
| High Dynamism | ||||||||||||||
| Schizophrenia Group () | Control Group () | |||||||||||||
| Surprise | Fear | Anger | Disgust | Joy | Sadness | Surprise | Fear | Anger | Disgust | Joy | Sadness | |||
| Surprise | 86.8 | 6.2 | 0.9 | 1.3 | 2.2 | 0.4 | 87.1 | 12.0 | 0.0 | 0.4 | 0.0 | 0.0 | ||
| Fear | 38.3 | 52.8 | 0.5 | 2.3 | 0.5 | 2.8 | 16.0 | 79.9 | 0.5 | 0.9 | 0.0 | 1.8 | ||
| Anger | 1.9 | 2.8 | 84.3 | 4.6 | 0.9 | 3.2 | 0.4 | 0.9 | 96.5 | 0.9 | 0.0 | 0.4 | ||
| Disgust | 4.0 | 6.3 | 6.7 | 75.0 | 0.4 | 3.1 | 0.5 | 2.3 | 1.9 | 94.9 | 0.0 | 0.0 | ||
| Joy | 2.9 | 1.3 | 1.7 | 3.8 | 79.5 | 0.4 | 0.5 | 0.0 | 0.0 | 0.0 | 98.1 | 0.0 | ||
| Sadness | 8.0 | 5.8 | 5.8 | 8.0 | 1.8 | 56.7 | 3.4 | 2.1 | 0.4 | 3.8 | 0.0 | 87.6 | ||
| Low Dynamism | ||||||||||||||
| Schizophrenia Group () | Control Group () | |||||||||||||
| Surprise | Fear | Anger | Disgust | Joy | Sadness | Surprise | Fear | Anger | Disgust | Joy | Sadness | |||
| Surprise | 88.7 | 4.1 | 0.0 | 0.9 | 2.3 | 2.3 | 95.8 | 3.7 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Fear | 34.2 | 38.9 | 1.7 | 3.8 | 0.9 | 12.4 | 13.1 | 73.4 | 0.0 | 0.4 | 0.0 | 12.7 | ||
| Anger | 1.3 | 3.4 | 82.3 | 5.6 | 0.4 | 2.6 | 0.9 | 1.8 | 91.9 | 4.1 | 0.0 | 1.4 | ||
| Disgust | 0.4 | 1.3 | 41.1 | 47.8 | 0.4 | 4.5 | 1.7 | 0.9 | 14.7 | 82.3 | 0.0 | 0.0 | ||
| Joy | 6.7 | 1.0 | 3.3 | 2.9 | 73.7 | 2.4 | 1.3 | 0.0 | 0.0 | 0.0 | 96.2 | 0.0 | ||
| Sadness | 5.4 | 10.7 | 4.5 | 5.4 | 0.9 | 63.8 | 1.9 | 7.0 | 0.5 | 3.3 | 0.9 | 82.7 | ||
| Frontal Views | ||||||||||||||
| Schizophrenia Group () | Control Group () | |||||||||||||
| Neutral | Surprise | Fear | Anger | Disgust | Joy | Sadness | Neutral | Surprise | Fear | Anger | Disgust | Joy | Sadness | |
| Neutral | 88.4 | 1.8 | 0.0 | 1.8 | 0.0 | 5.4 | 2.7 | 98.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 |
| Surprise | 1.8 | 89.3 | 4.9 | 0.4 | 0.4 | 1.8 | 1.3 | 0.4 | 91.0 | 8.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fear | 7.1 | 30.4 | 50.4 | 1.3 | 3.1 | 0.9 | 6.7 | 0.9 | 13.7 | 75.7 | 0.4 | 0.9 | 0.0 | 8.4 |
| Anger | 2.7 | 2.2 | 3.1 | 83.0 | 6.3 | 0.9 | 1.8 | 0.4 | 0.9 | 1.3 | 93.8 | 2.2 | 0.0 | 1.3 |
| Disgust | 4.0 | 2.2 | 4.5 | 26.3 | 59.4 | 0.4 | 3.1 | 0.4 | 0.4 | 1.8 | 8.5 | 88.8 | 0.0 | 0.0 |
| Joy | 9.8 | 4.5 | 1.3 | 1.8 | 3.1 | 78.6 | 0.9 | 1.8 | 0.9 | 0.0 | 0.0 | 0.0 | 97.2 | 0.0 |
| Sadness | 10.3 | 7.1 | 7.6 | 6.7 | 6.7 | 2.2 | 59.4 | 2.2 | 1.8 | 3.1 | 0.9 | 3.6 | 0.9 | 87.6 |
| Profile Views | ||||||||||||||
| Schizophrenia Group () | Control Group () | |||||||||||||
| Neutral | Surprise | Fear | Anger | Disgust | Joy | Sadness | Neutral | Surprise | Fear | Anger | Disgust | Joy | Sadness | |
| Neutral | 83.9 | 0.0 | 4.5 | 1.8 | 3.6 | 1.8 | 4.5 | 96.4 | 0.0 | 0.0 | 0.0 | 1.8 | 0.0 | 1.8 |
| Surprise | 2.2 | 86.2 | 5.4 | 0.4 | 1.8 | 2.7 | 1.3 | 0.4 | 91.6 | 7.6 | 0.0 | 0.4 | 0.0 | 0.0 |
| Fear | 4.0 | 42.0 | 40.6 | 0.9 | 3.1 | 0.4 | 8.9 | 0.5 | 15.3 | 77.5 | 0.0 | 0.5 | 0.0 | 6.3 |
| Anger | 4.0 | 0.9 | 3.1 | 83.5 | 4.0 | 0.4 | 4.0 | 0.5 | 0.5 | 1.4 | 94.6 | 2.7 | 0.0 | 0.5 |
| Disgust | 4.9 | 2.2 | 3.1 | 21.4 | 63.4 | 0.4 | 4.5 | 0.4 | 1.8 | 1.3 | 8.5 | 87.9 | 0.0 | 0.0 |
| Joy | 10.7 | 4.9 | 0.9 | 3.1 | 3.6 | 75.0 | 1.8 | 2.2 | 0.9 | 0.0 | 0.0 | 0.0 | 97.0 | 0.0 |
| Sadness | 12.9 | 6.3 | 8.9 | 3.6 | 6.7 | 0.4 | 61.2 | 4.0 | 3.6 | 5.8 | 0.0 | 3.6 | 0.0 | 83.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muros, N.I.; García, A.S.; Forner, C.; López-Arcas, P.; Lahera, G.; Rodriguez-Jimenez, R.; Nieto, K.N.; Latorre, J.M.; Fernández-Caballero, A.; Fernández-Sotos, P. Facial Affect Recognition by Patients with Schizophrenia Using Human Avatars. J. Clin. Med. 2021, 10, 1904. https://doi.org/10.3390/jcm10091904
Muros NI, García AS, Forner C, López-Arcas P, Lahera G, Rodriguez-Jimenez R, Nieto KN, Latorre JM, Fernández-Caballero A, Fernández-Sotos P. Facial Affect Recognition by Patients with Schizophrenia Using Human Avatars. Journal of Clinical Medicine. 2021; 10(9):1904. https://doi.org/10.3390/jcm10091904
Chicago/Turabian StyleMuros, Nora I., Arturo S. García, Cristina Forner, Pablo López-Arcas, Guillermo Lahera, Roberto Rodriguez-Jimenez, Karen N. Nieto, José Miguel Latorre, Antonio Fernández-Caballero, and Patricia Fernández-Sotos. 2021. "Facial Affect Recognition by Patients with Schizophrenia Using Human Avatars" Journal of Clinical Medicine 10, no. 9: 1904. https://doi.org/10.3390/jcm10091904
APA StyleMuros, N. I., García, A. S., Forner, C., López-Arcas, P., Lahera, G., Rodriguez-Jimenez, R., Nieto, K. N., Latorre, J. M., Fernández-Caballero, A., & Fernández-Sotos, P. (2021). Facial Affect Recognition by Patients with Schizophrenia Using Human Avatars. Journal of Clinical Medicine, 10(9), 1904. https://doi.org/10.3390/jcm10091904












