Quality of Life and Sleep in Patients with Pituitary Adenoma in Relation to Tumor Type and Compression of the Optic Chiasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Questionnaires
2.2.1. Short Form 36 Survey Version 2
2.2.2. Epworth Sleepiness Scale
2.2.3. Pittsburgh Sleep Quality Index
2.3. Statistical Analysis
3. Results
3.1. Surgical Outcomes
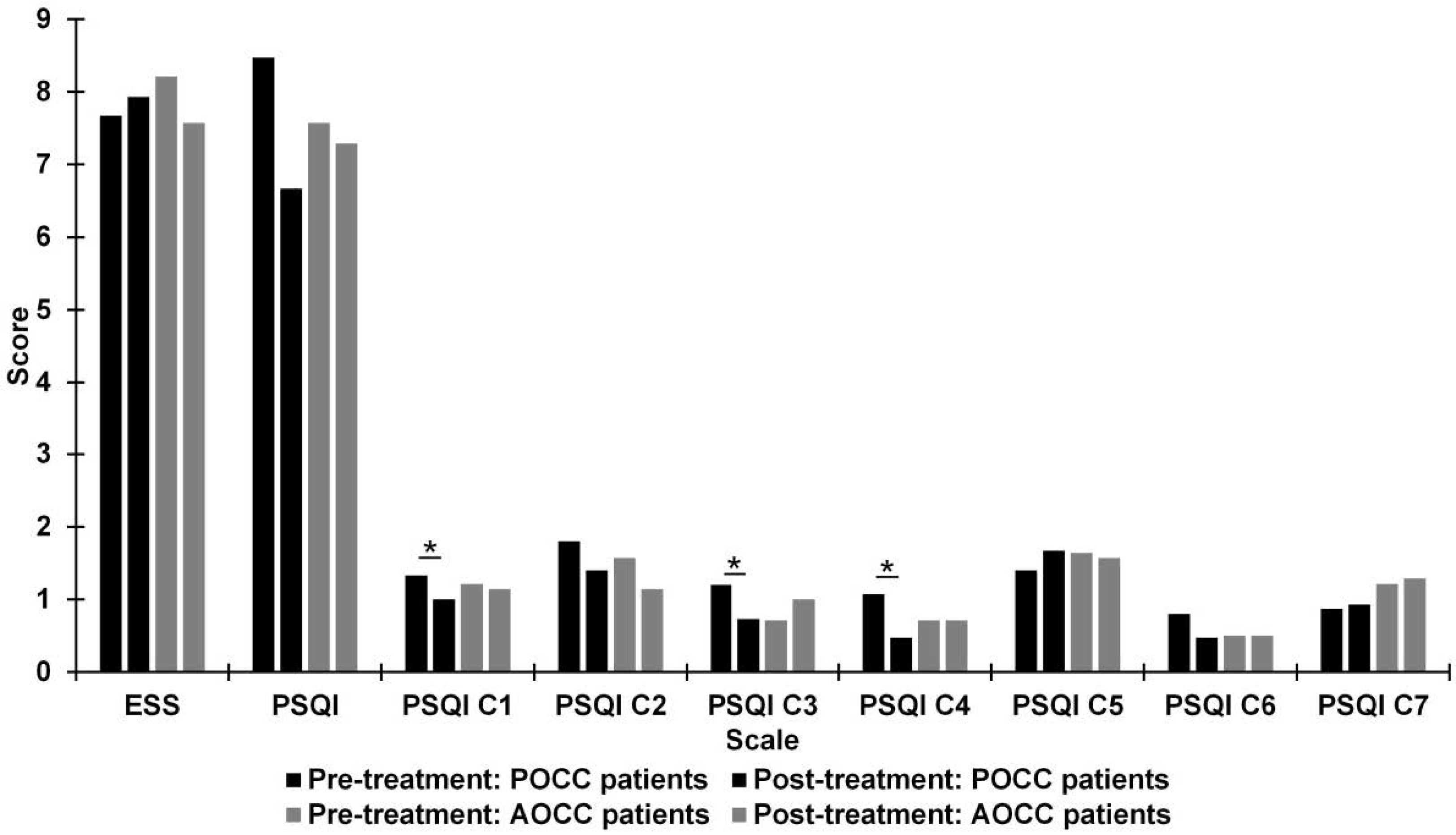
3.2. Pre- and Post-Treatment Comparison of Quality of Sleep
3.3. Pre- and Post-Treatment Comparison of Quality of Life
3.4. Pre-Treatment Relationship between Quality of Sleep and Quality of Life
3.5. Post-Treatment Relationship between Quality of Sleep and Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aflorei, E.D.; Korbonits, M. Epidemiology and etiopathogenesis of pituitary adenomas. J. Neuro-Oncol. 2014, 117, 379–394. [Google Scholar] [CrossRef]
- Ntali, G.; Wass, J.A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 2018, 21, 111–118. [Google Scholar] [CrossRef]
- Vermalle, M.; Alessandrini, M.; Graillon, T.; Paladino, N.C.; Baumstarck, K.; Sebag, F.; Dufour, H.; Brue, T.; Castinetti, F. Lack of functional remission in Cushing’s syndrome. Endocrine 2018, 61, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Van der Klaauw, A.A.; Pereira, A.M.; Biermasz, N.R.; Honkoop, P.J.; Roelfsema, F.; Smit, J.W.; Romijn, J.A. Quality of Life Is Decreased after Treatment for Nonfunctioning Pituitary Macroadenoma. J. Clin. Endocrinol. Metab. 2006, 91, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Biermasz, N.R.; van Thiel, S.W.; Pereira, A.M.; Hoftijzer, H.C.; van Hemert, A.M.; Smit, J.W.; Romijn, J.A.; Roelfsema, F. Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J. Clin. Endocrinol. Metab. 2004, 89, 5369–5376. [Google Scholar] [CrossRef]
- Capatina, C.; Christodoulides, C.; Fernandez, A.; Cudlip, S.; Grossman, A.B.; Wass, J.A.; Karavitaki, N. Current treatment protocols can offer a normal or near-normal quality of life in the majority of patients with non-functioning pituitary adenomas. Clin. Endocrinol. 2013, 78, 86–93. [Google Scholar] [CrossRef]
- Lindsay, J.R.; Nansel, T.; Baid, S.; Gumowski, J.; Nieman, L.K. Long-Term Impaired Quality of Life in Cushing’s Syndrome despite Initial Improvement after Surgical Remission. J. Clin. Endocrinol. Metab. 2006, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Van Der Klaauw, A.A.; Kars, M.; Biermasz, N.R.; Roelfsema, F.; Dekkers, O.M.; Corssmit, E.P.; Van Aken, M.O.; Havekes, B.; Pereira, A.M.; Pijl, H.; et al. Disease-specific impairments in quality of life during long-term follow-up of patients with different pituitary adenomas. Clin. Endocrinol. 2008, 69, 775–784. [Google Scholar] [CrossRef]
- Andela, C.D.; Scharloo, M.; Pereira, A.M.; Kaptein, A.A.; Biermasz, N.R. Quality of life (QoL) impairments in patients with a pituitary adenoma: A systematic review of QoL studies. Pituitary 2015, 18, 752–776. [Google Scholar] [CrossRef] [PubMed]
- Leistner, S.M.; Klotsche, J.; Dimopoulou, C.; Athanasoulia, A.P.; Roemmler-Zehrer, J.; Pieper, L.; Schopohl, J.; Wittchen, H.U.; Stalla, G.K.; Fulda, S.; et al. Reduced sleep quality and depression associate with decreased quality of life in patients with pituitary adenomas. Eur. J. Endocrinol. 2015, 172, 733–743. [Google Scholar] [CrossRef]
- van Schaik, J.; Pillen, S.; van Litsenburg, R.R.; Vandenbussche, N.L.; de Bont, J.M.; Schouten-van Meeteren, A.Y.; van Santen, H.M. The importance of specialized sleep investigations in children with a suprasellar tumor. Pituitary 2020, 23, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A. Pituitary diseases and sleep disorders. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. The diagnosis of Cushing’s syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Bolanowski, M.; Ruchała, M.; Zgliczyński, W.; Kos-Kudła, B.; Hubalewska-Dydejczyk, A.; Lewiński, A. Diagnostics and treatment of acromegaly-updated recommendations of the Polish Society of Endocrinology. Endokrynol. Pol. 2019, 70, 2–18. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Kosinski, M.; Bjorner, J.B.; Turner-Bowker, D.M.; Gandek, B.; Maruish, M.E. User’s Manual for the 36v2® Health Survey, 2nd ed.; Quality Metric Incorporated: Lincoln, RI, USA, 2007. [Google Scholar]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Rosenthal, R.; Rubin, D.B. Requivalent: A simple effect size indicator. Psychol. Methods 2003, 8, 492–496. [Google Scholar] [CrossRef]
- Shibata, S.; Moore, R.Y. Neuropeptide Y and optic chiasm stimulation affect suprachiasmatic nucleus circadian function in vitro. Brain Res. 1993, 615, 95–100. [Google Scholar] [CrossRef]
- Joustra, S.D.; Kruijssen, E.; Verstegen, M.J.; Pereira, A.M.; Biermasz, N.R. Determinants of altered sleep-wake rhythmicity in patients treated for nonfunctioning pituitary macroadenomas. J. Clin. Endocrinol. Metab. 2014, 99, 4497–4505. [Google Scholar] [CrossRef]
- Borgers, A.J.; Romeijn, N.; Van Someren, E.; Fliers, E.; Alkemade, A.; Bisschop, P.H. Compression of the optic chiasm is associated with permanent shorter sleep duration in patients with pituitary insufficiency. Clin. Endocrinol. 2011, 75, 347–353. [Google Scholar] [CrossRef]
- Al Lawati, N.M.; Patel, S.R.; Ayas, N.T. Epidemiology, Risk Factors, and Consequences of Obstructive Sleep Apnea and Short Sleep Duration. Prog. Cardiovasc. Dis. 2009, 51, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Vouzouneraki, K.; Franklin, K.A.; Forsgren, M.; Wärn, M.; Persson, J.T.; Wik, H.; Dahlgren, C.; Nilsson, A.S.; Alkebro, C.; Burman, P.; et al. Temporal relationship of sleep apnea and acromegaly: A nationwide study. Endocrine 2018, 62, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Chemla, D.; Attal, P.; Maione, L.; Veyer, A.S.; Mroue, G.; Baud, D.; Salenave, S.; Kamenicky, P.; Bobin, S.; Chanson, P. Impact of successful treatment of acromegaly on overnight heart rate variability and sleep apnea. J. Clin. Endocrinol. Metab. 2014, 99, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Wolters, T.L.; Roerink, S.H.; Drenthen, L.C.; van Haren-Willems, J.H.; Wagenmakers, M.A.; Smit, J.W.; Hermus, A.R.; Netea-Maier, R.T. The Course of Obstructive Sleep Apnea Syndrome in Patients with Acromegaly During Treatment. J. Clin. Endocrinol. Metab. 2020, 105, 290–304. [Google Scholar] [CrossRef]
- Santos, A.; Resmini, E.; Martínez Momblán, M.A.; Valassi, E.; Martel, L.; Webb, S.M. Quality of Life in Patients with Cushing’s Disease. Front. Endocrinol. 2019, 10, 862. [Google Scholar] [CrossRef]
- Pivonello, R.; De Leo, M.; Cozzolino, A.; Colao, A. The treatment of cushing’s disease. Endocr. Rev. 2015, 36, 385–486. [Google Scholar] [CrossRef]
- Broersen, L.H.; Andela, C.D.; Dekkers, O.M.; Pereira, A.M.; Biermasz, N.R. Improvement but no normalization of quality of life and cognitive functioning after treatment for Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 5325–5337. [Google Scholar] [CrossRef]
- Santos, A.; Granell, E.; Gómez-Ansón, B.; Crespo, I.; Pires, P.; Vives-Gilabert, Y.; Valassi, E.; Webb, S.M.; Resmini, E. Depression and Anxiety Scores Are Associated with Amygdala Volume in Cushing’s Syndrome: Preliminary Study. BioMed Res. Int. 2017, 2017, 2061935. [Google Scholar] [CrossRef] [PubMed]
- Berr, C.M.; Stieg, M.R.; Deutschbein, T.; Quinkler, M.; Schmidmaier, R.; Osswald, A.; Reisch, N.; Ritzel, K.; Dimopoulou, C.; Fazel, J.; et al. Persistence of myopathy in Cushing’s syndrome: Evaluation of the German Cushing’s Registry. Eur. J. Endocrinol. 2017, 176, 737–746. [Google Scholar] [CrossRef]
- Santos, A.; Resmini, E.; Martnez-Mombln, M.A.; Crespo, I.; Valassi, E.; Roset, M.; Badia, X.; Webb, S.M. Psychometric performance of the CushingQoL questionnaire in conditions of real clinical practice. Eur. J. Endocrinol. 2012, 167, 337–342. [Google Scholar] [CrossRef]
- Jawiarczyk-Przybyłowska, A.; Szcześniak, D.; Ciułkowicz, M.; Bolanowski, M.; Rymaszewska, J. Importance of Illness Acceptance among Other Factors Affecting Quality of Life in Acromegaly. Front. Endocrinol. 2020, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Caron, P.J.; Bevan, J.S.; Petersenn, S.; Houchard, A.; Sert, C.; Webb, S.M. Effects of lanreotide Autogel primary therapy on symptoms and quality-of-life in acromegaly: Data from the PRIMARYS study. Pituitary 2016, 19, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lewiński, A.; Smyczyńska, J.; Stawerska, R.; Hilczer, M.; Stasiak, M.; Bednarczuk, T.; Bolanowski, M.; Junik, R.; Ruchała, M.; Syrenicz, A.; et al. National Program of Severe Growth Hormone Deficiency Treatment in Adults and Adolescents after Completion of Growth Promoting Therapy. Endokrynol. Pol. 2018, 69, 468–524. [Google Scholar] [CrossRef]
- Valassi, E.; Brick, D.J.; Johnson, J.C.; Biller, B.M.; Klibanski, A.; Miller, K.K. Effect of growth hormone replacement therapy on the quality of life in women with growth hormone deficiency who have a history of acromegaly versus other disorders. Endocr. Pract. 2012, 18, 209–218. [Google Scholar] [CrossRef]
- van der Klaauw, A.A.; Biermasz, N.R.; Hoftijzer, H.C.; Pereira, A.M.; Romijn, J.A. Previous radiotherapy negatively influences quality of life during 4 years of follow-up in patients cured from acromegaly. Clin. Endocrinol. 2008, 69, 123–128. [Google Scholar] [CrossRef]
- Wolf, A.; Goncalves, S.; Salehi, F.; Bird, J.; Cooper, P.; Van Uum, S.; Lee, D.H.; Rotenberg, B.W.; Duggal, N. Quantitative evaluation of headache severity before and after endoscopic transsphenoidal surgery for pituitary adenoma. J. Neurosurg. 2016, 124, 1627–1633. [Google Scholar] [CrossRef]
- Wolf, A.; Coros, A.; Bierer, J.; Goncalves, S.; Cooper, P.; Van Uum, S.; Lee, D.H.; Proulx, A.; Nicolle, D.; Fraser, J.A.; et al. Quantitative evaluation of vision-related and health-related quality of life after endoscopic transsphenoidal surgery for pituitary adenoma. J. Neurosurg. 2017, 127, 409–416. [Google Scholar] [CrossRef]
- Tanemura, E.; Nagatani, T.; Aimi, Y.; Kishida, Y.; Takeuchi, K.; Wakabayashi, T. Quality of life in nonfunctioning pituitary macroadenoma patients before and after surgical treatment. Acta Neurochir. 2012, 154, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Sommerfelt, H.; Sagberg, L.M.; Solheim, O. Impact of transsphenoidal surgery for pituitary adenomas on overall health-related quality of life: A longitudinal cohort study. Br. J. Neurosurg. 2019, 33, 635–640. [Google Scholar] [CrossRef]
- Dolci, R.L.; de Moraes, L.T.; de Carvalho, A.C.; Rickli, J.C.; de Souza, J.L.; Encinas, W.E.; Junior, J.V.; Scalissi, N.M.; Dos Santos, A.R.; Lazarini, P.R. Quality-of-life evaluation for patients submitted to nasal endoscopic surgery for resection of pituitary tumours. Eur. Arch. Oto-Rhino-Laryngol. 2020, 278, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Li, J.; Wei, L.; Wang, R. Olfactory function and quality of life following microscopic endonasal transsphenoidal pituitary surgery. Medicine 2015, 94, e465. [Google Scholar] [CrossRef] [PubMed]
- Vega-Beyhart, A.; Enriquez-Estrada, V.M.; Bello-Chavolla, O.Y.; Torres-Victoria, T.R.; Martínez-Sánchez, F.D.; López-Navarro, J.M.; Pérez-Guzmán, M.C.; Hinojosa-Amaya, J.M.; León-Suárez, A.; Espinoza-Salazar, H.D.; et al. Quality of life is significantly impaired in both secretory and non-functioning pituitary adenomas. Clin. Endocrinol. 2018, 90, 457–467. [Google Scholar] [CrossRef] [PubMed]
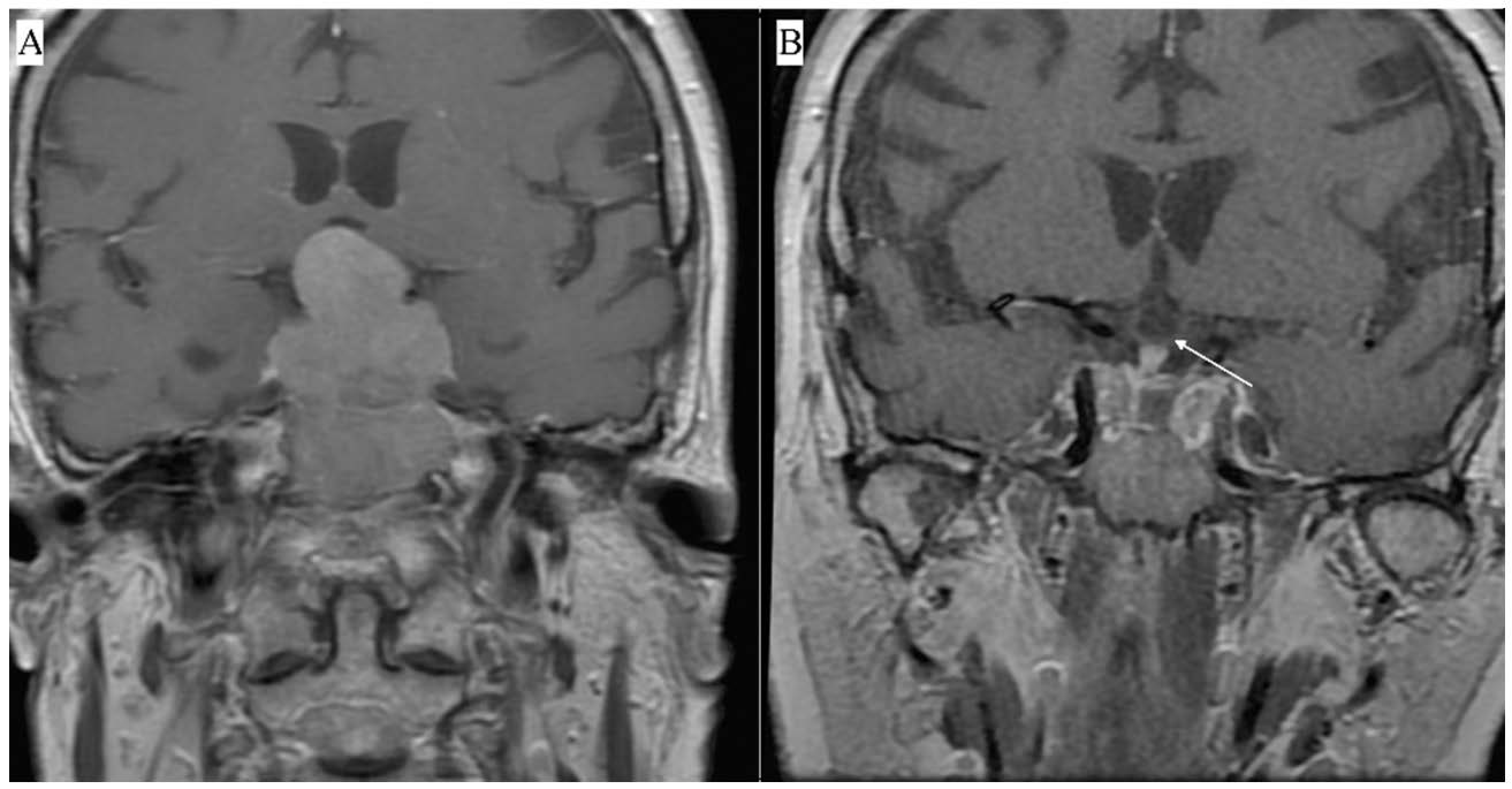



| FPA: n (%) | 17 (59%) |
| Acromegaly: n (%) | 8 (28%) |
| Cushing’s disease: n (%) | 8 (28%) |
| Gonadotropinoma: n (%) | 1 (3.4%) |
| NFPA: n (%) | 12 (41%) |
| Hypopituitarism: n (%) | 18 (62%) |
| Macroadenoma: n (%) | 26 (90%) |
| Below < 4 cm: n | 24 |
| Above > 4 cm (Giant adenoma): n | 2 |
| Microadenoma: n (%) | 3 (10%) |
| Suprasellar tumors with optic chiasm compression (grading according to the Hardy classification system): n (%) | 15 (52%) |
| Grade A | 6 (21%) |
| Grade B | 5 (17%) |
| Grade C | 2 (7%) |
| Grade D | 2 (7%) |
| Knosp scale: n (%) | |
| Grade 0 | 2 (6.9%) |
| Grade 1 | 4 (20%) |
| Grade 2 | 10 (34%) |
| Grade 3 | 8 (28%) |
| Grade 4 | 5 (17%) |
| Age: M (SD) | 52.7 (15.8) |
| Sex | |
| Male: n (%) | 15 (52%) |
| Female: n (%) | 14 (48%) |
| SF-36v2 RP | SF-36v2 BP | SF-36v2 GH | SF-36v2 PF | SF-36v2 VT | SF-36v2 SF | SF-36v2 RE | SF-36v2 MH | |
|---|---|---|---|---|---|---|---|---|
| Patients with acromegaly | ||||||||
| ESS | 0.28 | 0.14 | −0.12 | 0.21 | −0.49 | −0.14 | −0.25 | −0.31 |
| PSQI | −0.35 | −0.78 * | −0.74 * | −0.57 | −0.34 | −0.78 * | −0.76 * | −0.30 |
| Patients with Cushing’s disease | ||||||||
| ESS | 0.14 | −0.59 | −0.16 | −0.09 | −0.11 | −0.60 | −0.39 | −0.56 |
| PSQI | 0.59 | 0.10 | −0.72 * | −0.75 * | 0.33 | −0.39 | 0.08 | 0.09 |
| Patients with non-functioning pituitary adenomas (NFPA) | ||||||||
| ESS | −0.10 | −0.32 | −0.20 | −0.16 | −0.47 | −0.27 | −0.09 | −0.66 * |
| PSQI | −0.37 | −0.62 * | 0.45 | 0.00 | −0.11 | −0.35 | −0.29 | −0.05 |
| Patients with presence of optic chiasm compression (POCC) | ||||||||
| ESS | −0.19 | −0.53 * | −0.33 | 0.17 | −0.46 | −0.41 | −0.30 | −0.62 * |
| PSQI | −0.28 | 0.50 | 0.49 | −0.16 | −0.03 | −0.34 | −0.25 | 0.15 |
| Patients with absence of optic chiasm compression (AOCC) | ||||||||
| ESS | 0.21 | −0.14 | −0.14 | −0.06 | −0.20 | −0.09 | −0.02 | −0.45 |
| PSQI | 0.01 | −0.42 | −0.68 * | −0.78 * | 0.09 | −0.62 * | −0.45 | −0.31 |
| SF-36v2 RP | SF-36v2 BP | SF-36v2 GH | SF-36v2 PF | SF-36v2 VT | SF-36v2 SF | SF-36v2 RE | SF-36v2 MH | |
|---|---|---|---|---|---|---|---|---|
| Patients with acromegaly | ||||||||
| ESS | 0.07 | −0.05 | −0.37 | 0.04 | −0.36 | −0.09 | 0.01 | −0.46 |
| PSQI | −0.24 | −0.02 | −0.74 * | −0.18 | −0.30 | −0.35 | −0.55 | −0.66 |
| Patients with Cushing’s disease | ||||||||
| ESS | −0.35 | −0.70 | −0.04 | −0.15 | 0.14 | 0.16 | −0.11 | 0.33 |
| PSQI | −0.21 | −0.27 | 0.66 | −0.35 | −0.01 | −0.30 | −0.16 | 0.12 |
| Patients with non-functioning pituitary adenomas (NFPA) | ||||||||
| ESS | 0.20 | −0.25 | −0.32 | 0.31 | 0.13 | 0.12 | 0.14 | 0.03 |
| PSQI | −0.21 | −0.31 | −0.18 | −0.32 | −0.11 | −0.15 | −0.18 | −0.01 |
| Patients with presence of optic chiasm compression (POCC) | ||||||||
| ESS | 0.12 | −0.36 | −0.15 | 0.25 | −0.23 | −0.23 | 0.01 | −0.40 |
| PSQI | −0.57 * | −0.20 | −0.02 | −0.49 | −0.12 | −0.15 | −0.45 | 0.05 |
| Patients with absence of optic chiasm compression (AOCC) | ||||||||
| ESS | 0.05 | −0.44 | −0.24 | −0.01 | 0.08 | 0.24 | 0.09 | 0.09 |
| PSQI | −0.03 | −0.10 | −0.43 | −0.21 | −0.13 | −0.16 | −0.14 | −0.55 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagan, K.P.; Andrysiak-Mamos, E.; Tyburski, E.; Sagan, L.M.; Syrenicz, A. Quality of Life and Sleep in Patients with Pituitary Adenoma in Relation to Tumor Type and Compression of the Optic Chiasm. J. Clin. Med. 2021, 10, 1879. https://doi.org/10.3390/jcm10091879
Sagan KP, Andrysiak-Mamos E, Tyburski E, Sagan LM, Syrenicz A. Quality of Life and Sleep in Patients with Pituitary Adenoma in Relation to Tumor Type and Compression of the Optic Chiasm. Journal of Clinical Medicine. 2021; 10(9):1879. https://doi.org/10.3390/jcm10091879
Chicago/Turabian StyleSagan, Karol Piotr, Elżbieta Andrysiak-Mamos, Ernest Tyburski, Leszek Michał Sagan, and Anhelli Syrenicz. 2021. "Quality of Life and Sleep in Patients with Pituitary Adenoma in Relation to Tumor Type and Compression of the Optic Chiasm" Journal of Clinical Medicine 10, no. 9: 1879. https://doi.org/10.3390/jcm10091879
APA StyleSagan, K. P., Andrysiak-Mamos, E., Tyburski, E., Sagan, L. M., & Syrenicz, A. (2021). Quality of Life and Sleep in Patients with Pituitary Adenoma in Relation to Tumor Type and Compression of the Optic Chiasm. Journal of Clinical Medicine, 10(9), 1879. https://doi.org/10.3390/jcm10091879






