Pathophysiology and Treatment Strategies of Acute Myopathy and Muscle Wasting after Sepsis
Abstract
1. Introduction
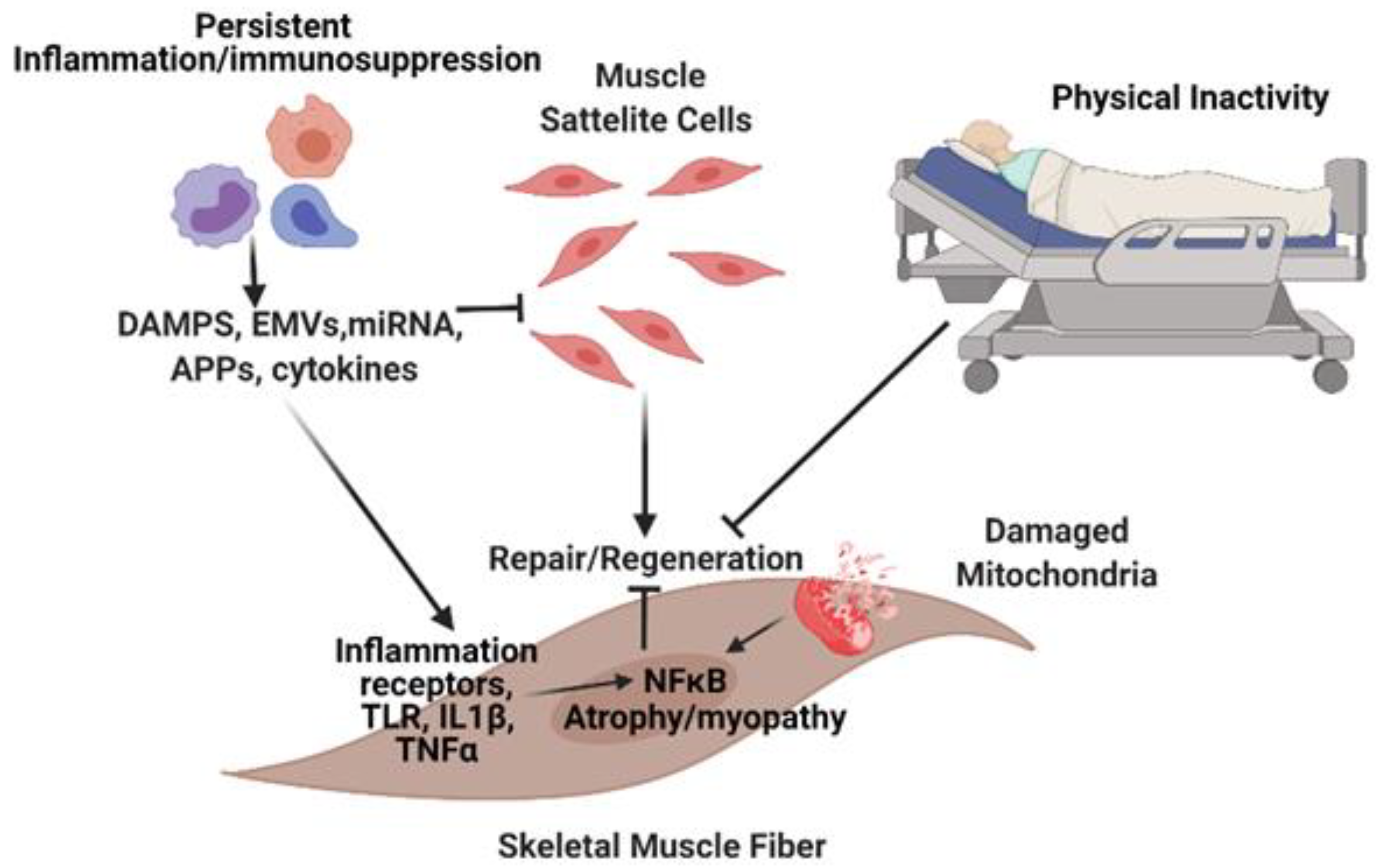
2. Pathobiology of Muscle Dysfunction in Sepsis
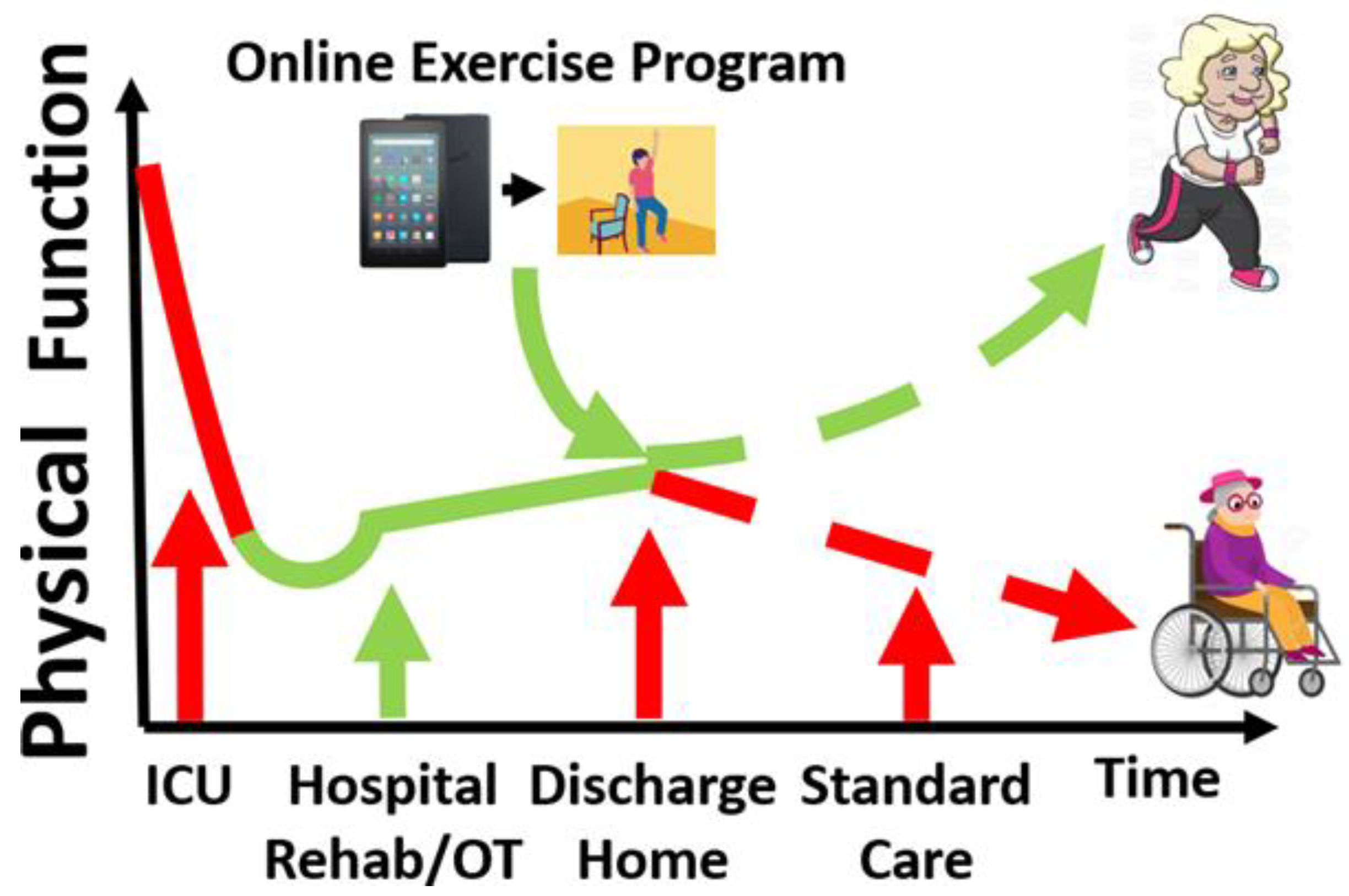
3. Skeletal Muscle Rehabilitation in Sepsis (in-Hospital and Post-Discharge)
4. Other Interventions to Prevent Muscle Wasting in Sepsis Survivors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prescott, H.C.; Angus, D.C. Enhancing Recovery from Sepsis. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Stortz, J.A.; Mira, J.C.; Raymond, S.L.; Loftus, T.J.; Ozrazgat-Baslanti, T.; Wang, Z.; Ghita, G.L.; Leeuwenburgh, C.; Segal, M.S.; Bihorac, A.; et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J. Trauma Acute Care Surg. 2018, 84, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.; Kellum, J.A.; Talisa, V.B.; Palmer, O.M.P.; Chang, C.-C.H.; Filbin, M.R.; Shapiro, N.I.; Hou, P.C.; Venkat, A.; Lovecchio, F.; et al. Long-term Host Immune Response Trajectories Among Hospitalized Patients With Sepsis. JAMA Netw. Open 2019, 2, e198686. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Blatchford, P.J.; Trzeciak, S.; Hollander, J.E.; Birkhahn, R.; Otero, R.; Osborn, T.M.; Moretti, E.; Nguyen, H.B.; Gunnerson, K.J.; et al. Age-Related Differences in Biomarkers of Acute Inflammation During Hospitalization for Sepsis. Shock 2014, 42, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Horiguchi, H.; Loftus, T.J.; Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Hollen, M.K.; Weiss, B.P.; Miller, E.S.; Bihorac, A.; Larson, S.D.; et al. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front. Immunol. 2018, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Mathias, B.; Delmas, A.L.; Ozrazgat-Baslanti, T.; Vanzant, E.L.; Szpila, B.E.; Mohr, A.M.; Moore, F.A.; Brakenridge, S.C.; Brumback, B.A.; Moldawer, L.L.; et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Ann. Surg. 2017, 265, 827–834. [Google Scholar] [CrossRef]
- Hollen, M.K.; Stortz, J.A.; Darden, D.; Dirain, M.L.; Nacionales, D.C.; Hawkins, R.B.; Cox, M.C.; Lopez, M.-C.; Rincon, J.C.; Ungaro, R.; et al. Myeloid-derived suppressor cell function and epigenetic expression evolves over time after surgical sepsis. Crit. Care 2019, 23, 1–16. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Cox, M.C.; Mohr, A.M.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older Sepsis Survivors Suffer Persistent Disability Burden and Poor Long-Term Survival. J. Am. Geriatr. Soc. 2020, 68, 1962–1969. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Darden, D.B.; Bihorac, A.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; Moore, F.A. Older adults demonstrate biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) after sepsis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Rocheteau, P.; Chatre, L.; Briand, D.; Mebarki, M.; Jouvion, G.; Bardon, J.; Crochemore, C.; Serrani, P.; Lecci, P.P.; Latil, M.; et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat. Commun. 2015, 6, 10145. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Hussain, S.N.A.; Mathur, S.; Picard, M.; Herridge, M.; Correa, J.; Bain, A.; Guo, Y.; Advani, A.; Advani, S.L.; et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay. A Pilot Study. Am. J. Respir. Crit. Care Med. 2016, 194, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.; Supinski, G.S. Sepsis-induced myopathy. Crit. Care Med. 2009, 37, S354–S367. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Efron, P.A.; Mohr, A.M.; Bihorac, A.; Horiguchi, H.; Hollen, M.K.; Segal, M.S.; Baker, H.V.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery 2018, 164, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.K.; Ghita, G.L.; Wang, Z.; Ozrazgat-Baslanti, T.; Raymond, S.L.; Mankowski, R.T.; Brumback, B.A.; Efron, P.A.; Bihorac, A.; Moore, F.A.; et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival among Early Survivors of Sepsis in Surgical ICUs. Crit. Care Med. 2019, 47, 566–573. [Google Scholar] [CrossRef]
- Choi, S.-J. Age-related functional changes and susceptibility to eccentric contraction-induced damage in skeletal muscle cell. Integr. Med. Res. 2016, 5, 171–175. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Deutschman, C.S.; Pinsky, M.R.; Zuckerbraun, B.; Schumacker, P.T.; Gomez, H.; Gomez, A.; Murray, P.; Kellum, J.A. Mitochondrial Function in Sepsis. Shock 2016, 45, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80. [Google Scholar] [CrossRef]
- Lepper, C.; Partridge, T.A.; Fan, C.-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Developement 2011, 138, 3639–3646. [Google Scholar] [CrossRef]
- Mierzejewski, B.; Archacka, K.; Grabowska, I.; Florkowska, A.; Ciemerych, M.A.; Brzoska, E. Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin. Cell Dev. Biol. 2020, 104, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Relaix, F.; Zammit, P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development 2012, 139, 2845–2856. [Google Scholar] [CrossRef]
- Lewis, M.I.; Lorusso, T.J.; Zhan, W.-Z.; Sieck, G.C. Interactive effects of denervation and malnutrition on diaphragm structure and function. J. Appl. Physiol. 1996, 81, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv. Exp. Med. Biol. 2018, 1088, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Monici, M.; Aguennouz, M.; Mazzeo, A.; Messina, C.; Vita, G. Activation of nuclear factor- B in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 2003, 60, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Krook, A. Innate immune receptors in skeletal muscle metabolism. Exp. Cell Res. 2017, 360, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, K.R.; Goli, P.; Roy, A.; Sharma, A.K.; Xiong, G.; Gallot, Y.S.; Kumar, A. The Toll-Like Receptor/MyD88/XBP1 Signaling Axis Mediates Skeletal Muscle Wasting during Cancer Cachexia. Mol. Cell. Biol. 2019, 39. [Google Scholar] [CrossRef]
- McKenzie, A.I.; Briggs, R.A.; Barrows, K.M.; Nelson, D.S.; Kwon, O.S.; Hopkins, P.N.; Higgins, T.F.; Marcus, R.L.; Drummond, M.J. A pilot study examining the impact of exercise training on skeletal muscle genes related to the TLR signaling pathway in older adults following hip fracture recovery. J. Appl. Physiol. 2017, 122, 68–75. [Google Scholar] [CrossRef]
- Sachdev, U.; Cui, X.; McEnaney, R.; Wang, T.; Benabou, K.; Tzeng, E. TLR2 and TLR4 Mediate Differential Responses to Limb Ischemia through MyD88-Dependent and Independent Pathways. PLoS ONE 2012, 7, e50654. [Google Scholar] [CrossRef]
- Kwon, O.S.; Tanner, R.E.; Barrows, K.M.; Runtsch, M.; Symons, J.D.; Jalili, T.; Bikman, B.T.; McClain, D.A.; O’Connell, R.M.; Drummond, M.J. MyD88 regulates physical inactivity-induced skeletal muscle inflammation, ceramide biosynthesis signaling, and glucose intolerance. Am. J. Physiol. Metab. 2015, 309, E11–E21. [Google Scholar] [CrossRef] [PubMed]
- Laitano, O.; Robinson, G.P.; Murray, K.O.; Garcia, C.K.; Mattingly, A.J.; Morse, D.; King, M.A.; Iwaniec, J.D.; Alzahrani, J.M.; Clanton, T.L. Skeletal muscle fibers play a functional role in host defense during sepsis in mice. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laitano, O.; Robinson, G.P.; Garcia, C.K.; Mattingly, A.J.; Sheikh, L.H.; Murray, K.O.; Iwaniec, J.D.; Alzahrani, J.; Morse, D.; Hidalgo, J.; et al. Skeletal Muscle Interleukin-6 Contributes to the Innate Immune Response in Septic Mice. Shock 2021, 55, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nat. Cell Biol. 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Kim, J.; Kostin, S.; Lepper, C.; Fan, C.-M.; Braun, T. Myf5-Positive Satellite Cells Contribute to Pax7-Dependent Long-Term Maintenance of Adult Muscle Stem Cells. Cell Stem Cell 2013, 13, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Llano-Diez, M.; Renaud, G.; Andersson, M.; Marrero, H.; Cacciani, N.; Engquist, H.; Corpeño, R.; Artemenko, K.; Bergquist, J.; Larsson, L. Mechanisms underlying ICU muscle wasting and effects of passive mechanical loading. Crit. Care 2012, 16, R209. [Google Scholar] [CrossRef]
- Pannérec, A.; Formicola, L.; Besson, V.; Marazzi, G.; Sassoon, D.A. Defining skeletal muscle resident progenitors and their cell fate potentials. Developement 2013, 140, 2879–2891. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Chatre, L.; Verdonk, F.; Rocheteau, P.; Crochemore, C.; Chrétien, F.; Ricchetti, M. A novel paradigm links mitochondrial dysfunction with muscle stem cell impairment in sepsis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.; Kowaltowski, A.J. Satellite cell self-renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption. J. Cachex. Sarcopenia Muscle 2020, 11, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Stiller, K. Physiotherapy in Intensive Care. Chest 2013, 144, 825–847. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ayala, E.; Khan, B.A.; Farber, M.O.; Ely, E.W.; Boustani, M.A. Interventions to Improve the Physical Function of ICU Survivors. Chest 2013, 144, 1469–1480. [Google Scholar] [CrossRef]
- Tipping, C.J.; Harrold, M.; Holland, A.; Romero, L.; Nisbet, T.; Hodgson, C.L. The effects of active mobilisation and rehabilitation in ICU on mortality and function: A systematic review. Intensiv. Care Med. 2017, 43, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Taito, S.; Taito, M.; Banno, M.; Tsujimoto, H.; Kataoka, Y.; Tsujimoto, Y. Rehabilitation for patients with sepsis: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0201292. [Google Scholar] [CrossRef] [PubMed]
- Fuest, K.; Schaller, S.J. Recent evidence on early mobilization in critical-Ill patients. Curr. Opin. Anaesthesiol. 2018, 31, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Fuke, R.; Hifumi, T.; Kondo, Y.; Hatakeyama, J.; Takei, T.; Yamakawa, K.; Inoue, S.; Nishida, O. Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: A systematic review and meta-analysis. BMJ Open 2018, 8, e019998. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, Z.; Zhang, C.; Yao, L.; Yang, L.; Jiang, B.; Wu, Y.; Jiang, L.; Tian, J. What is the optimum time for initiation of early mobilization in mechanically ventilated patients? A network meta-analysis. PLoS ONE 2019, 14, e0223151. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Unoki, T.; Matsuishi, Y.; Egawa, Y.; Hayashida, K.; Inoue, S. Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: A systematic review and meta-analysis. J. Intensiv. Care 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, W.; Cai, Z.; Liu, J.; Wu, J.; Deng, Y.; Yu, K.; Chen, X.; Zhu, L.; Ma, J.; et al. Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0223185. [Google Scholar] [CrossRef] [PubMed]
- Vanhorebeek, I.; Latronico, N.; Berghe, G.V.D. ICU-acquired weakness. Intensiv. Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Anekwe, D.E.; Biswas, S.; Bussières, A.; Spahija, J. Early rehabilitation reduces the likelihood of developing intensive care unit-acquired weakness: A systematic review and meta-analysis. Physiotherapy 2020, 107, 1–10. [Google Scholar] [CrossRef]
- Menges, D.; Seiler, B.; Tomonaga, Y.; Schwenkglenks, M.; Puhan, M.A.; Yebyo, H.G. Systematic early versus late mobilization or standard early mobilization in mechanically ventilated adult ICU patients: Systematic review and meta-analysis. Crit. Care 2021, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Mackney, J.; Harrold, M.; Jenkins, S.; Fehlberg, R.; Thomas, L.; Havill, K.; Jacques, A.; Hill, K. Survivors of Acute Lung Injury Have Greater Impairments in Strength and Exercise Capacity Than Survivors of Other Critical Illnesses as Measured Shortly After ICU Discharge. J. Intensiv. Care Med. 2020, 2020. [Google Scholar] [CrossRef]
- Denehy, L.; Skinner, E.H.; Edbrooke, L.; Haines, K.; Warrillow, S.; Hawthorne, G.; Gough, K.; Hoorn, S.V.; Morris, M.E.; Berney, S. Exercise rehabilitation for patients with critical illness: A randomized controlled trial with 12 months of follow-up. Crit. Care 2013, 17, R156. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Nordon-Craft, A.; Malone, D.C.; Van Pelt, D.; Frankel, S.K.; Warner, M.L.; Kriekels, W.; McNulty, M.; Fairclough, D.L.; Schenkman, M. A Randomized Trial of an Intensive Physical Therapy Program for Patients with Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2016, 193, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.E.; Thomas, K.; Watson, G.; Baker, C.; Bryant, A.; Chadwick, T.J.; Shen, J.; Wood, R.; Wilkinson, J.; Mansfield, L.; et al. Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): A multicentre, parallel-group, randomised controlled trial. Thorax 2017, 73, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Song, J.E.; Ann, H.W.; Jeon, Y.; Ahn, M.Y.; Jung, I.Y.; Kim, M.H.; Jeong, W.; Jeong, S.J.; Ku, N.S.; et al. Effects of Early Exercise Rehabilitation on Functional Recovery in Patients with Severe Sepsis. Yonsei Med. J. 2018, 59, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Eggmann, S.; Verra, M.L.; Luder, G.; Takala, J.; Jakob, S.M. Effects of early, combined endurance and resistance training in mechanically ventilated, critically ill patients: A randomised controlled trial. PLoS ONE 2018, 13, e0207428. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Schaller, S.J.; Anstey, M.; Blobner, M.; Edrich, T.; Grabitz, S.D.; Gradwohl-Matis, I.; Heim, M.; Houle, T.; Kurth, T.; Latronico, N.; et al. Early, goal-directed mobilisation in the surgical intensive care unit: A randomised controlled trial. Lancet 2016, 388, 1377–1388. [Google Scholar] [CrossRef]
- Routsi, C.; Gerovasili, V.; Vasileiadis, I.; Karatzanos, E.; Pitsolis, T.; Tripodaki, E.S.; Markaki, V.; Zervakis, D.; Nanas, S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: A randomized parallel intervention trial. Crit. Care 2010, 14, R74. [Google Scholar] [CrossRef] [PubMed]
- Kayambu, G.; Boots, R.; Paratz, J. Early physical rehabilitation in intensive care patients with sepsis syndromes: A pilot randomised controlled trial. Intensiv. Care Med. 2015, 41, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-Y.; Lee, C.-H.; Lin, R.-L.; Cheng, K.-H. Electric Muscle Stimulation for Weaning from Mechanical Ventilation in Elder Patients with Severe Sepsis and Acute Respiratory Failure—A Pilot Study. Int. J. Gerontol. 2017, 11, 41–45. [Google Scholar] [CrossRef]
- Kuhn, K.F.; Schaller, S.J. Comment on Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: A systematic review and meta-analysis (Okada et al., Journal of Intensive Care 2019). J. Intensiv. Care 2020, 8, 21. [Google Scholar] [CrossRef]
- Burke, D.; Gorman, E.; Stokes, D.; Lennon, O. An evaluation of neuromuscular electrical stimulation in critical care using the ICF framework: A systematic review and meta-analysis. Clin. Respir. J. 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Zayed, Y.; Kheiri, B.; Barbarawi, M.; Chahine, A.; Rashdan, L.; Chintalapati, S.; Bachuwa, G.; Al-Sanouri, I. Effects of neuromuscular electrical stimulation in critically ill patients: A systematic review and meta-analysis of randomised controlled trials. Aust. Crit. Care 2020, 33, 203–210. [Google Scholar] [CrossRef]
- Jackson, J.C.; Ely, E.W.; Morey, M.C.; Anderson, V.M.; Denne, L.B.; Clune, J.K.; Siebert, C.S.; Archer, K.R.; Torres, R.C.; Janz, D.R.; et al. Cognitive and physical rehabilitation of intensive care unit survivors. Crit. Care Med. 2012, 40, 1088–1097. [Google Scholar] [CrossRef]
- Gustavson, A.M.; Falvey, J.R.; Forster, J.E.; Stevens-Lapsley, J.E. Predictors of Functional Change in a Skilled Nursing Facility Population. J. Geriatr. Phys. Ther. 2019, 42, 189–195. [Google Scholar] [CrossRef]
- Wang, J.; Liebel, D.V.; Yu, F.; Caprio, T.V.; Shang, J. Inverse Dose-Response Relationship Between Home Health Care Services and Rehospitalization in Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Costa, D.K. Improving Long-Term Outcomes after Sepsis. Crit. Care Clin. 2018, 34, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Webber, S.C.; John, P.D.S. Changes in Intensity and Duration of Walking among Older Adults from In-Patient Geriatric Rehabilitation to Home. Physiother. Can. 2018, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.E.; Loyd, B.J.; Falvey, J.R.; Figiel, G.J.; Kittelson, A.J.; Cumbler, E.U.; Mangione, K.K. Progressive multi-component home-based physical therapy for deconditioned older adults following acute hospitalization: A pilot randomized controlled trial. Clin. Rehabilitation 2016, 30, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Ashe, M.C.; Winters, M.; Hoppmann, C.A.; Dawes, M.G.; Gardiner, P.A.; Giangregorio, L.M.; Madden, K.M.; McAllister, M.M.; Wong, G.; Puyat, J.H.; et al. “Not just another walking program”: Everyday Activity Supports You (EASY) model—a randomized pilot study for a parallel randomized controlled trial. Pilot Feasibility Stud. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Gibbs, B.B.; Brach, J.S.; Byard, T.; Creasy, S.; Davis, K.K.; McCoy, S.; Peluso, A.; Rogers, R.J.; Rupp, K.; Jakicic, J.M. Reducing Sedentary Behavior Versus Increasing Moderate-to-Vigorous Intensity Physical Activity in Older Adults. J. Aging Health 2017, 29, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.R.; Kuo, Y.-F.; Sharma, G.; Raji, M.A.; Kumar, A.; Goodwin, J.S.; Ostir, G.V.; Ottenbacher, K.J. Mobility After Hospital Discharge as a Marker for 30-Day Readmission. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2012, 68, 805–810. [Google Scholar] [CrossRef]
- Flynn, S.; Oviedo, V.; Hoffmann, W.; Stevens-Lapsley, J.; Roberts, P.; Bini, S. Reliability, Validity and Usability of a digital education and self-assessment app for adults with knee osteoarthritis. Gerontechnology 2018, 17, 158–159. [Google Scholar] [CrossRef]
- Hall, C.D.; Rouse, S.B.; Flynn, S.M.; Hoffmann, W.N. Development of Rock Steady 1.0—A Mobile, Gamified Vestibular Rehabilitation Therapy App. International Conference for Vestibular Rehabilitation; East Tennessee State University: Chicago, IL, USA, 2018. [Google Scholar]
- Walker, B.A.; Hoke, K.; Manley, M.; Flynn, S.; Johnson, R. Establishing the Reliability and Validity of Health in Motion© Automated Falls Screening Tool. Adv. Aging Res. 2018, 7, 39–51. [Google Scholar] [CrossRef][Green Version]
- Flynn, S.M.O.V.; Hoffmann, W.N.; Grindo, T.; Limberg, T.; Niemeier, J.P.; Robinson, K.; Stearns, M.; Smith, B.K. Initial Usability and Val-idation of Inspiration Point: A Digital Pulmonary Rehabilitation Tool for Use with Self-Management Interventions; American Association of Cardiovascular and Pulmonary Rehabilitation: Chicago, IL, USA, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mankowski, R.T.; Laitano, O.; Clanton, T.L.; Brakenridge, S.C. Pathophysiology and Treatment Strategies of Acute Myopathy and Muscle Wasting after Sepsis. J. Clin. Med. 2021, 10, 1874. https://doi.org/10.3390/jcm10091874
Mankowski RT, Laitano O, Clanton TL, Brakenridge SC. Pathophysiology and Treatment Strategies of Acute Myopathy and Muscle Wasting after Sepsis. Journal of Clinical Medicine. 2021; 10(9):1874. https://doi.org/10.3390/jcm10091874
Chicago/Turabian StyleMankowski, Robert T., Orlando Laitano, Thomas L. Clanton, and Scott C. Brakenridge. 2021. "Pathophysiology and Treatment Strategies of Acute Myopathy and Muscle Wasting after Sepsis" Journal of Clinical Medicine 10, no. 9: 1874. https://doi.org/10.3390/jcm10091874
APA StyleMankowski, R. T., Laitano, O., Clanton, T. L., & Brakenridge, S. C. (2021). Pathophysiology and Treatment Strategies of Acute Myopathy and Muscle Wasting after Sepsis. Journal of Clinical Medicine, 10(9), 1874. https://doi.org/10.3390/jcm10091874





