High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial)
Abstract
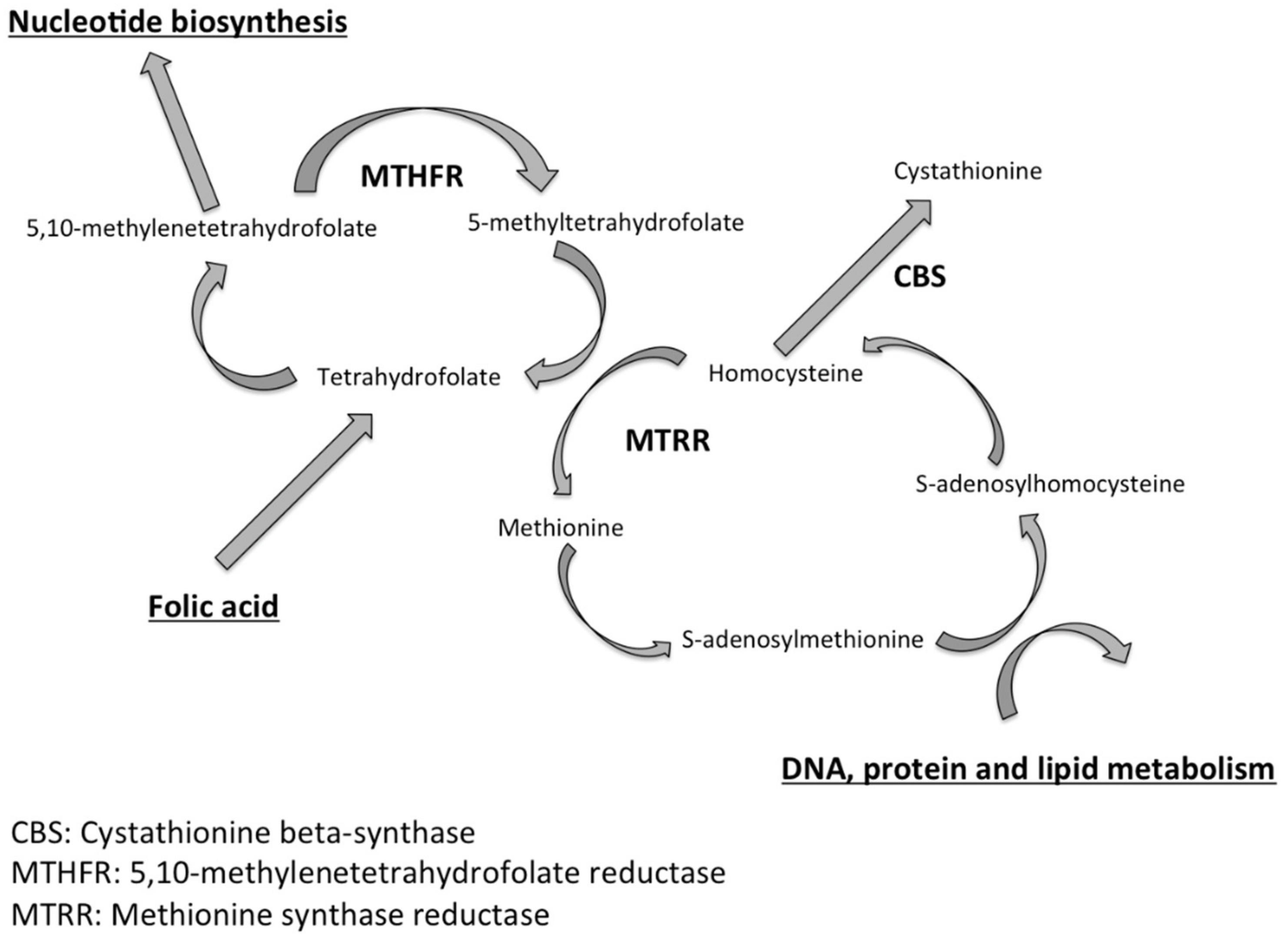
1. Introduction
2. Materials and Methods
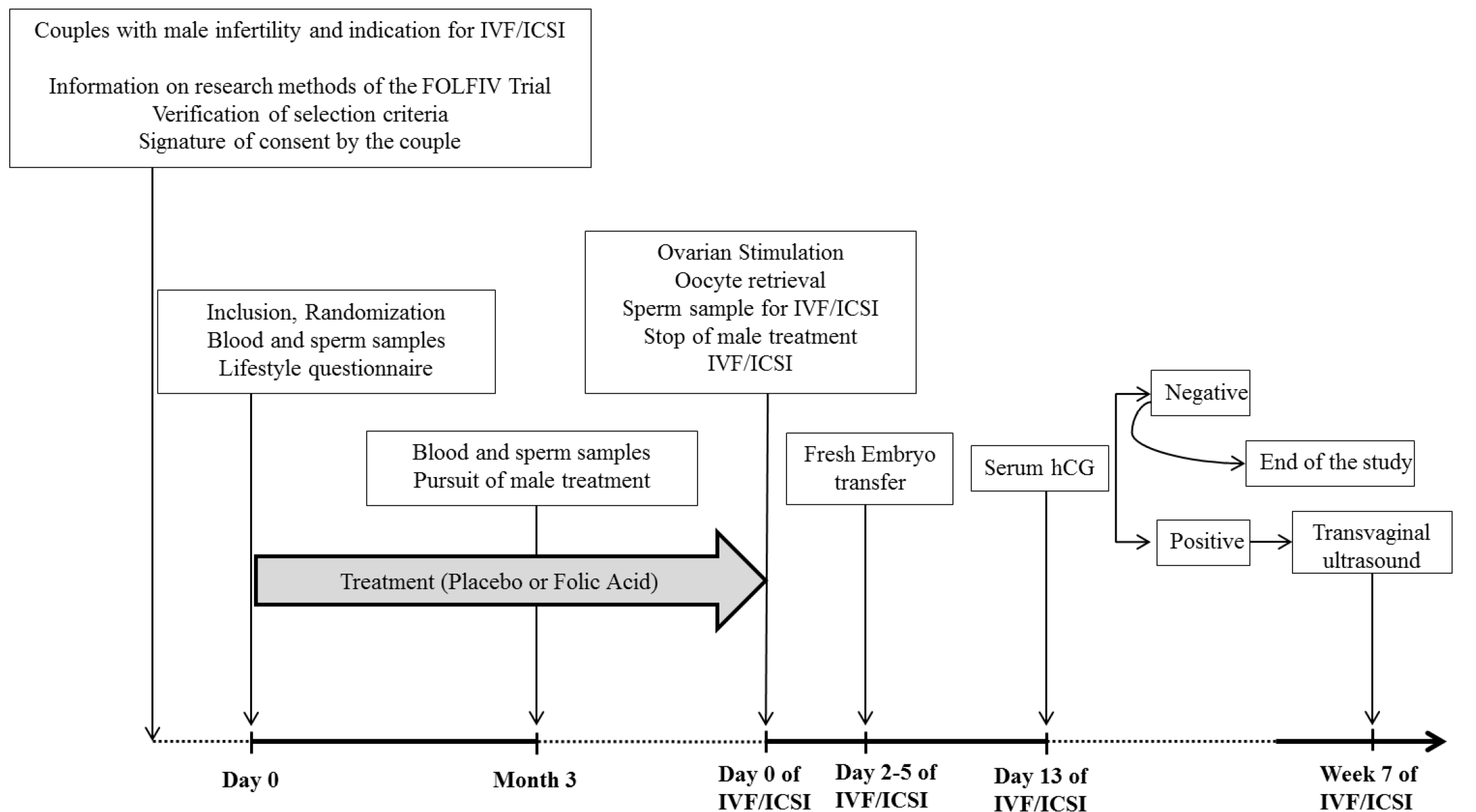
2.1. Study Design
2.2. Participants
2.3. Protocol Procedures
2.3.1. Semen Analysis
2.3.2. Evaluation of DNA Sperm Fragmentation by TUNEL Assay
2.3.3. Blood Samples
2.4. Outcome Measures
- The biochemical pregnancy rate was assessed by the serum hCG level after the embryo transfer and considered positive when >100 IU/mL;
- The clinical pregnancy rate was assessed by ultrasonography at the 7th WG and considered positive when a gestational sac with at least one fetus with positive heart activity was observed.
- Variations in sperm characteristics evaluated by comparing samples on Day 0 and at M3: volume of ejaculate, sperm concentration, motility, vitality;
- Variation in sperm DNA fragmentation was assessed on Day 0 and at M3.
2.5. Randomization
2.6. Statistical Analysis
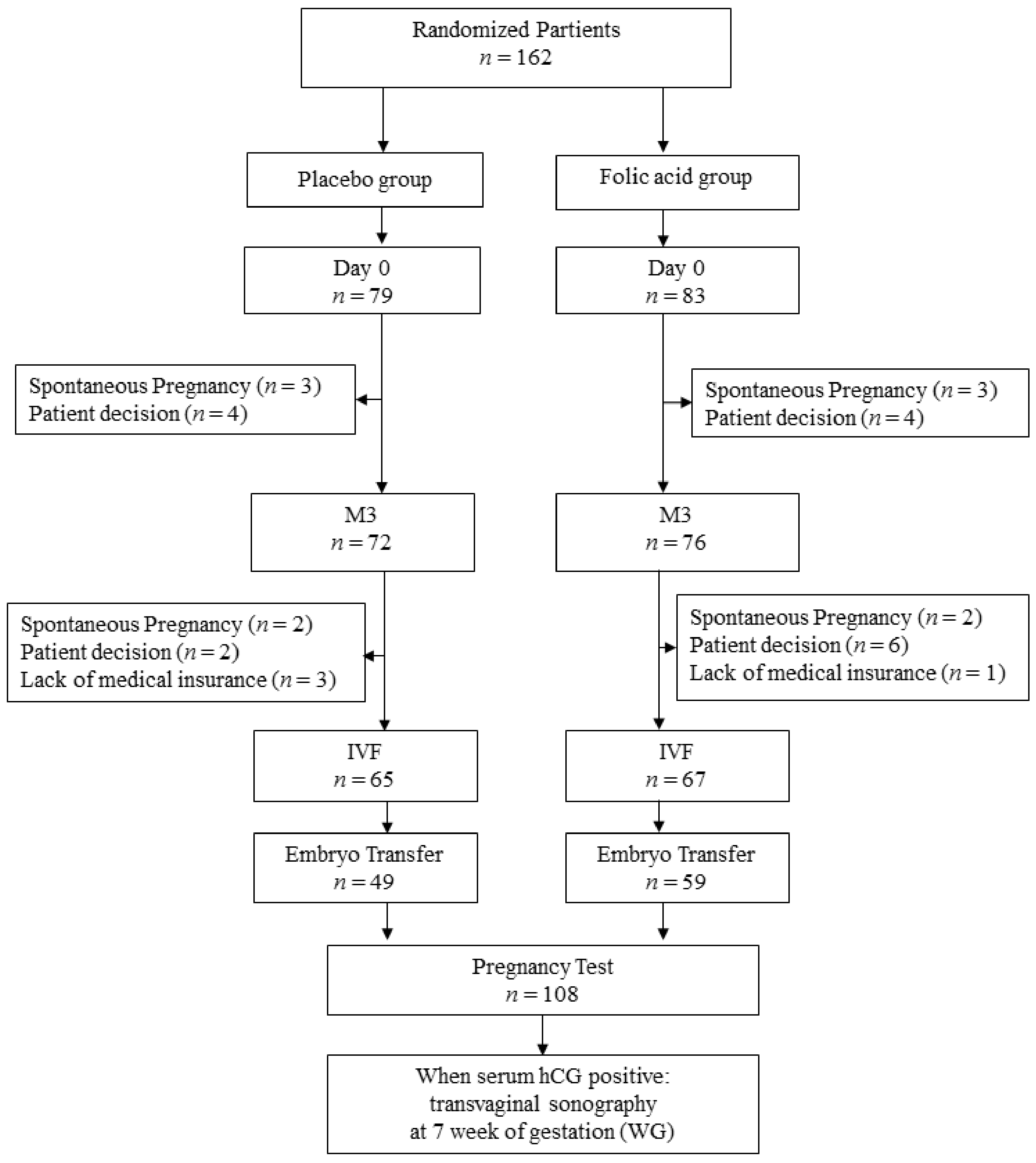
3. Results
3.1. Epidemiologic Characteristics of the Population
3.2. Semen Characteristics and Folic Acid Serum Levels on Day 0, at M3 and on the Day of IVF-ICSI
3.3. Comparison between Baseline and M3
3.4. Protocols of Ovarian Stimulation for IVF-ICSI
3.5. Fertility Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, P.; Aitken, R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014, CD007411. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, R.; Shen, M.; Ye, J.; Li, X.; Huang, Y.; Hua, L.; Wang, Z.; Li, J. Role of genetic mutations in folate-related enzyme genes on Male Infertility. Sci. Rep. 2015, 5, 15548. [Google Scholar] [CrossRef]
- Bezold, G.; Lange, M.; Peter, R.U. Homozygous methylenetetrahydrofolate reductase C677T mutation and male infertility. N. Engl. J. Med. 2001, 344, 1172–1173. [Google Scholar] [CrossRef]
- Chern, C.L.; Huang, R.F.; Chen, Y.H.; Cheng, J.T.; Liu, T.Z. Folate deficiency-induced oxidative stress and apoptosis are mediated via homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-kappaB in human Hep G2 cells. Biomed. Pharmacother. 2001, 55, 434–442. [Google Scholar] [CrossRef]
- Ly, L.; Chan, D.; Aarabi, M.; Landry, M.; Behan, N.A.; MacFarlane, A.J.; Trasler, J. Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation. MHR Basic Sci. Reprod. Med. 2017, 23, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.-F.; Zhao, K.; Zang, Y.; Liu, C.-Y.; Hu, Z.-Y.; Wei, J.-J.; Zhou, T.; Li, Y.; Zhang, H.-P. Effect of folate deficiency on promoter methylation and gene expression of Esr1, Cav1, and Elavl1, and its influence on spermatogenesis. Oncotarget 2017, 8, 24130–24141. [Google Scholar] [CrossRef]
- Eskenazi, B.; Kidd, S.A.; Marks, A.R.; Sloter, E.; Block, G.; Wyrobek, A.J. Antioxidant intake is associated with semen quality in healthy men. Hum. Reprod. 2005, 20, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, G.; Melica, F.; Cristoforoni, P. Folinic acid in the treatment of human male infertility. Fertil. Steril. 1993, 60, 698–701. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.G.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- ALPHA Scientists in Reproductive Medicine; ESHRE Special Interest Group Embryology. Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Reprod. Biomed. Online 2011, 22, 632–646. [Google Scholar] [CrossRef]
- Ravel, C.; Chantot-Bastaraud, S.; Chalmey, C.; Barreiro, L.; Aknin-Seifer, I.; Pfeffer, J.; Berthaut, I.; Mathieu, E.E.; Mandelbaum, J.; Siffroi, J.-P.; et al. Lack of association between genetic polymorphisms in enzymes associated with folate metabolism and unexplained reduced sperm counts. PLoS ONE 2009, 4, e6540. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef]
- Ross, C.; Morriss, A.; Khairy, M.; Khalaf, Y.; Braude, P.; Coomarasamy, A.; El-Toukhy, T. A systematic review of the effect of oral antioxidants on male infertility. Reprod. Biomed. Online 2010, 20, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. J. Am. Med. Assoc. 2020, 323, 35–48. [Google Scholar] [CrossRef]
- Wong, W.Y.; Merkus, H.M.W.M.; Thomas, C.M.G.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Effects of folic acid and zinc sulfate on male factor subfertility: A double-blind, randomized, placebo-controlled trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Thomas, C.M.G.; Peters, W.H.M.; Braat, D.D.M.; Steegers-Theunissen, R.P.M. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Update 2007, 13, 163–174. [Google Scholar] [CrossRef]
- Aarabi, M.; Gabriel, M.C.S.; Chan, D.; Behan, N.A.; Caron, M.; Pastinen, T.; Bourque, G.; MacFarlane, A.J.; Zini, A.; Trasler, J. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum. Mol. Genet. 2015, 24, 6301–6313. [Google Scholar] [CrossRef]
- Irani, M.; Amirian, M.; Sadeghi, R.; Lez, J.L.; Roudsari, R.L. The Effect of Folate and Folate Plus Zinc Supplementation on Endocrine Parameters and Sperm Characteristics in Sub-Fertile Men: A Systematic Review and Meta-Analysis. Urol. J. 2017, 14, 4069–4078. [Google Scholar] [PubMed]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Gardner, D.K.; Schoolcraft, W.B.; Moffatt, O.; Sakkas, D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril. 2004, 82, 378–383. [Google Scholar] [CrossRef]
- Simon, L.; Brunborg, G.; Stevenson, M.; Lutton, D.; McManus, J.; Lewis, S.E.M. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum. Reprod. 2010, 25, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.M.; Pember, S.M.; Jequier, A.M.; Yovich, J.L.; Hartmann, P.E. A test of the human sperm acrosome reaction following ionophore challenge. Relationship to fertility and other seminal parameters. J. Androl. 1991, 12, 98–103. [Google Scholar] [PubMed]
- Esteves, S.C.; Agarwal, A. Novel concepts in male infertility. Int. Braz. J. Urol. 2011, 37, 5–15. [Google Scholar] [CrossRef]
- Condorelli, R.A.; la Vignera, S.; Mongioì, L.M.; Vitale, S.G.; Laganà, A.S.; Cimino, L.; Calogero, A.E. Myo-inositol as a male fertility molecule: Speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 30–35. [Google Scholar] [PubMed]
- Facchinetti, F.; Espinola, M.S.B.; Dewailly, D.; Ozay, A.C.; Prapas, N.; Vazquez-Levin, M.; Wdowiak, A.; Unfer, V. Expert Group on Inositols in Preclinical and Clinical Research, Breakthroughs in the Use of Inositols for Assisted Reproductive Treatment (ART). Trends Endocrinol. Metab. 2020, 31, 570–579. [Google Scholar] [CrossRef]
- Boxmeer, J.C.; Smit, M.; Utomo, E.; Romijn, J.C.; Eijkemans, M.J.C.; Lindemans, J.; Laven, J.S.E.; Macklon, N.S.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertil. Steril. 2009, 92, 548–556. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of the Men | Placebo Group (n = 79) | Folic Acid Group (n = 83) | p | ||
|---|---|---|---|---|---|
| n * | n * | ||||
| Age (year), mean (SD) | 79 | 36.5 (±6.2) | 83 | 37.1 (±6.7) | 0.5128 |
| BMI (kg/m2), median (IQR) | 74 | 25.3 (22.8–27.7) | 73 | 26.5 (23.4–28.7) | 0.2902 |
| Ethnicity | 79 | 83 | 0.4989 | ||
| North African, n (%) | 18 (22.7) | 29 (35) | |||
| Black African, n (%) | 7 (8.9) | 10 (12.1) | |||
| South American, n (%) | 1 (1.3) | 1 (1.2) | |||
| Asian n (%) | 3 (3.8) | 2 (2.4) | |||
| Caucasian n (%) | 44 (55.7) | 36 (43.4) | |||
| Others n (%) | 6 (7.5) | 5 (6) | |||
| Medical history | |||||
| Family cryptorchidism 1, n (%) | 76 | 1 (1.3) | 78 | 1 (1.3) | 0.8593 |
| Pregnancy with previous partners, n (%) | 77 | 3 (3.9) | 83 | 5 (6.0) | 0.3792 |
| Mumps orchitis, n (%) | 74 | 0 (0) | 81 | 1 (1.2) | 0.2682 |
| Genital infection, n (%) | 74 | 2 (2.7) | 81 | 3 (3.7) | 0.5371 |
| Cryptorchidism, n (%) | 74 | 0 | 80 | 0 | 0.5861 |
| Testicular trauma, n (%) | 75 | 3 (4.0) | 81 | 3 (3.7) | 0.7412 |
| Treated for varicocele, n (%) | 77 | 4 (5.2) | 82 | 7 (8.5) | 0.6035 |
| History of testicular surgery 2, n (%) | 77 | 4 (5.2) | 82 | 2 (2.4) | 0.4858 |
| FSH serum level (IU/mL), median (IQR) | 42 | 4 (0.0–6.7) | 49 | 4 (0.0–7.9) | 0.8179 |
| Characteristics of Women | Placebo Group (n = 79) | Folic Acid Group (n = 83) | p | ||
|---|---|---|---|---|---|
| n * | n * | ||||
| Age (years) Mean (SD) | 79 | 31.7 ± 4.1 | 83 | 31.3 ± 4.5 | 0.5514 |
| BMI (kg/m2) median (IQR) | 78 | 23.3 (20.9–26.6) | 83 | 24.1 (21.5–26.1) | 0.7331 |
| Ethnicity | 78 | 83 | 0.3254 | ||
| North African n (%) | 19 (24) | 26 (31.3) | |||
| Black African n (%) | 8 (10.3) | 14 (16.9) | |||
| South American n (%) | 2 (2.6) | 1 (1.2) | |||
| Asian n (%) | 5 (6.4) | 1 (1.2) | |||
| Caucasian n (%) | 39 (50.0) | 35 (42.2) | |||
| Others n (%) | 6 (7.7) | 6 (7.2) | |||
| Endometriosis n (%) | 78 | 1 (1.3) | 83 | 0 | 0.2363 |
| Polycystic ovary syndrome n (%) | 78 | 4 (5.1) | 82 | 5 (6.1) | 1 |
| FSH serum level (IU/mL), median (IQR) | 70 | 4.7 (0.0–6.3) | 74 | 1.5 (0.0–6.8) | 0.7594 |
| AMH serum level (ng/mL) median (IQR) | 73 | 3.5 (2.1–5.4) | 73 | 3.8 (2.1–5.7) | 0.7571 |
| AFC median (IQR) | 63 | 14.0 (12–17) | 60 | 13 (9.5–17.5) | 0.4115 |
| Characteristics of Couples | Placebo Group (n = 79) | Folic Acid Group (n = 83) | p | ||
|---|---|---|---|---|---|
| n * | n * | ||||
| Primary infertility, n (%) | 78 | 53(67.9) | 83 | 56 (67.5) | 0.9468 |
| Etiology of the infertility: | 79 | 83 | 0.9226 | ||
| Male Infertility, n (%) | 61 (77.2) | 65 (78.3) | |||
| Mixed Infertility, n (%) | 17 (21.5) | 18 (21.7) | |||
| Duration of infertility (years) median (IQR) | 79 | 3.0 (2.0–5.0) | 83 | 3.0 (2.0–5.0) | 0.4947 |
| Previous infertility treatment: | 0.8900 | ||||
| Intra-uterine insemination, n (%) | 3 (3.8) | 5 (6) | |||
| IVF, n (%) | 2 (2.5) | 3 (3.6) | |||
| ICSI, n (%) | 22 (27.9) | 26 (31.3) | |||
| Placebo Group (n = 79) | Folic Acid Group (n = 83) | p-Value | |||
|---|---|---|---|---|---|
| n * | n * | ||||
| Spermatozoa concentration (Millions/mL) | |||||
| Day 0, median (IQR) mean ± SD | 79 | 7.0 (3.0–18.0) 12.0 ± 13.3 | 83 | 5.5 (2.0–28.0) 21.9 ± 50.0 | 0.9360 |
| M3, median (IQR) mean ± SD | 72 | 7.7 (2.4–17.0) 11.0 ± 10.1 | 76 | 7.6 (2.5–24.0) 21.9 ± 38.6 | 0.6349 |
| M3-M0, median (IQR) mean ± SD | 72 | 0.0 (−4–3.1) −1.2 ± 11.3 | 76 | −0.1 (−4.1–5.2) −1.2 ± 25.7 | 0.8088 |
| Total motility (a + b + c) | |||||
| Day 0, median (IQR) mean ± SD | 78 | 40.0 (25.0–50.0) 38.0 ± 18.4 | 82 | 45.0 (25.0–50.0) 38.3 ± 19.3 | 0.7457 |
| M3, median (IQR) mean ± SD | 72 | 40.0 (25.0–50.0) 38.4 ± 19.7 | 76 | 40.0 (20.0–55.0) 39.1 ± 20.3 | 1 |
| M3-M0, median (IQR) mean ± SD | 71 | 0.0 (−10.0–10.0) 0.0 ± 17.6 | 75 | 0.0 (−10.0–8.0) 0.1 ± 12.5 | 0.8830 |
| Progressive motility (a) % | |||||
| Day 0, median (IQR) mean ± SD | 66 | 0.0 (0.0–5.0) 4.3 ± 6.7 | 72 | 0.0 (0.0–10.0) 5.8 ± 8.4 | 0.4390 |
| M3, median (IQR) mean ± SD | 62 | 0.0 (0.0–10.0) 5.8 ± 8.6 | 67 | 0.0 (0.0–10.0) 6.4 ± 8.5 | 0.4390 |
| M3-M0, median (IQR) mean ± SD | 60 | 0.0 (0.0–5) 0.8 ± 6.9 | 66 | 0.0 (0.0–2) 0.6 ± 7.5 | 0.7871 |
| Spermatozoa Morphology, % normal | |||||
| Day 0, median (IQR) mean ± SD | 74 | 5.0 (2.0–10.0) 9.0 ± 11.7 | 80 | 6.0 (1.0–14.5) 9.9 ± 11.5 | 0.7513 |
| M3, median (IQR) mean ± SD | 70 | 5.0 (3.0–14.0) 9.7 ± 11.1 | 72 | 6.0 (1.0–16.0) 10.5 ± 12.2 | 0.8605 |
| M3–M0, median (IQR) mean ± SD | 66 | 0.0 (−2–4) 0.9 ± 7.3 | 71 | 0.0 (−2–4) 1.7 ± 10.0 | 0.6212 |
| DNA fragmentation index (%) | |||||
| Day 0, median (IQR) mean ± SD | 53 | 7.0 (5.5–10.5) 15.0 ± 6.5 | 52 | 8.0 (5.5–10) 8.4 ± 4.6 | 0.6963 |
| M3, median (IQR) mean ± SD | 53 | 5.5 (4.0–10.0) 7.4 ± 4.6 | 51 | 5.0 (3.0–9.0) 6.5 ± 4.6 | 0.1838 |
| M3–M0, median (IQR) mean ± SD | 53 | −1.0 (−2.5–1) −65 ± 3.8 | 51 | −2.0 (−4.5–0) −2.1 ± 3.3 | 0.0488 |
| Placebo Group | Folic Acid Group | p-Value | |
|---|---|---|---|
| Number of patients, n | 65 | 67 | |
| Attempt rank, n (%) | 0.1402 | ||
| 1 | 44 (67.69) | 43 (64.8) | |
| 2 | 5 (7.69) | 8 (11.94) | |
| 3 | 7 (10.77) | 13 (19.0) | |
| 4 | 9 (13.85) | 3 (4.48) | |
| IVF, n (%) | 1 (1.54) | 6 (8.96) | 0.1153 |
| IVF + ICSI n (%) | 64 (98.46) | 61 (91.04) | |
| Stimulation protocol, n (%) | 0.6679 | ||
| Long Agonist | 14 (21.54) | 20 (29.85) | |
| Short Agonist | 9 (13.85) | 10 (14.93) | |
| Flare Up agonist | 2 (3.08) | 1 (1.49) | |
| Antagonist | 40 (61.54) | 36 (53.73) | |
| Gonadotropin doses (IU), mean (SD) | 1947.88 (±867.62) | 2103.52 (±1267.95) | 0.9059 |
| Ovarian stimulation time (day), mean (SD) | 11.38 (±1.57) | 10.98 (±2.46) | 0.5206 |
| No. of retrieved oocytes, mean (SD) | 12.7 (±7.1) | 11.0 (±6.8) | 0.2226 |
| No. M II oocytes, mean (SD) | 8.59 (±5.8) | 7.6 (±5.32) | 0.3735 |
| No. of embryos obtained (Day1), mean (SD) | 5.07 (±3.3) | 4.83 (±3.17) | 0.7255 |
| Fertilization rate, mean (SD) | 65.8 ± 39.3 | 68.0 ± 33.7 | 0.7541 |
| No. of transfers, n (%) | 49 (80.3) | 59 (89.4) | 0.1524 |
| Day of fresh transfer, n (%) | 0.7942 | ||
| D1 | 1 (2) | 1 (1.7) | |
| D2–D3 | 45 (91.8) | 56 (94) | |
| D5 | 1 (2) | 1 (1.69) | |
| No. of transferred embryos, mean (SD) | 1.65 (±0.52) | 1.58 (±0.5) | 0.4803 |
| No. of frozen embryos, mean (SD) | 1.98 (±2.16) | 1.81 (±2.3) | 0.1402 |
| Pregnancy Rate | Placebo Group | Folic Acid Group | p-Value |
|---|---|---|---|
| Biochemical pregnancy rate per oocyte retrieval, n (%) * | 11 (16.9) | 26 (38.8) | 0.005 |
| Biochemical pregnancy per embryo transfer, n (%) ** | 11 (22.4) | 26 (44.1) | 0.018 |
| Clinical pregnancy (7WG) per embryo transfer, n (%) ** | 10 (20.4) | 21 (35.6) | 0.082 |
| Miscarriage rate per embryo transfer, n (%) ** | 1 (2) | 5 (8.5) | 0.146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathieu d’Argent, E.; Ravel, C.; Rousseau, A.; Morcel, K.; Massin, N.; Sussfeld, J.; Simon, T.; Antoine, J.-M.; Mandelbaume, J.; Daraï, E.; et al. High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial). J. Clin. Med. 2021, 10, 1876. https://doi.org/10.3390/jcm10091876
Mathieu d’Argent E, Ravel C, Rousseau A, Morcel K, Massin N, Sussfeld J, Simon T, Antoine J-M, Mandelbaume J, Daraï E, et al. High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial). Journal of Clinical Medicine. 2021; 10(9):1876. https://doi.org/10.3390/jcm10091876
Chicago/Turabian StyleMathieu d’Argent, Emmanuelle, Celia Ravel, Alexandra Rousseau, Karine Morcel, Nathalie Massin, Julie Sussfeld, Tabassome Simon, Jean-Marie Antoine, Jacqueline Mandelbaume, Emile Daraï, and et al. 2021. "High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial)" Journal of Clinical Medicine 10, no. 9: 1876. https://doi.org/10.3390/jcm10091876
APA StyleMathieu d’Argent, E., Ravel, C., Rousseau, A., Morcel, K., Massin, N., Sussfeld, J., Simon, T., Antoine, J.-M., Mandelbaume, J., Daraï, E., & Kolanska, K. (2021). High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial). Journal of Clinical Medicine, 10(9), 1876. https://doi.org/10.3390/jcm10091876







