Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis—A Narrative Literature Review
Abstract
1. Introduction
2. Materials and Results
3. Prognostic Factors and Electrophysiological Abnormalities in Patients with Cardiac Amyloidosis
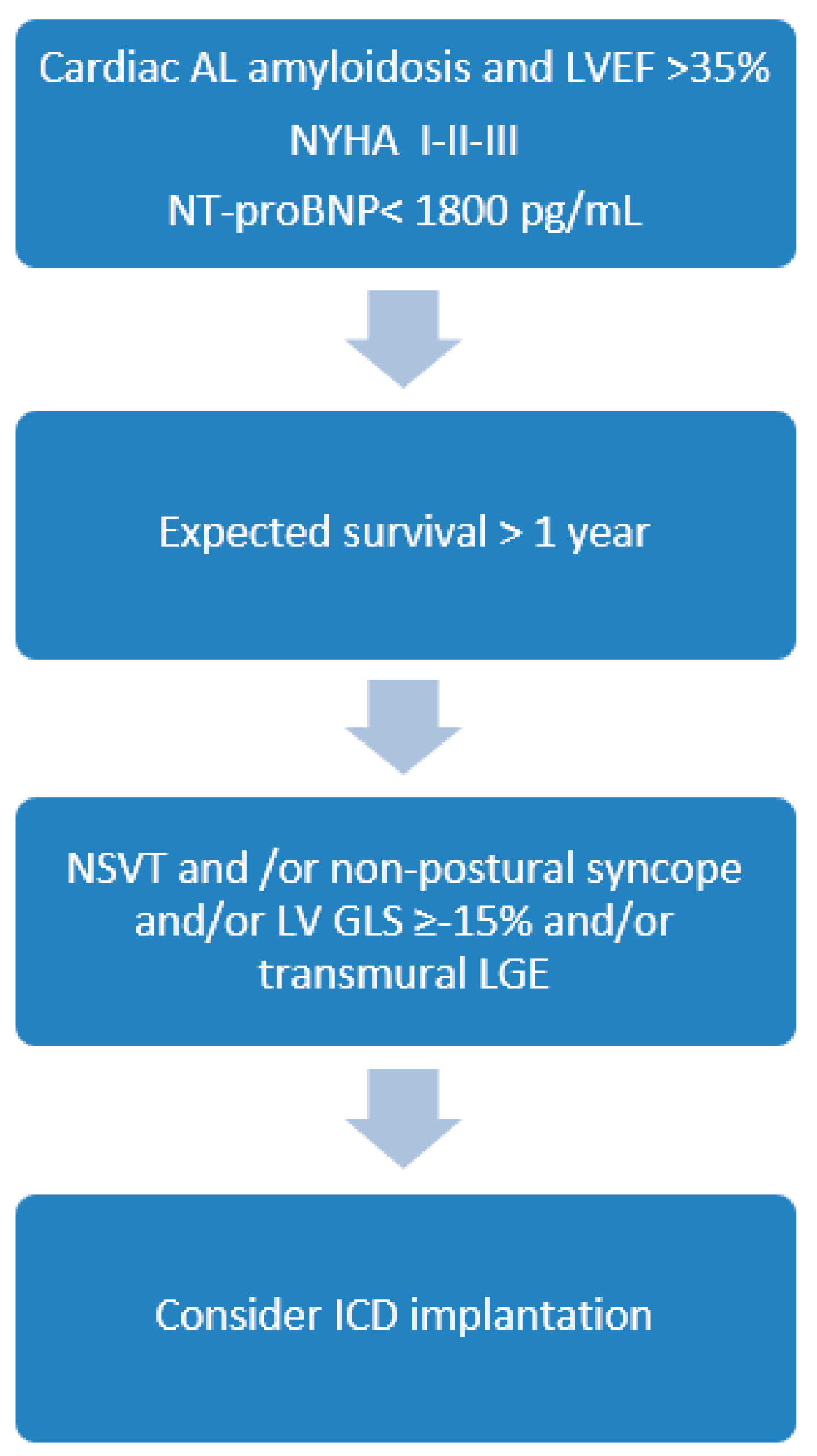
4. The Role of ICD Therapy
5. Future Perspective of Studies on SCD Risk in Patients with Cardiac Amyloidosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falk, R.H. Diagnosis and management of the cardiac amyloidoses. Circulation 2005, 112, 2047–2060. [Google Scholar] [CrossRef]
- Oerlemans, M.I.F.J.; Rutten, K.H.G.; Minnema, M.C.; Raymakers, R.A.P.; Asselbergs, F.W.; de Jonge, N. Cardiac amyloidosis: The need for early diagnosis. Neth. Heart J. 2019, 27, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Witteles, R.M.; Liedtke, M. AL Amyloidosis for the cardiologist and oncologist: Epidemiology, diagnosis, and management. JACC Cardio Oncol. 2019, 1, 117–130. [Google Scholar] [CrossRef]
- Emdin, M.; Aimo, A.; Rapezzi, C.; Fontana, M.; Perfetto, F.; Seferović, P.M.; Barison, A.; Castiglione, V.; Vergaro, G.; Giannoni, A.; et al. Treatment of cardiac transthyretin amyloidosis: An update. Eur. Heart J. 2019, 1, 40, 3699–3706. [Google Scholar] [CrossRef]
- Müller, M.L.; Butler, J.; Heidecker, B. Emerging therapies in transthyretin amyloidosis—A new wave of hope after years of stagnancy? Eur. J. Heart Fail. 2020, 22, 39–53. [Google Scholar] [CrossRef]
- Escher, F.; Senoner, M.; Doerler, J.; Zaruba, M.M.; Messner, M.; Mussner-Seeber, C.; Ebert, M.; Ensinger, C.; Mair, A.; Kroiss, A.; et al. When and how do patients with cardiac amyloidosis die? Clin. Res. Cardiol. 2020, 109, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, J.; Dubrey, S.W.; Lavalley, M.; Skinner, M.; Falk, R.H. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J. Am. Coll. Cardiol. 1997, 30, 1046–1051. [Google Scholar] [CrossRef]
- D’Errico, S.; Mazzanti, A.; Baldari, B.; Maiese, A.; Frati, P.; Fineschi, V. Sudden death in lambda light chain AL cardiac amyloidosis: A review of literature and update for clinicians and pathologists. Int. J. Clin. Exp. Pathol. 2020, 13, 1474–1482. [Google Scholar]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef]
- Wechalekar, A.D.; Schonland, S.O.; Kastritis, E.; Gillmore, J.D.; Dimopoulos, M.A.; Lane, T.; Foli, A.; Foard, D.; Milani, P.; Rannigan, L.; et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013, 25, 3420–3427. [Google Scholar] [CrossRef]
- Lilleness, B.; Ruberg, F.L.; Mussinelli, R.; Doros, G.; Sanchorawala, V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood 2019, 133, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Scott, C.G.; Kyle, R.A.; Zeldenrust, S.R.; Gertz, M.A.; Lin, G.; Klarich, K.W.; Miller, W.L.; Maleszewski, J.J.; Dispenzieri, A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J. Am. Coll. Cardiol. 2016, 6, 1014–1020. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Damy, T.; Fontana, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 7, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Varr, B.C.; Zarafshar, S.; Coakley, T.; Liedtke, M.; Lafayette, R.A.; Arai, S.; Schrier, S.L.; Witteles, R.M. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014, 11, 158–162. [Google Scholar] [CrossRef]
- Fluechter, S.; Kuschyk, J.; Wolpert, C.; Doesch, C.; Veltmann, C.; Haghi, D.; Schoenberg, S.O.; Sueselbeck, T.; Germans, T.; Streitner, F.; et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2010, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 20, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordi, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Heerajnarain Bulluck, V.; et al. Myocardial edema and prognosis in amyloidosis. JACC 2018, 71, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Peck, M.M.; Maram, R.; Mohamed, A.; Ochoa Crespo, D.; Kaur, G.; Malik, B.H. Association of Arrhythmias in Cardiac Amyloidosis and Cardiac Sarcoidosis. Cureus 2020, 12, e9842. [Google Scholar] [CrossRef]
- John, R.M. Arrhythmias in Cardiac Amyloidosis. J. Innov. Card. Rhythm Manag. 2018, 9, 3051–3057. [Google Scholar] [CrossRef]
- Orini, M.; Graham, A.J.; Martinez-Naharro, A.; Andrews, C.M.; de Marvao, A.; Statton, B.; Cook, S.; O’Regan, D.P.; Hawkins, P.N.; Rudy, Y.; et al. Noninvasive Mapping of the Electrophysiological Substrate in Cardiac Amyloidosis and Its Relationship to Structural Abnormalities. J. Am. Heart Assoc. 2019, 8, e012097. [Google Scholar] [CrossRef]
- Ramírez, J.; Orini, M.; Mincholé, A.; Monasterio, V.; Cygankiewicz, I.; Bayés de Luna, A.; Martínez, J.P.; Pueyo, E.; Laguna, P. T-Wave Morphology Restitution Predicts Sudden Cardiac Death in Patients With Chronic Heart Failure. J. Am. Heart Assoc. 2017, 6, e005310. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, C.R.; Kumar, S.; Baldinger, S.H.; Michaud, G.F.; Stevenson, W.G.; Falk, R.; John, R.M. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm. 2016, 13, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Lo, P.; Cho, K.; Subbiah, R. Ventricular Arrhythmias in Cardiac Amyloidosis: A Review of Current Literature. Clin. Med. Insights Cardiol. 2020, 29, 14:1179546820963055. [Google Scholar] [CrossRef] [PubMed]
- Giancaterino, S.; Urey, M.A.; Darden, D.; Hsu, J.C. Management of Arrhythmias in Cardiac Amyloidosis. JACC Clin. Electrophysiol. 2020, 6, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef]
- Kristen, A.V.; Dengler, T.J.; Hegenbart, U.; Schonland, S.O.; Goldschmidt, H.; Sack, F.U.; Voss, F.; Becker, R.; Katus, H.A.; Bauer, A. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008, 5, 235–240. [Google Scholar] [CrossRef]
- Harmon, D.; Algalarrondo, V.; Gandjbakhch, E.; Extramiana, F.; Marijon, E.; Elbaz, N.; Selhane, D.; Dubois-Rande, J.L.; Teiger, E.; Plante-Bordeneuve, V.; et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int. J. Cardiol. 2016, 222, 562–568. [Google Scholar] [CrossRef]
- Kim, E.J.; Holmes, B.B.; Huang, S.; Lugo, R.; Al Aboud, A.; Goodman, S.; Hung, R.R.; Slosky, D.; Stevenson, W.G.; Michaud, G.F.; et al. Outcomes in patients with cardiac amyloidosis and implantable cardioverter-defibrillator. Europace 2020, 22, 1216–1223. [Google Scholar] [CrossRef]
- Lin, G.; Dispenzieri, A.; Kyle, R.; Grogan, M.; Brady, P.A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 2013, 24, 793–798. [Google Scholar] [CrossRef]
- Kojima, T.; Imai, Y.; Fujiu, K.; Suzuki, T.; Sugiyama, H.; Asada, K.; Ajiki, K.; Hayami, N.; Murakawa, Y.; Nagai, R. Anti-arrhythmic device therapy has limits in improving the prognosis of patients with cardiac amyloidosis. J. Arrhythmia 2012, 28, 242–246. [Google Scholar] [CrossRef]
- Rezk, T.; Whelan, C.J.; Lachmann, H.J.; Fontana, M.; Sachchithanantham, S.; Mahmood, S.; Khan, F.; Khiani, R.; Tomson, J.; Youngstein, T.; et al. Role of implantable intracardiac defibrillators in patients with cardiac immunoglobulin light chain amyloidosis. Br. J. Haematol. 2018, 182, 145–148. [Google Scholar] [CrossRef]
- Chuzi, S.; Rosenblatt, A.; Knight, B.P.; Anderson, A.S. Abstract 18064: Outcome of implantable defibrillator therapy in patients with cardiac amyloidosis. Circulation 2018, 136, 1. [Google Scholar]
- Higgins, A.Y.; Annapureddy, A.R.; Wang, Y.; Minges, K.E.; Lampert, R.; Rosenfeld, L.E.; Jacoby, D.L.; Curtis, J.P.; Miller, E.J.; Freeman, J.V. Survival Following Implantable Cardioverter-Defibrillator Implantation in Patients With Amyloid Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e016038. [Google Scholar] [CrossRef]
- Halawa, A.; Woldu, H.G.; Kacey, K.G.; Alpert, M.A. Effect of ICD implantation on cardiovascular outcomes in patients with cardiac amyloidosis: A systematic review and meta-anaylsis. J. Cardiovasc. Electrophysiol. 2020, 31, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Dubrey, S.W.; Cha, K.; Anderson, J.; Chamarthi, B.; Reisinger, J.; Skinner, M.; Falk, R.H. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998, 91, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.M.; Saliba, W.I.; Baranowski, B.; Hanna, M.; Martyn, M.; Patel, D.; Trulock, K.; Menon, V.; Hussein, A.; et al. Cardiac devices in patients with transthyretin amyloidosis: Impact on functional class, left ventricular function, mitral regurgitation, and mortality. J. Cardiovasc. Electrophysiol. 2019, 30, 2427–2432. [Google Scholar] [CrossRef]
- Gonzalez-Duarte, A.; Valdés-Ferre, S.; Cantú-Brito, C. Characteristics and natural history of autonomic involvement in hereditary ATTR amyloidosis: A systematic review. Clin. Auton. Res. 2019, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reyners, A.K.; Hazenberg, B.P.; Reitsma, W.D.; Smit, A.J. Heart rate variability as a predictor of mortality in patients with AA and AL amyloidosis. Eur. Heart J. 2002, 23, 157–161. [Google Scholar] [CrossRef]
- Kokotis, P.; Manios, E.; Schmelz, M.; Fotiou, D.; Dialoupi, I.; Gavriatopoulou, M.; Roussou, M.; Lykka, A.; Dimopoulos, M.A.; Kastritis, E. Involvement of small nerve fibres and autonomic nervous system in AL amyloidosis: Comprehensive characteristics and clinical implications. Amyloid 2020, 27, 103–110. [Google Scholar] [CrossRef]
- Merchant, F.M.; Sayadi, O.; Moazzami, K.; Puppala, D.; Armoundas, A.A. T-wave alternans as an arrhythmic risk stratifier: State of the art. Curr. Cardiol. Rep. 2013, 15, 398. [Google Scholar] [CrossRef] [PubMed]
| Study/Year Published | CA Patients with ICD (n) | AL Amyloi-dosis | ICD in Primary Prevention (n/%) | Criteria for ICD Implantation in Primary Prevention | LVEF | History of Syncope (n/%) | Appropriate ICD Therapy (n/%) | Inappropriate ICD Therapy (n/%) | Survival Directly Post-ICD Therapy (n/%) | Survival during Follow-Up after Appropriate ICD Therapy (n/%) | Overall Survival (n/%) | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kristen et al. (2008) [27] | 19 | 19/19 (100%) | 19 (100%) | Syncope and/or frequent PVBs | ≤45% in 5 pts | 4 (21%) | 2 (11%) | 2 (11%) | 1/2 (50%) | 1/2(50%) | 10/19 (53%) | 811 ± 151 days |
| Kojima et al. (2012) [31] | 3 | 3/3 (100%) | 2 (67%) | NSVT | >55% | 267%) | 3 (100%) | 0 | 3/3 (100%) | 0/3 (0%) | 0/3 (0%) | 7 months (median) |
| Lin et al. (2013) [30] | 53 | 33/53 (62%) | 41 (77%) | LVEF ≤35% or syncope or NSVT | 48 ± 17% | UN | 15 (28%) | 6 (11%) | UN | UN | 21/53(40%) | 23.25 ± 21.45 months |
| Varr et al. (2014) [14] | 19 | 15/19 (79%) | 15 (79%) | Not specified | ≤45% in 5 pts | UN | 5 (26%) | UN | 4/5 (80%) | 1/4 (25%) | UN | 6–23 months |
| Harmon et al. (2016) [28] | 45 | 12/45 (27%) | 38 (84%) | LVEF ≤35% or pacing indication and LV GLS ≥−15% and/or NSVT/frequent PVBs with syncope or planned HTX | <50% in 31 pts <35% in 14 pts | 2 (4%) | 12 (27%) | 2 (4%) | 11/12 (92%) | UN | 27/45 (60%) | 17 ± 14 months |
| Chuzi et al. (2018) [33] | 31 | 14/31 (45%) | 25 (80%) | Not specified | 43 ± 14% | UN | 2 (6%) | 2 (6%) | 2/2 (100%) | UN | 19/31 (61%) | 15 ± 11 months |
| Rezc et al. (2018) [33] | 15 | 15/15 (100%) | 14 (93%) | NSVT and syncope/presyncope | 53% | 4 (27%) | 4 (27%) | UN | 3/4 (75%) | 2/4 (50%) | 13/15 (87%) | 49 months (median) |
| Kim et al. (2019) [30] | 23 | 7/23 (30%) | 23 (100%) | LVEF ≤35% or NSVT and/or syncope | 36 ± 14% | UN | 6 (26%) | 1 (4%) | 6/6 (100%) | 0/6 (0%) | 14/23 (61%) | 3.24 years (median) |
| Donellan et al. (2019) [37] | 38 | 0/38 (0%) | 35 (92%) | Not specified | - | UN | 8 (21%) | UN | UN | 2/8 (25%) | UN | 42 ± 26 months |
| Higgins et al. (2020) [34] | 472 | UN | 356 (75%) | Not specified | ≤30%in 236 pts; >30–40% in 99 pts; <40% in 119 pts | 116 (25%) | UN | UN | UN | UN | 345/472 (73%) | 42 months (median) |
| All studies | 718 | 118/246 (48%) | 569/718 (79%) | - | - | 128/554 (23%) | 57/246(23%) | 13/174 (7%) | 30/34 (88%) | 6/27 (22%) | 449/661 (68%) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liżewska-Springer, A.; Sławiński, G.; Lewicka, E. Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis—A Narrative Literature Review. J. Clin. Med. 2021, 10, 1858. https://doi.org/10.3390/jcm10091858
Liżewska-Springer A, Sławiński G, Lewicka E. Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis—A Narrative Literature Review. Journal of Clinical Medicine. 2021; 10(9):1858. https://doi.org/10.3390/jcm10091858
Chicago/Turabian StyleLiżewska-Springer, Aleksandra, Grzegorz Sławiński, and Ewa Lewicka. 2021. "Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis—A Narrative Literature Review" Journal of Clinical Medicine 10, no. 9: 1858. https://doi.org/10.3390/jcm10091858
APA StyleLiżewska-Springer, A., Sławiński, G., & Lewicka, E. (2021). Arrhythmic Sudden Cardiac Death and the Role of Implantable Cardioverter-Defibrillator in Patients with Cardiac Amyloidosis—A Narrative Literature Review. Journal of Clinical Medicine, 10(9), 1858. https://doi.org/10.3390/jcm10091858







