Thrombotic Complications in Patients with Immune-Mediated Hemolysis
Abstract
1. Introduction
2. Incidence and Risk Factors for Venous or Arterial Thrombosis in Immune Mediated Hemolysis
3. Pathophysiology of Thrombosis in Immune Mediated Hemolysis
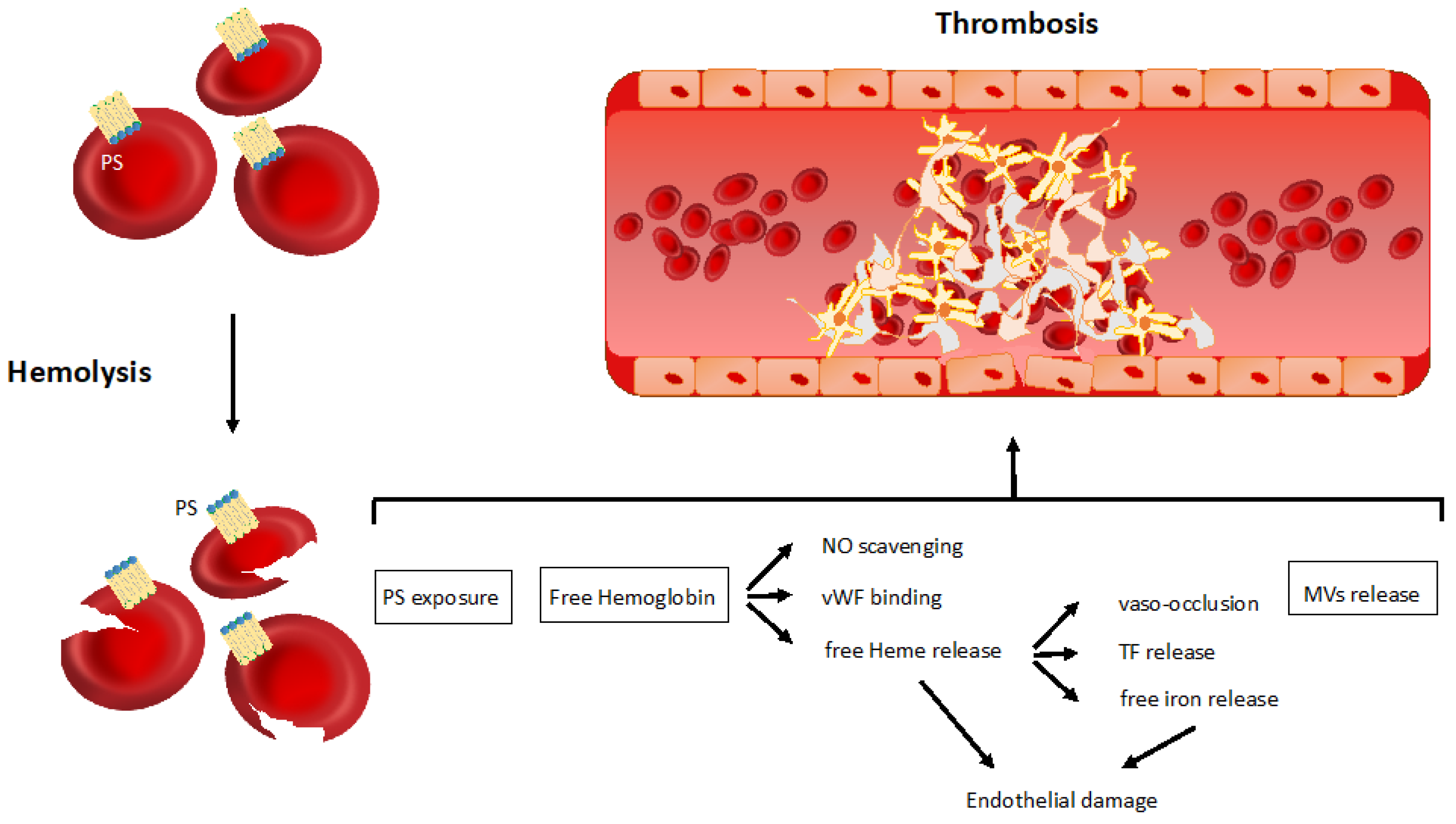
3.1. Non-Immune Related Mechanisms of Thrombosis in Hemolytic Anemia
3.1.1. Phosphatidylserine Exposure
3.1.2. Free Hemoglobin and Heme Release
3.1.3. Microvesicles Shedding
3.2. Immune-Mediated Mechanisms of Thrombosis in Hemolytic Anemia
3.2.1. Autoimmune Hemolytic Anemia
3.2.2. Paroxysmal Nocturnal Hemoglobinuria
4. Thrombophilia in Immune-Mediated Hemolysis
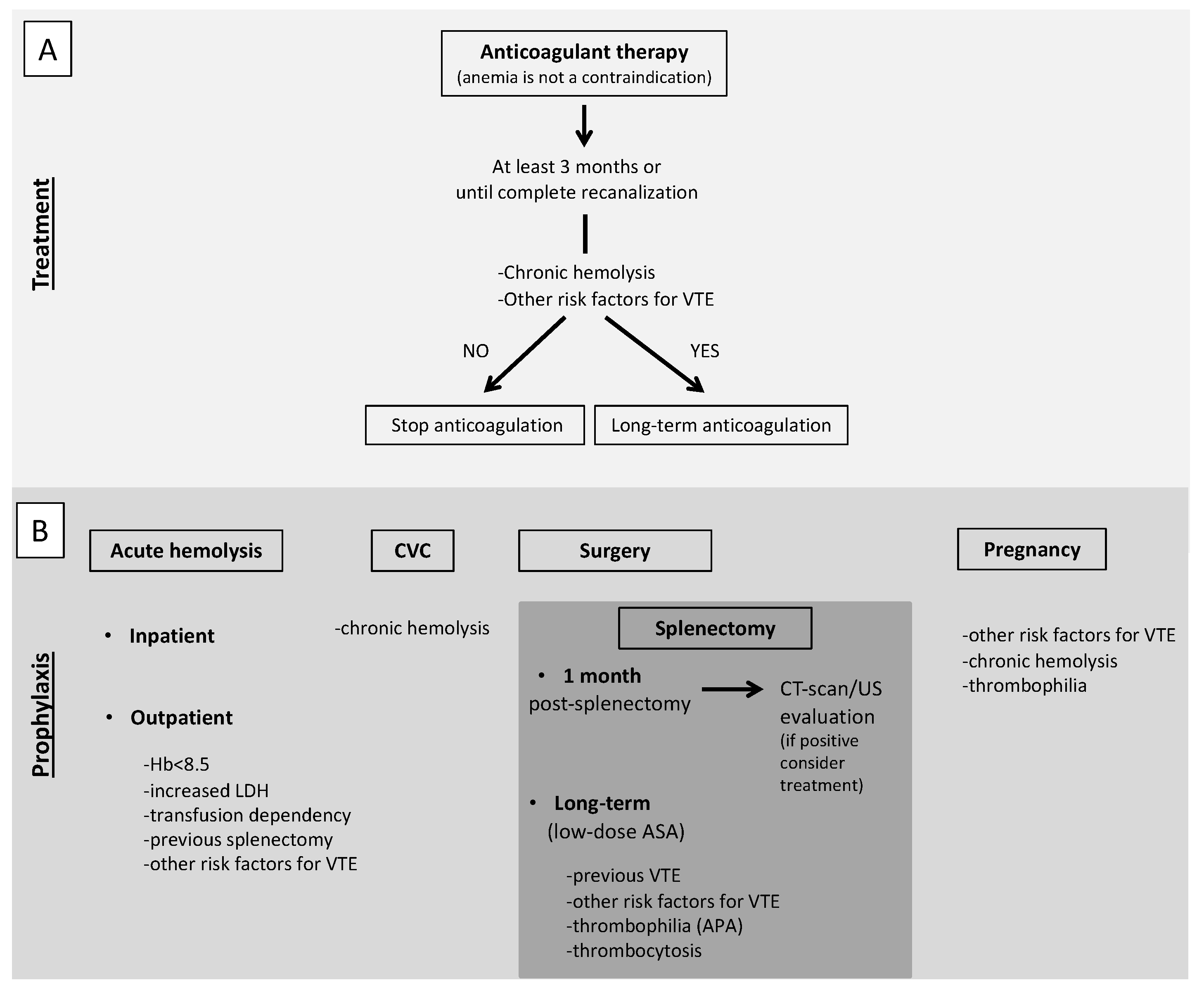
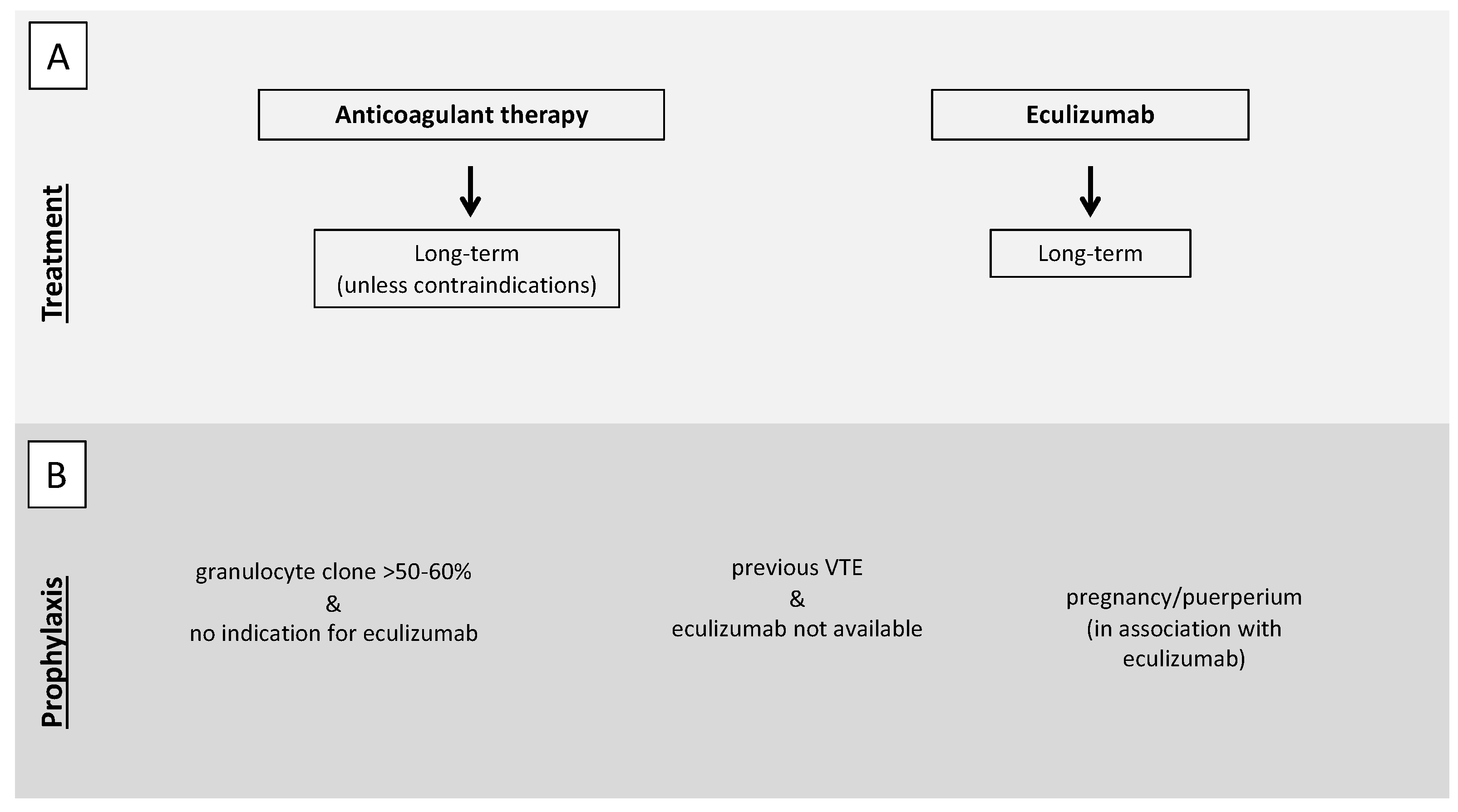
5. Diagnostic-Therapeutic Approach for Thrombosis in Immune-Mediate Hemolysis: Expert Opinion from a Tertiary Referral Center
5.1. Treatment of VTE
5.2. Antithrombotic Prophylaxis
5.2.1. Particular Situation: Splenectomy
5.2.2. Particular Situation: Pregnancy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pullarkat, V.; Ngo, M.; Iqbal, S.; Espina, B.; Liebman, H.A. Detection of lupus anticoagulant identifies patients with autoimmune hae-molytic anaemia at increased risk for venous thromboembolism. Br. J. Haematol. 2002, 118, 1166–1169. [Google Scholar] [CrossRef]
- L’Acqua, C.; Hod, E. New perspectives on the thrombotic complications of haemolysis. Br. J. Haematol. 2015, 168, 175–185. [Google Scholar] [CrossRef]
- Hansen, D.L.; Möller, S.; Andersen, K.; Gaist, D.; Frederiksen, H. Increasing incidence and prevalence of acquired hemolytic anemias in Denmark, 1980–2016. Clin. Epidemiol. 2020, 12, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Allgood, J.W.; Chaplin, H. Idiopathic acquired autoimmune hemolytic anemia. A review of forty-seven cases treated from 1955 through 1965. Am. J. Med. 1967, 43, 254–273. [Google Scholar] [CrossRef]
- Chapin, J.; Terry, H.S.; Kleinert, D.; Laurence, J. The role of complement activation in thrombosis and hemolytic anemias. Transfus. Apher. Sci. 2016, 54, 191–198. [Google Scholar] [CrossRef]
- Hendrick, A. Auto-immune haemolytic anaemia—A high-risk disorder for thromboembolism? Hematology 2003, 8, 53–56. [Google Scholar] [CrossRef] [PubMed]
- de Latour, R.P.; Mary, J.Y.; Salanoubat, C.; Terriou, L.; Etienne, G.; Mohty, M.; Roth, S.; de Guibert, S.; Maury, S.; Cahn, J.Y.; et al. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood 2008, 112, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, H.; Röth, A.; Araten, D.J.; Kanakura, Y.; Larratt, L.; Shammo, J.M.; Wilson, A.; Shayan, G.; Maciejewski, J.P. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): Updated analysis from the International PNH Registry. Ann. Hematol. 2020, 99, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Fattizzo, B.; Zaninoni, A.; Radice, T.; Nichele, I.; Di Bona, E.; Lunghi, M.; Tassinari, C.; Alfinito, F.; Ferrari, A.; et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: A GIMEMA study of 308 patients. Blood 2014, 124, 2930–2936. [Google Scholar] [CrossRef]
- Lecouffe-Desprets, M.; Neel, A.; Graveleau, J.; Leux, C.; Perrin, F.; Visomblain, B.; Artifoni, M.; Masseau, A.; Connault, J.; Pottier, P.; et al. Venous thromboembolism related to warm auto-immune hemolytic anemia: A case-control study. Autoimmun. Rev. 2015, 14, 1023–1028. [Google Scholar] [CrossRef]
- Audia, S.; Bach, B.; Samson, M.; Lakomy, D.; Bour, J.-B.; Burlet, B.; Guy, J.; Duvillard, L.; Branger, M.; Leguy-Seguin, V.; et al. Venous thromboembolic events during warm autoimmune hemolytic anemia. PLoS ONE 2018, 13, e0207218. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Zaninoni, A.; Fattizzo, B.; Giannotta, J.A.; Lunghi, M.; Ferrari, A.; Leporace, A.P.; Maschio, N.; Scaramucci, L.; Cantoni, S.; et al. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am. J. Hematol. 2018, 93, E243–E246. [Google Scholar] [CrossRef]
- Bylsma, L.C.; Ording, A.G.; Rosenthal, A.; Öztürk, B.; Fryzek, J.P.; Arias, J.M.; Röth, A.; Berentsen, S. Occurrence, thromboembolic risk, and mortality in Danish patients with cold agglutinin disease. Blood Adv. 2019, 3, 2980–2985. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Brunson, A.; Keegan, T.H.; Wun, T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with autoimmune hemolytic anemia. Blood Cells Mol. Dis. 2020, 81, 102388. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.M.; Cunningham, J.M.; Mullins, M.; Jiang, X.; Bylsma, L.C.; Fryzek, J.P.; Rosenthal, A. Increased risk of thrombotic events in cold agglutinin disease: A 10-year retrospective analysis. Res. Pract. Thromb. Haemost. 2020, 4, 628–635. [Google Scholar] [CrossRef]
- Hall, C.; Richards, S.; Hillmen, P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood 2003, 102, 3587–3591. [Google Scholar] [CrossRef]
- Ruggeri, M.; Rodeghiero, F. Thrombotic risk in patients with immune haemolytic anaemia. Br. J. Haematol. 2015, 172, 144–146. [Google Scholar] [CrossRef]
- Solari, D.; Alberio, L.; Ribi, C.; Grandoni, F.; Stalder, G. Autoimmune hemolytic anemia and pulmonary embolism: An association to consider. TH Open 2021, 5, e8–e13. [Google Scholar] [CrossRef]
- Röth, A.; Bommer, M.; Hüttmann, A.; Herich-Terhürne, D.; Kuklik, N.; Rekowski, J.; Lenz, V.; Schrezenmeier, H.; Dührsen, U. Eculizumab in cold agglutinin disease (DECADE): An open-label, prospective, bicentric, nonrandomized phase 2 trial. Blood Adv. 2018, 2, 2543–2549. [Google Scholar] [CrossRef]
- Parker, C.; Omine, M.; Richards, S.; Nishimura, J.I.; Bessler, M.; Ware, R.; Hillmen, P.; Luzzatto, L.; Young, N.; Kinoshita, T.; et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005, 106, 3699–3709. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Kelly, R.J.; Hillmen, P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013, 121, 4985–4996. [Google Scholar] [CrossRef]
- Yenerel, M.N.; Muus, P.; Wilson, A.; Szer, J. Clinical course and disease burden in patients with paroxysmal nocturnal hemoglobinuria by hemolytic status. Blood Cells Mol. Dis. 2017, 65, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ziakas, P.D.; Poulou, L.S.; Rokas, G.I.; Bartzoudis, D.; Voulgarelis, M. Thrombosis in paroxysmal nocturnal hemoglobinuria: Sites, risks, outcome. An overview. J. Thromb. Haemost. 2007, 5, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Reid, S.A.; Rother, R.P.; Gladwin, M.T.; Collinson, P.O.; Gaze, D.C.; Lowe, A.; Guthrie, A.; Sivananthan, M.U.; Hillmen, P. High definition contrast-enhanced MR imaging in paroxysmal nocturnal hemoglobinuria (PNH) suggests a high frequency of subclinical thrombosis. Blood 2006, 108, 979. [Google Scholar] [CrossRef]
- Hillmen, P.; Muus, P.; Dührsen, U.; Risitano, A.M.; Schubert, J.; Luzzatto, L.; Schrezenmeier, H.; Szer, J.; Brodsky, R.A.; Hill, A.; et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007, 110, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Caby-Tosi, M.; Marantz, J.L.; Cole, A.; Bedrosian, C.L.; Gasteyger, C.; Mujeebuddin, A.; Hillmen, P.; Walle, J.V.; Haller, H. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br. J. Haematol. 2019, 185, 297–310. [Google Scholar] [CrossRef]
- Brodsky, R.A. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2021, 137, 1304–1309. [Google Scholar] [CrossRef]
- Loschi, M.; Porcher, R.; Barraco, F.; Terriou, L.; Mohty, M.; De Guibert, S.; Mahe, B.; Lemal, R.; Dumas, P.-Y.; Etienne, G.; et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: A treatment versus no-treatment study. Am. J. Hematol. 2016, 91, 366–370. [Google Scholar] [CrossRef]
- Kokori, S.I.; Ioannidis, J.P.; Voulgarelis, M.; Tzioufas, A.G.; Moutsopoulos, H.M. Autoimmune hemolytic anemia in patients with systemic lupus erythematosus. Am. J. Med. 2000, 108, 198–204. [Google Scholar] [CrossRef]
- Sanchez, C.J. Venous and arterial thrombosis: A continuous spectrum of the same disease? Eur. Heart J. 2004, 26, 3–4. [Google Scholar] [CrossRef]
- Lentz, B.R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003, 42, 423–438. [Google Scholar] [CrossRef]
- Wesseling, M.C.; Wagner-Britz, L.; Huppert, H.; Hanf, B.; Hertz, L.; Nguyen, D.B.; Bernhardt, I. Phosphatidylserine exposure in human red blood cells depending on cell age. Cell. Physiol. Biochem. 2016, 38, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Cappellini, M.D.; Rachmilewitz, E.A. β-Thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br. J. Haematol. 2007, 139, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bartolmäs, T.; Mayer, B.; Balola, A.H.; Salama, A. Eryptosis in autoimmune haemolytic anaemia. Eur. J. Haematol. 2017, 100, 36–44. [Google Scholar] [CrossRef]
- Jeffers, A.; Gladwin, M.T.; Kim-Shapiro, D.B. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic. Biol. Med. 2006, 41, 1557–1565. [Google Scholar] [CrossRef]
- Helms, C.C.; Marvel, M.; Zhao, W.; Stahle, M.; Vest, R.; Kato, G.J.; Lee, J.S.; Christ, G.; Gladwin, M.T.; Hantgan, R.R.; et al. Mechanisms of hemolysis-associated platelet activation. J. Thromb. Haemost. 2013, 11, 2148–2154. [Google Scholar] [CrossRef]
- Radomski, M.; Palmer, R.M.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 330, 1057–1058. [Google Scholar] [CrossRef]
- Gambaryan, S.; Subramanian, H.; Kehrer, L.; Mindukshev, I.; Sudnitsyna, J.; Reiss, C.; Rukoyatkina, N.; Friebe, A.; Sharina, I.; Martin, E.; et al. Erythrocytes do not activate purified and platelet soluble guanylate cyclases even in conditions favourable for NO synthesis. Cell Commun. Signal. 2016, 14, 16. [Google Scholar] [CrossRef]
- Yetik-Anacak, G.; Catravas, J.D. Nitric oxide and the endothelium: History and impact on cardiovascular disease. Vasc. Pharmacol. 2006, 45, 268–276. [Google Scholar] [CrossRef]
- Yang, Y.; Loscalzo, J. Regulation of tissue factor expression in human microvascular endothelial cells by nitric oxide. Circulation 2000, 101, 2144–2148. [Google Scholar] [CrossRef]
- Da, Q.; Teruya, M.; Guchhait, P.; Teruya, J.; Olson, J.S.; Cruz, M.A. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: Implications for circulatory devices. Blood 2015, 126, 2338–2341. [Google Scholar] [CrossRef]
- Zhou, Z.; Hyojeong, H.; Cruz, M.A.; Lopez, J.A.; Dong, J.-F.; Guchhait, P. Hemoglobin blocks von willebrand factor proteolysis by ADAMTS-13: A mechanism associated with acquired ADAMTS-13 deficiency in patients with sickle cell disease. Blood 2008, 112, 3919. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Bornmann, S.; Jägers, J.; Patyk, V.; Kirsch, M. Microvascular stasis and hemolysis: Coincidence or causality? J. Inflamm. Res. 2019, 12, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Setty, B.N.Y.; Betal, S.G.; Zhang, J.; Stuart, M.J. Heme induces endothelial tissue factor expression: Potential role in hemostatic activation in patients with hemolytic anemia. J. Thromb. Haemost. 2008, 6, 2202–2209. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler. Thromb. Vasc. Biol. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Lindenblatt, N.; Bordel, R.; Schareck, W.; Menger, M.; Vollmar, B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arter. Thromb. Vasc. Biol. 2004, 24, 601–606. [Google Scholar] [CrossRef]
- Peng, L.; Mundada, L.; Stomel, J.M.; Liu, J.J.; Sun, J.; Yet, S.-F.; Fay, W.P. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxid. Redox Signal. 2004, 6, 729–735. [Google Scholar] [CrossRef]
- Ronson, R.S.; Nakamura, M.; Vinten-Johansen, J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc. Res. 1999, 44, 47–59. [Google Scholar] [CrossRef]
- Lizarralde-Iragorri, M.A.; Shet, A.S. Sickle cell disease: A paradigm for venous thrombosis pathophysiology. Int. J. Mol. Sci. 2020, 21, 5279. [Google Scholar] [CrossRef]
- Kimball, A.S.; Obi, A.T.; Diaz, J.A.; Henke, P.K. The emerging role of NETs in venousthrombosis and immunothrombosis. Front. Immunol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Ofori-Acquah, S.F. Erythroid DAMPs drive inflammation in SCD. Blood 2014, 123, 3689–3690. [Google Scholar] [CrossRef]
- Westerman, M.; Pizzey, A.; Hirschman, J.; Cerino, M.; Weil-Weiner, Y.; Ramotar, P.; Eze, A.; Lawrie, A.; Purdy, G.; Mackie, I.; et al. Microvesicles in haemoglobinopathies offer in-sights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br. J. Haematol. 2008, 142, 126–135. [Google Scholar] [CrossRef]
- van Beers, E.J.; Schaap, M.C.; Berckmans, R.J.; Nieuwland, R.; Sturk, A.; van Doormaal, F.F.; Meijers, J.C.; Biemond, B.J. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica 2009, 94, 1513–1519. [Google Scholar] [CrossRef]
- Barcellini, W.; Zaninoni, A.; Giannotta, J.A.; Merati, G.; Capecchi, M.; Fattizzo, B.; Trombetta, E.; Artoni, A. Circulating extracellular vesicles and cytokines in congenital and acquired hemolytic anemias. Am. J. Hematol. 2021, 96. [Google Scholar] [CrossRef]
- Diamant, M.; Tushuizen, M.E.; Sturk, A.; Nieuwland, R. Cellular microparticles: New players in the field of vascular disease? Eur. J. Clin. Investig. 2004, 34, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Rubin, O.; Crettaz, D.; Tissot, J.-D.; Lion, N. Microparticles in stored red blood cells: Submicron clotting bombs? Blood Transfus. 2010, 8, s31. [Google Scholar] [PubMed]
- Zöller, B.; Li, X.; Sundquist, J.; Sundquist, K. Autoimmune diseases and venous thromboembolism: A review of the literature. Am. J. Cardiovasc. Dis. 2012, 2, 171–183. [Google Scholar]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef]
- Kamphuisen, P.W.; Lensen, R.; Houwing-Duistermaat, J.J.; Eikenboom, J.C.J.; Harvey, M.; Bertina, R.M.; Rosendaal, F.R. Heritability of elevated factor VIII antigen levels in factor V Leiden families with thrombophilia. Br. J. Haematol. 2000, 109, 519–522. [Google Scholar] [CrossRef]
- Lindemann, S.; Krämer, B.; Seizer, P.; Gawaz, M. Platelets, inflammation and atherosclerosis. J. Thromb. Haemost. 2007, 5, 203–211. [Google Scholar] [CrossRef]
- Medcalf, R.L. Fibrinolysis, inflammation, and regulation of the plasminogen activating system. J. Thromb. Haemost. 2007, 5, 132–142. [Google Scholar] [CrossRef]
- Zwaal, R.F.A.; Schroit, A.J. Pathophysiologic Implications of membrane phospholipid asymmetry in blood cells. Blood 1997, 89, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Tanratana, P.; Srivali, N. Autoimmune hemolytic anemia and venous thromboembolism: A systematic review and meta-analysis. Thromb. Res. 2015, 136, 1013–1017. [Google Scholar] [CrossRef]
- Schreiber, K.; Sciascia, S.; De Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Prim. 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rottem, M.; Krause, I.; Fraser, A.; Stojanovich, L.; Rovensky, J.; Shoenfeld, Y. Autoimmune hemolytic anaemia in the antiphospholipid syndrome. Lupus 2006, 15, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.; Merashli, M.; Bucci, M.M.T.; Pastori, D.; Pignatelli, P.; Arcaro, A.; Gentile, F. Antiphospholipid antibodies and autoimmune haemolytic anaemia: A systematic review and meta-analysis. Int. J. Mol. Sci. 2020, 21, 4120. [Google Scholar] [CrossRef] [PubMed]
- Roumier, M.; Loustau, V.; Guillaud, C.; Languille, L.; Mahevas, M.; Khellaf, M.; Limal, N.; Noizat-Pirenne, F.; Godeau, B.; Michel, M. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: New insights based on a single-center experience with 60 patients. Am. J. Hematol. 2014, 89, E150–E155. [Google Scholar] [CrossRef]
- Foley, J.H. Examining coagulation-complement crosstalk: Complement activation and thrombosis. Thromb. Res. 2016, 141, S50–S54. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part I—Molecular mechanisms of activation and regulation. Front. Immunol. 2015, 6, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, T.; Esmon, C.T.; Sims, P.J. On the mechanism by which complement proteins C5b-9 increase platelet prothrombinase activity. J. Biol. Chem. 1986, 261, 14587–14592. [Google Scholar] [CrossRef]
- Ritis, K.; Doumas, M.; Mastellos, D.; Micheli, A.; Giaglis, S.; Magotti, P.; Rafail, S.; Kartalis, G.; Sideras, P.; Lambris, J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006, 177, 4794–4802. [Google Scholar] [CrossRef]
- Hattori, R.; Hamilton, K.K.; McEver, R.P.; Sims, P.J. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989, 264, 9053–9060. [Google Scholar] [CrossRef]
- Foreman, K.E.; Vaporciyan, A.A.; Bonish, B.K.; Jones, M.L.; Johnson, K.J.; Glovsky, M.M.; Eddy, S.M.; Ward, P.A. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Investig. 1994, 94, 1147–1155. [Google Scholar] [CrossRef]
- Krarup, A.; Wallis, R.; Presanis, J.S.; Gál, P.; Sim, R.B. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE 2007, 2, e623. [Google Scholar] [CrossRef]
- Wojta, J.; Kaun, C.; Zorn, G.; Ghannadan, M.; Hauswirth, A.W.; Sperr, W.R.; Fritsch, G.; Printz, D.; Binder, B.R.; Schatzl, G.; et al. C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood 2002, 100, 517–523. [Google Scholar] [CrossRef]
- Berentsen, S. Role of complement in autoimmune hemolytic anemia. Transfus. Med. Hemother. 2015, 42, 303–310. [Google Scholar] [CrossRef]
- Berentsen, S. Complement activation and inhibition in autoimmune hemolytic anemia: Focus on cold agglutinin disease. Semin. Hematol. 2018, 55, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gralnick, H.R.; Vail, M.; McKeown, L.P.; Merryman, P.; Wilson, O.; Chu, I.; Kimball, J. Activated platelets in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 1995, 91, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Peerschke, E.I.B.; Andemariam, B.; Yin, W.; Bussel, J.B. Complement activation on platelets correlates with a decrease in circulating im-mature platelets in patients with immune thrombocytopenic purpura. Br. J. Haematol. 2010, 148, 638–645. [Google Scholar] [CrossRef]
- Devine, D.V.; Siegel, R.S.; Rosse, W.F. Interactions of the platelets in paroxysmal nocturnal hemoglobinuria with complement. Relationship to defects in the regulation of complement and to platelet survival in vivo. J. Clin. Investig. 1987, 79, 131–137. [Google Scholar] [CrossRef]
- Wiedmer, T.; Esmon, C.T.; Sims, P.J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 1986, 68, 875–880. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Hugel, B.; Socié, G.; Vu, T.; Toti, F.; Gluckman, E.; Freyssinet, J.-M.; Scrobohaci, M.-L. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood 1999, 93, 3451–3456. [Google Scholar] [CrossRef]
- van Bijnen, S.T.A.; van Heerde, W.L.; Muus, P. Mechanisms and clinical implications of thrombosis in paroxysmal nocturnal hemoglobinuria. J. Thromb. Haemost. 2012, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Grahl, L.; Von Bruehl, M.-L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Sloand, E.M.; Pfannes, L.; Scheinberg, P.; More, K.; Wu, C.O.; Horne, M.; Young, N.S. Increased soluble urokinase plasminogen activator receptor (suPAR) is associated with thrombosis and inhibition of plasmin generation in paroxysmal nocturnal hemoglobinuria (PNH) patients. Exp. Hematol. 2008, 36, 1616–1624. [Google Scholar] [CrossRef]
- Ninomiya, H.; Hasegawa, Y.; Nagasawa, T.; Abe, T. Excess soluble urokinase-type plasminogen activator receptor in the plasma of patients with paroxysmal nocturnal hemoglobinuria inhibits cell-associated fibrinolytic activity. Int. J. Hematol. 1997, 65, 285–291. [Google Scholar] [CrossRef]
- Griffin, M.; Hillmen, P.; Munir, T.; Richards, S.; Arnold, L.; Riley, K.; Hill, A. Significant hemolysis is not required for thrombosis in paroxysmal nocturnal hemoglobinuria. Haematologica 2019, 104, e94–e96. [Google Scholar] [CrossRef]
- Connors, J.M. Thrombophilia testing and venous thrombosis. N. Engl. J. Med. 2017, 377, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.J.; Ortel, T.L.; Pengo, V.; De Laat, B. Laboratory criteria for antiphospholipid syndrome: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Nafa, K.; Bessler, M.; Mason, P.; Vulliamy, T.; Hillmen, P.; Castro-Malaspina, H.; Luzzatto, L. Factor V Leiden mutation investigated by amplification created restriction enzyme site (ACRES) in PNH patients with and without thrombosis. Haematologica 1996, 81, 540–542. [Google Scholar] [PubMed]
- Grünewald, M.; Siegemund, A.; Grünewald, A.; Schmid, A.; Koksch, M.; Schöpflin, C.; Schauer, S.; Griesshammer, M. Plasmatic coagulation and fibrinolytic system alterations in PNH: Relation to clone size. Blood Coagul. Fibrinolysis 2003, 14, 685–695. [Google Scholar] [CrossRef]
- Dragoni, F.; Iori, A.P.; Pignoloni, P.; Minotti, C.; Chiarotti, F.; Mazzucconi, M.G.; Mengarelli, A.; Arcese, W.; Foà, R.; Avvisati, G. Thrombophilic screening in patients with paroxysmal nocturnal haemoglobinuria: A pilot study. Br. J. Haematol. 2010, 150, 492–494. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Chen, F.; Zhou, W.; Li, H.; Long, Z.; Yang, C.; Chen, M.; Han, B. Prediction of thrombosis risk in patients with paroxysmal nocturnal hemoglobinuria. Ann. Hematol. 2019, 98, 2283–2291. [Google Scholar] [CrossRef]
- Bode, C.; Olivier, C.B.; Duerschmied, D. Anticoagulation and anaemia: Old opponents from the era of VKA? Eur. Heart J. 2019, 40, 3791–3792. [Google Scholar] [CrossRef]
- Stolz, E.; Valdueza, J.M.; Grebe, M.; Schlachetzki, F.; Schmitt, E.; Madlener, K.; Rahimi, A.; Kempkes-Matthes, B.; Blaes, F.; Gerriets, T.; et al. Anemia as a risk factor for cerebral venous thrombosis? An old hypothesis revisited: Results of a prospective study. J. Neurol. 2007, 254, 729–734. [Google Scholar] [CrossRef]
- Hung, S.-H.; Lin, H.-C.; Chung, S.-D. Association between venous thromboembolism and iron-deficiency anemia. Blood Coagul. Fibrinolysis 2015, 26, 368–372. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Patriquin, C.J.; Kiss, T.; Caplan, S.; Chin-Yee, I.; Grewal, K.; Grossman, J.; Larratt, L.; Marceau, D.; Nevill, T.; Sutherland, D.R.; et al. How we treat paroxysmal nocturnal hemoglobinuria: A consensus statement of the Canadian PNH Network and review of the national registry. Eur. J. Haematol. 2018, 102, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Brodsky, R.A. Successful discontinuation of anticoagulation following eculizumab administration in paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 2009, 84, 699–701. [Google Scholar] [CrossRef]
- Jäger, U.; Barcellini, W.; Broome, C.M.; Gertz, M.A.; Hill, A.; Hill, Q.A.; Jilma, B.; Kuter, D.J.; Michel, M.; Montillo, M.; et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020, 41, 100648. [Google Scholar] [CrossRef] [PubMed]
- Hill, Q.A.; Stamps, R.; Massey, E.; Grainger, J.D.; Provan, D.; Hill, A. The diagnosis and management of primary autoimmune haemolytic anaemia. Br. J. Haematol. 2016, 176, 395–411. [Google Scholar] [CrossRef]
- Weinkle, T.K.; Center, S.A.; Randolph, J.F.; Warner, K.L.; Barr, S.C.; Erb, H.N. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993–2002). J. Am. Vet. Med. Assoc. 2005, 226, 1869–1880. [Google Scholar] [CrossRef]
- Moyo, V.M.; Mukhina, G.L.; Garrett, E.S.; Brodsky, R.A. Natural history of paroxysmal nocturnal haemoglobinuria using modern diag-nostic assays. Br. J. Haematol. 2004, 126, 133–138. [Google Scholar] [CrossRef]
- Schrezenmeier, H.; Muus, P.; Socié, G.; Szer, J.; Urbano-Ispizua, A.; Maciejewski, J.P.; Brodsky, R.A.; Bessler, M.; Kanakura, Y.; Rosse, W.; et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 2014, 99, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Schoonen, W.M.; Farkas, D.K.; Riis, A.; Fryzek, J.P.; Sørensen, H.T. Risk of venous thromboembolism in splenectomized patients compared with the general population and appendectomized patients: A 10-year nationwide cohort study. J. Thromb. Haemost. 2010, 8, 1413–1416. [Google Scholar] [CrossRef]
- Stuck, A.; Spirk, D.; Schaudt, J.; Kucher, N. Risk assessment models for venous thromboembolism in acutely ill medical patients: A systematic review. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 769–770. [Google Scholar] [CrossRef]
- Van’t Riet, M.; Burger, J.W.A.; Van Muiswinkel, J.M.; Kazemier, G.; Schipperus, M.R.; Bonjer, H.J. Diagnosis and treatment of portal vein thrombosis following splenectomy. Br. J. Surg. 2000, 87, 1229–1233. [Google Scholar] [CrossRef]
- Mohren, M.; Markmann, I.; Dworschak, U.; Franke, A.; Maas, C.; Mewes, S.; Jentsch-Ullrich, K. Thromboembolic complications after splenectomy for hematologic diseases. Am. J. Hematol. 2004, 76, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Höchsmann, B.; Szer, J.; Kulasekararaj, A.; De Guibert, S.; Röth, A.; Weitz, I.C.; Armstrong, E.; Risitano, A.M.; Patriquin, C.J.; et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2015, 373, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
| Study | Design | Patients | Thrombotic Events | Prothrombotic Risk Factors | Antithrombotic Prohpylaxis |
|---|---|---|---|---|---|
| Pullarkat et al., 2002 [1] | Multicenter Prospective | 30 AIHA from 1995 to 2001 | 8 (27%) with at least one event (7 DVT, 3 PE, 1 PE + portal vein thrombosis) | Lupus anticoagulant Active hemolysis (4/8) | NA |
| Hendrick et al., 2003 [6] | Single-center Retrospective | 28 (23 AIHA + 5 CAD) from 1986 to 2001 | 6 (21%) (4 PE, 1 MI, 1 CVT) | NA | 21 (1 of whom developed a fatal PE) |
| Barcellini et al., 2014 [9] | Multicenter Retrospective | 308 (224 AIHA + 84 CAD) from 1978 to 2013 | 33 (11%) with at least one event (13 DVT, 11 PE, 5 splanchnic, 3 strokes, 2 TIA, 3 cardiac ischemic events, 1 DIC) | Hemoglobin < 6 g/dL Intravascular hemolysis Previous splenectomy | NA |
| Lecouffe-Desprets et al., 2015 [10] | Single-center Retrospective | 40 AIHA from 2009 to 2013 | 8 (20%) (4 PE, 4 DVT + PE) | Low hemoglobin levels Active hemolysis (7/8) | 0/8 |
| Audia et al., 2018 [11] | Single-center Retrospective | 48 AIHA from 2006 to 2016 | 11 (23%) (5 DVT, 1 PE, 5 DVT + PE) | Active hemolysis (10/11) | 2/11 (LMWH prophylaxis, VKA) |
| Barcellini et al., 2018 [12] | Multicenter Retrospective | 378 (271 AIHA + 107 CAD) | 58 (15%) (14 with CAD) | NA | NA |
| Bylsma et al., 2019 [13] | Multicenter Retrospective (population-based registry) | 72 CAD from 1999 to 2013 | 7 (10%) with at least one event (venous or arterial) | NA | NA |
| Ho G et al., 2020 [14] | Multicenter Retrospective (population-based registry) | 4756 AIHA from 1991 to 2014 | 259 (5%) VTE (61 splanchnic) | Splenectomy | NA |
| Broome et al., 2020 [15] | Multicenter Retrospective (population-based registry) | 608 CAD from 2006 to 2016 | 180 (30%) (19 DVT, 30 PE, 12 splanchnic, 57 other VTE, 85 cerebral events, 13 arterial thrombosis, 34 MI) | NA | NA |
| Hall et al., 2003 [16] | Single-center Prospective | 163 PNH | 29 (18%) with at least one event (20 splanchnic, 4 DVT, 3 PE, 3 CVT, 1 retinal, 1 catheter related thrombosis) | NA | 32 patients on primary prophylaxis with warfarin (no VTE) |
| De Latour et al., 2008 [7] | Multicenter Retrospective | 454 PNH from 1950 to 2005 | 116 (26%) with at least one event (31 DVT, 35 central nervous system, 49 Budd-Chiari syndrome, 29 others) | Age >55 years Use of transfusion Previous thrombosis Warfarin as primary prophylaxis | 18 patients on primary prophylaxis with warfarin (10 VTE) 19 patients on secondary prophylaxis with warfarin (8 VTE) |
| Schrezenmeier et al., 2020 [8] | Multicenter prospective registry | 4134 PNH from 2007 to 2017 | 779 (19%) (SVT, DVT, splanchnic vein or arterial thrombosis, acute peripheral vascular disease occlusion, CVT, PE, MI, TIA, stroke) | Hemolysis, regardless of clone size | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capecchi, M.; Ciavarella, A.; Artoni, A.; Abbattista, M.; Martinelli, I. Thrombotic Complications in Patients with Immune-Mediated Hemolysis. J. Clin. Med. 2021, 10, 1764. https://doi.org/10.3390/jcm10081764
Capecchi M, Ciavarella A, Artoni A, Abbattista M, Martinelli I. Thrombotic Complications in Patients with Immune-Mediated Hemolysis. Journal of Clinical Medicine. 2021; 10(8):1764. https://doi.org/10.3390/jcm10081764
Chicago/Turabian StyleCapecchi, Marco, Alessandro Ciavarella, Andrea Artoni, Maria Abbattista, and Ida Martinelli. 2021. "Thrombotic Complications in Patients with Immune-Mediated Hemolysis" Journal of Clinical Medicine 10, no. 8: 1764. https://doi.org/10.3390/jcm10081764
APA StyleCapecchi, M., Ciavarella, A., Artoni, A., Abbattista, M., & Martinelli, I. (2021). Thrombotic Complications in Patients with Immune-Mediated Hemolysis. Journal of Clinical Medicine, 10(8), 1764. https://doi.org/10.3390/jcm10081764







