Patterns, Predictors, and Prognostic Value of Skeletal Muscle Mass Loss in Patients with Locally Advanced Head and Neck Cancer Undergoing Cisplatin-Based Chemoradiotherapy
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Therapy
2.4. Skeletal Muscle Measurements
2.5. Skeletal Muscle Mass Changes
- Stable changes in SMM: No change ±1 SD from baseline;
- Moderate gain in SMM: ≥1 SD to <2 SD of gain from baseline;
- Moderate loss in SMM: ≥1 SD to <2 SD of loss from baseline;
- Large gain in SMM: ≥2 SD from baseline;
- Large loss in SMM: ≥2 SD from baseline.
2.6. Survival
2.7. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Predictors of Loss in Skeletal Muscle Mass
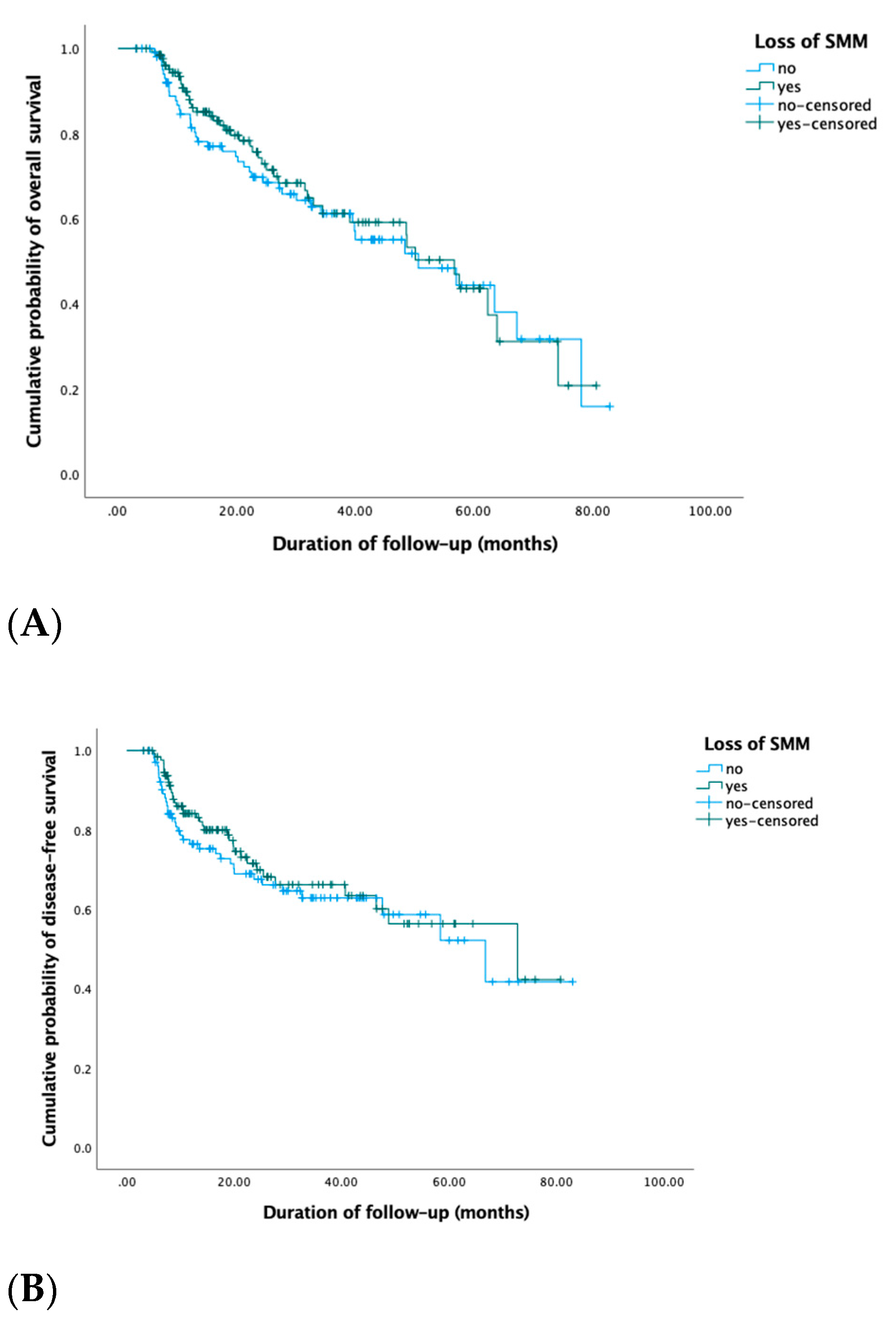
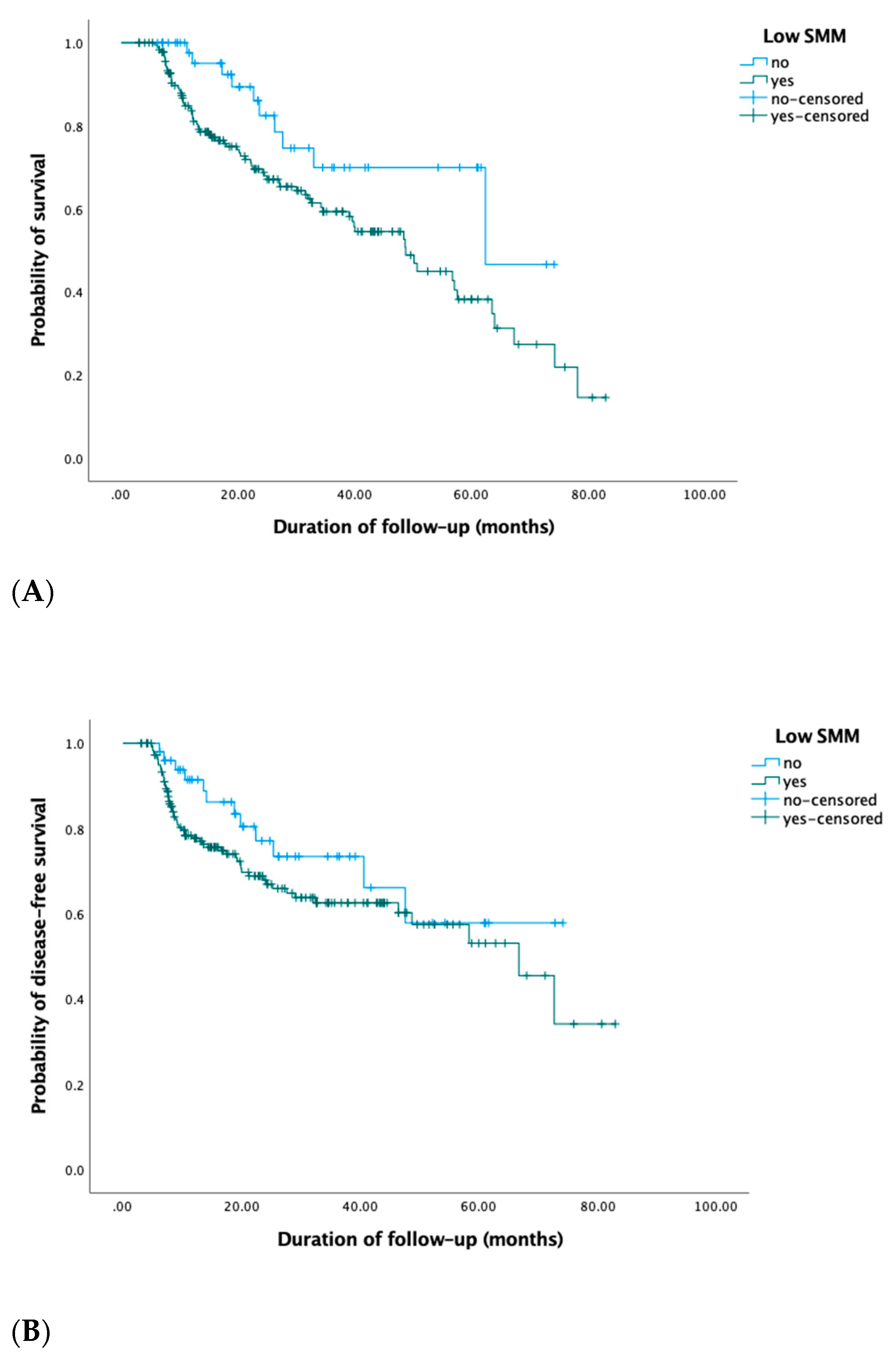
3.3. Survival: Overall Survival and Disease-Free Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| LA-HNC | Locally advanced head and neck cancer |
| SMA | Skeletal muscle area |
| SMM | Skeletal muscle mass |
| LSMI | Lumbar skeletal muscle index |
| C3 | Third cervical vertebra |
| L3 | Third lumbar vertebra |
| HU | Hounsfield unit |
| BMI | Body mass index |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| ICC | Intraclass correlation coefficient |
| SD | Standard deviation |
| IQR | Interquartile range |
| ACE–27 | Adult Comorbidity Evaluation 27 |
| ECOG | Eastern Cooperative Oncology Group |
| TNM | Tumor, node, metastasis |
| CI | Confidence interval |
| HR | Hazard ratio |
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study Global Burden of Disease Cancer Collaboration. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Helfenstein, S.; Riesterer, O.; Meier, U.R.; Papachristofilou, A.; Kasenda, B.; Pless, M.; Rothschild, S.I. 3-weekly or weekly cisplatin concurrently with radiotherapy for patients with squamous cell carcinoma of the head and neck—A multicentre, retrospective analysis. Radiation Oncology. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Muzumder, S.; Srikantia, N.; Vashishta, G.D.; Udayashankar, A.H.; Raj, J.M.; Sebastian, M.G.J.; Kainthaje, P.B. Compliance, toxicity and efficacy in weekly versus 3-weekly cisplatin concurrent chemoradiation in locally advanced head and neck cancer. J. Radiother. Pract. 2019, 18, 26–31. [Google Scholar] [CrossRef]
- Schüttrumpf, L.; Marschner, S.; Scheu, K.; Hess, J.; Rietzler, S.; Walch, A.; Baumeister, P.; Kirchner, T.; Ganswindt, U.; Zitzelsberger, H.; et al. Definitive chemoradiotherapy in patients with squamous cell cancers of the head and neck—Results from an unselected cohort of the clinical cooperation group “personalized Radiotherapy in Head and Neck Cancer”. Radiat. Oncol. 2020, 15, 7. [Google Scholar] [CrossRef]
- Hua, X.; Liu, S.; Liao, J.-F.; Wen, W.; Long, Z.-Q.; Lu, Z.-J.; Guo, L.; Lin, H.-X. When the Loss Costs Too Much: A Systematic Review and Meta-Analysis of Sarcopenia in Head and Neck Cancer. Front. Oncol. 2020, 9, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kurk, S.; Peeters, P.; Stellato, R.; Dorresteijn, B.; De Jong, P.; Jourdan, M.; Creemers, G.; Erdkamp, F.; De Jongh, F.; Kint, P.; et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 803–813. [Google Scholar] [CrossRef]
- Ali, R.; Baracos, V.E.; Sawyer, M.B.; Bianchi, L.; Roberts, S.; Assenat, E.; Mollevi, C.; Senesse, P. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016, 5, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Sjøblom, B.; Benth, J.Š.; Grønberg, B.H.; Baracos, V.E.; Sawyer, M.B.; Fløtten, Ø.; Hjermstad, M.J.; Aass, N.; Jordhøy, M. Drug Dose Per Kilogram Lean Body Mass Predicts Hematologic Toxicity From Carboplatin-Doublet Chemotherapy in Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, e129–e136. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
- Sealy, M.J.; Dechaphunkul, T.; van der Schans, C.P.; Krijnen, W.P.; Roodenburg, J.L.; Walker, J.; Jager-Wittenaar, H.; Baracos, V.E. Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin. Nutr. 2020, 39, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; De Graeff, A.; Smid, E.J.; De Bree, R.; Pothen, A.J. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef]
- Garcia, J.M.; Scherer, T.; Chen, J.-A.; Guillory, B.; Nassif, A.; Papusha, V.; Smiechowska, J.; Asnicar, M.; Buettner, C.; Smith, R.G. Inhibition of cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice. Endocrinology 2013, 154, 3118–3129. [Google Scholar] [CrossRef]
- Daly, L.E.; Ní Bhuachalla, É.B.; Power, D.G.; Cushen, S.J.; James, K.; Ryan, A.M. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 315–325. [Google Scholar] [CrossRef]
- Kurk, S.A.; Peeters, P.H.M.; Dorresteijn, B.; De Jong, P.A.; Jourdan, M.; Creemers, G.-J.M.; Erdkamp, F.L.G.; De Jongh, F.E.; Kint, P.A.M.; Poppema, B.J.; et al. Loss of skeletal muscle index and survival in patients with metastatic colorectal cancer: Secondary analysis of the phase 3 CAIRO3 trial. Cancer Med. 2020, 9, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, P.; Salandini, M.; Chiari, D.; Pecorelli, N.; Cristel, G.; Damascelli, A.; Ronzoni, M.; Massimino, L.; De Cobelli, F.; Braga, M. Changes in body composition during neoadjuvant therapy can affect prognosis in rectal cancer patients: An exploratory study. Curr. Probl. Cancer 2020, 44, 100510. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Corvaja, C.; Caccialanza, R.; Aprile, G. Sarcopenia: Looking to muscle mass to better manage pancreatic cancer patients. Curr. Opin. Support. Palliat. Care 2019, 13, 279–285. [Google Scholar] [CrossRef]
- Stone, L.; Olson, B.; Mowery, A.; Krasnow, S.; Jiang, A.; Li, R.; Schindler, J.; Wax, M.K.; Andersen, P.; Marks, D.; et al. Association between Sarcopenia and Mortality in Patients Undergoing Surgical Excision of Head and Neck Cancer. In JAMA Otolaryngology—Head and Neck Surgery; American Medical Association: Chicago, IL, USA, 2019; Volume 145, pp. 647–654. [Google Scholar] [CrossRef]
- Chargi, N.; Bril, S.; Swartz, J.; Wegner, I.; Willems, S.; de Bree, R. Skeletal muscle mass is an imaging biomarker for decreased survival in patients with oropharyngeal squamous cell carcinoma. Oral Oncol. 2020, 101. [Google Scholar] [CrossRef]
- Ansari, E.; Chargi, N.; van Gemert, J.; van Es, R.; Dieleman, F.; Rosenberg, A.; Van Cann, E.; de Bree, R. Low skeletal muscle mass is a strong predictive factor for surgical complications and a prognostic factor in oral cancer patients undergoing mandibular reconstruction with a free fibula flap. Oral Oncol. 2020, 101. [Google Scholar] [CrossRef] [PubMed]
- Chargi, N.; Bril, S.I.; Emmelot-Vonk, M.H.; De Bree, R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur. Arch. Oto-Rhino-Laryngology 2019, 276, 1475–1486. [Google Scholar] [CrossRef]
- Huang, X.; Ma, J.; Li, L.; Zhu, X. Severe muscle loss during radical chemoradiotherapy for non-metastatic nasopharyngeal carcinoma predicts poor survival. Cancer Med. 2019, 8, 6604–6613. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver Validation of Skeletal Muscle Measurement by Magnetic Resonance Imaging and Computerized Tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Chargi, N.; Ansari, E.; Huiskamp, L.; Bol, G.; De Bree, R. Agreement between skeletal muscle mass measurements using computed tomography imaging and magnetic resonance imaging in head and neck cancer patients. Oral Oncol. 2019, 99, 104341. [Google Scholar] [CrossRef]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef]
- Williams, B.A.; Mandrekar, J.N.; Mandrekar, S.J.; Cha, S.S.; Furth, A.F. Finding Optimal Cutpoints for Continuous Covariates with Binary and Time-to-Event Outcomes; Division of Biostatistics, Mayo Clinic: Rochester, MN, USA, 2006. [Google Scholar]
- Brown, J.C.; Caan, B.J.; Meyerhardt, J.A.; Weltzien, E.; Xiao, J.; Feliciano, E.M.C.; Kroenke, C.H.; Castillo, A.; Kwan, M.L.; Prado, C.M. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: A population-based cohort study (C-SCANS). J. Cachexia Sarcopenia Muscle 2018, 9, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer Results From the C SCANS Study Supplemental content. JAMA Oncol. 2017, 3, 172319. [Google Scholar] [CrossRef]
- Cole, C.L.; Kleckner, I.R.; Jatoi, A.; Schwarz, E.; Dunne, R.F. The Role of Systemic Inflammation in Cancer-Associated Muscle Wasting and Rationale for Exercise as a Therapeutic Intervention. JCSM Clin. Rep. 2018, 3. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Faithfull, S.; Turner, L.; Poole, K.; Joy, M.; Manders, R.; Weprin, J.; Winters-Stone, K.; Saxton, J. Prehabilitation for adults diagnosed with cancer: A systematic review of long-term physical function, nutrition and patient-reported outcomes. Eur. J. Cancer Care 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Palma, S.; Hasenoehrl, T.; Jordakieva, G.; Ramazanova, D.; Crevenna, R. High-intensity interval training in the prehabilitation of cancer patients—a systematic review and meta-analysis. Support. Care Cancer 2021, 29, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 235) | Low SMM (n = 185) | Without low SMM (n = 50) | p-Value |
|---|---|---|---|---|
| N (%) | N (78.7%) | N (21.3%) | ||
| Gender Male Female | 164 (69.8) 71 (30.2) | 115 (62.2) 70 (37.8) | 49 (98) 1 (2) | <0.01 |
| Age diagnosis (years) (mean, SD) | 58.6 (8.0) | 59.1 (7.9) | 56.8 (8.2) | 0.07 |
| BMI (kg/m2) <18.5 18.5–24.9 25.0–29.9 ≥30 | 21 (8.9) 117 (49.8) 65 (27.7) 32 (13.6) | 21 (11.4) 113 (61.1) 40 (21.6) 11 (5.9) | 0 (0) 4 (8) 25 (50) 21 (42) | <0.01 |
| ACE-27 score None Mild Moderate Severe | 50 (21.3) 115 (48.9) 53 (22.6) 17 (7.2) | 42 (22.7) 87 (47) 43 (23.2) 13 (7) | 8 (16) 28 (56) 10 (20) 4 (8) | 0.64 |
| Performance status ECOG 0 ECOG 1 ECOG ≥ 2 Missing | 63 (26.8) 111 (47.2) 27 (11.5) 34 (14.5) | 49 (26.5) 89 (48.1) 21 (11.4) 26 (14.1) | 14 (28) 22 (44) 6 (12) 8 (16) | 0.96 |
| Smoker No Current/former | 43 (18.3) 192 (81.7) | 30 (16.2) 155 (83.8) | 13 (26) 37(74) | 0.15 |
| Alcohol use No Yes Smoker and alcohol use No Yes | 40 (17) 195 (83) 60 (25.5) 175 (74.5) | 32 (17.3) 153 (82.7) 44 (23.8) 141 (76.2) | 8 (16) 42 (84) 16 (32) 34 (68) | 0.84 0.27 |
| Albumin (g/L) (mean, SD) | 39.8 (4.6) | 40.0 (4.7) | 39.8 (4.6) | 0.8 |
| Tumor site Oral cavity Oropharynx HPV– HPV+ HPV unknown Nasopharynx Hypopharynx Larynx Paranasal sinus Unknown primary | 83 (35.3) 73 (31.1) 44 (60.3) 21 (28.8) 8 (11.0) 19 (8.1) 32 (13.6) 10 (4.3) 10 (4.3) 8 (3.4) | 66 (35.7) 57 (30.8) 36 (63.2) 14 (24.6) 7 (12.3) 14 (7.6) 27 (14.6) 8 (4.3) 7 (3.8) 6 (3.2) | 17 (34) 16 (32) 8 (50.0) 7 (9.6) 1 (6.3) 5 (10) 5 (10) 2 (4) 3 (6) 2 (4) | 0.98 |
| TNM stage III IV | 40 (17.0) 195 (83.0) | 31 (16.8) 154 (83.2) | 9 (18.0) 41 (82) | 0.84 |
| CRT setting Primary Adjuvant | 166 (70.6) 69 (29.4) | 126 (68.1) 59 (31.9) | 40 (80) 10 (20.0) | 0.12 |
| Stable | Moderate Loss | Moderate Gain | Large Loss | Large gain | |
|---|---|---|---|---|---|
| Change in SD | ±1 SD | ≥1 SD to <2 SD | ≥1 SD to <2 SD | ≥2 SD | ≥2 SD |
| SMA range (cm2) | >24.33 to <42.45 | ≤24.33 to >18.22 | ≥42.45 to <51.56 | ≤18.22 | ≥51.56 |
| n (%) | 91 (38.7) | 129 (54.9) | 13 (5.5) | 1 (0.4) | 1 (0.4) |
| Characteristics | Stable n = 91 41.2% | Muscle Loss n = 130 90.3% | Muscle Gain n = 14 9.7% | p-Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Gender Male Female | 63 (69.2) 28 (30.8) | 89 (68.5) 41 (31.5) | 12 (85.7) 2 (14.3) | 0.4 |
| Age > 60 years No Yes BMI (kg/m2) <18.5 18.5–24.9 25.0–29.9 ≥30 ACE-27 score None Mild Moderate Severe Performance ECOG 0 ECOG 1 ECOG ≥ 2 Unknown Smoking No Former Current Alcohol No Current/former | 45 (49.5) 46 (50.5) 14 (15.4) 50 (54.9) 19 (20.9) 8 (8.8) 18 (19.8) 48 (52.7) 19 (20.9) 6 (6.6) 24 (26.4) 48 (52.7) 8 (8.8) 11 (12.1) 18 (19.8) 25 (27.5) 48 (52.7) 18 (19.8) 73 (80.2) | 72 (55.4) 58 (44.6) 6 (4.6) 60 (46.2) 40 (30.8) 24 (18.5) 29 (22.3) 64 (49.2) 30 (23.1) 7 (5.4) 37 (28.5) 53 (40.8) 19 (14.6) 21 (16.2) 24 (18.5) 37 (28.5) 69 (53.1) 2 (14.3) 12 (85.7) | 7 (50) 7 (50) 7 (50) 1 (7.1) 6 (42.9) 0 (0) 3 (21.4) 3 (21.4) 4 (28.6) 4 (28.6) 2 (14.3) 10 (71.4) 0 (0) 2 (14.3) 1 (7.1) 3 (21.4) 10 (71.4) 20 (15.4) 110 (84.6) | 0.7 0.01 0.7 0.2 0.7 0.7 |
| Tumor site Oral cavity Oropharynx Nasopharynx Hypopharynx Larynx Paranasal sinus Unknown primary TNM stage III IV | 30 (33) 22 (24.2) 9 (9.9) 16 (17.6) 4 (4.4) 3 (3.3) 7 (7.7) 14 (15.4) 77 (84.6) | 46 (35.4) 46 (35.4) 10 (7.7) 14 (10.8) 6 (4.6) 7 (5.4) 1 (0.8) 26 (20) 104 (80) | 7 (50) 5 (35.7) 0 (0) 2 (14.3) 0 (0) 0 (0) 0 (0) 0 (0) 14 (100) | 0.2 0.3 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 1.0 (1.0–1.0) | 0.8 | ||
| Gender Female Male | Ref. 0.9 (0.5–1.5) | 0.6 | ||
| BMI (kg/m2) 18.5–24.9 <18.5 25–29.9 ≥30 Smoking No Yes Alcohol use No Yes Performance status ECOG 0 ECOG 1 ECOG ≥ 2 Unknown Albumin (mmol/L) | Ref. 0.4 (0.1–1.0) 1.5 (0.8–2.8) 2.9 (1.2–6.9) Ref. 1.0 (0.5–1.9) Ref. 1.3 (0.7–2.6) Ref. 0.6 (0.3–1.2) 1.7 (0.6–4.4) 1.1 (0.5–2.7) 1.1 (1.0–1.1) | 0.06 0.2 0.02 0.9 0.5 0.2 0.3 0.8 0.2 | Ref. 0.4 (0.1–2.0) 1.3 (0.7–2.5) 3.6 (1.4–9.3) | 0.1 0.4 <0.01 |
| ACE-27 None Mild Moderate Severe Tumor localization Oral cavity Oropharynx Nasopharynx Hypopharynx Larynx Paranasal sinus Unknown primary HPV status Negative Positive Unknown CRT setting Primary Adjuvant Dose-limiting toxicity No Yes Cumulative chemotherapy dose <300 mg ≥300 mg Weight loss during CRT None <10% ≥10% | Ref. 1.0 (0.6–1.8) 0.9 (0.4–2.1) 0.5 (0.2–1.5) Ref. 1.4 (0.7–2.6) 0.9 (0.3–2.4) 0.6 (0.3–1.4) 1.2 (0.3–4.6) 1.9 (0.5–7.8) 0.1 (0.01–0.9) Ref. 0.8 (0.3–2.2) 1.7 (0.3–9.5) Ref. 1.2 (0.7–2.1) Ref. 1.2 (0.7–2.0) Ref. 0.9 (0.5–1.6) Ref. 1.4 (0.7–2.5) 2.8 (0.9–8.8) | 0.8 0.9 0.2 0.4 0.8 0.3 0.8 0.4 0.047 0.6 0.5 0.6 0.5 0.6 0.3 0.08 | Ref. 1.7 (0.9–1.4) 0.9 (0.3–2.6) 0.7 (0.3–1.7) 0.9 (0.2–3.6) 1.9 (0.4–8.4) 0.1 (0.1–1.0) Ref. 1.3 (0.7–2.4) 2.6 (0.8–8.5) | 0.1 0.8 0.5 0.9 0.4 0.05 0.5 0.1 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (years) | 1.0 (1.0–1.0) | 0.2 | ||
| Low SMM No Yes | Ref. 2.1 (1.1–4.1) | 0.03 | Ref. 4.3 (0.6–32.6) | 0.2 |
| Gender Female Male | Ref. 1.1 (0.7–1.7) | 0.7 | ||
| BMI (kg/m2) 18.5–24.9 <18.5 25–29.9 ≥30 | Ref. 1.1 (0.5–2.2) 0.7 (0.4–1.1) 0.9 (0.5–1.7) | 0.9 0.1 0.7 | ||
| ACE–27 None Mild Moderate Severe | Ref. 1.1 (0.6–1.8) 1.5 (0.8–2.8) 1.1 (0.4–2.6) | 1.0 0.2 0.9 | ||
| Tumor localization Oral cavity Oropharynx Nasopharynx Hypopharynx Larynx Paranasal sinus Unknown primary | Ref. 1.1 (0.7–1.9) 0.6 (0.2–1.5) 1.2 (0.7–2.2) 0.6 (0.2–2.1) 0.2 (0.03–1.4) 1.1 (0.3–4.6) | 0.7 0.3 0.5 0.5 0.1 0.9 | ||
| HPV status Negative Positive Unknown | Ref. 0.1 (0.01–0.9) 0.3 (0.04–2.2) | 0.04 0.2 | Ref. 0.1 (0.02–1.0) 0.4 (0.05–2.7) | 0.06 0.3 |
| CRT setting Primary Adjuvant | Ref. 1.3 (0.9–2.1) | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chargi, N.; Wegner, I.; Markazi, N.; Smid, E.; de Jong, P.; Devriese, L.; de Bree, R. Patterns, Predictors, and Prognostic Value of Skeletal Muscle Mass Loss in Patients with Locally Advanced Head and Neck Cancer Undergoing Cisplatin-Based Chemoradiotherapy. J. Clin. Med. 2021, 10, 1762. https://doi.org/10.3390/jcm10081762
Chargi N, Wegner I, Markazi N, Smid E, de Jong P, Devriese L, de Bree R. Patterns, Predictors, and Prognostic Value of Skeletal Muscle Mass Loss in Patients with Locally Advanced Head and Neck Cancer Undergoing Cisplatin-Based Chemoradiotherapy. Journal of Clinical Medicine. 2021; 10(8):1762. https://doi.org/10.3390/jcm10081762
Chicago/Turabian StyleChargi, Najiba, Inge Wegner, Navid Markazi, Ernst Smid, Pim de Jong, Lot Devriese, and Remco de Bree. 2021. "Patterns, Predictors, and Prognostic Value of Skeletal Muscle Mass Loss in Patients with Locally Advanced Head and Neck Cancer Undergoing Cisplatin-Based Chemoradiotherapy" Journal of Clinical Medicine 10, no. 8: 1762. https://doi.org/10.3390/jcm10081762
APA StyleChargi, N., Wegner, I., Markazi, N., Smid, E., de Jong, P., Devriese, L., & de Bree, R. (2021). Patterns, Predictors, and Prognostic Value of Skeletal Muscle Mass Loss in Patients with Locally Advanced Head and Neck Cancer Undergoing Cisplatin-Based Chemoradiotherapy. Journal of Clinical Medicine, 10(8), 1762. https://doi.org/10.3390/jcm10081762








