Coronary Stent Strut Fractures: Classification, Prevalence and Clinical Associations
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective of the Study
2.2. Patients
2.3. Definitions
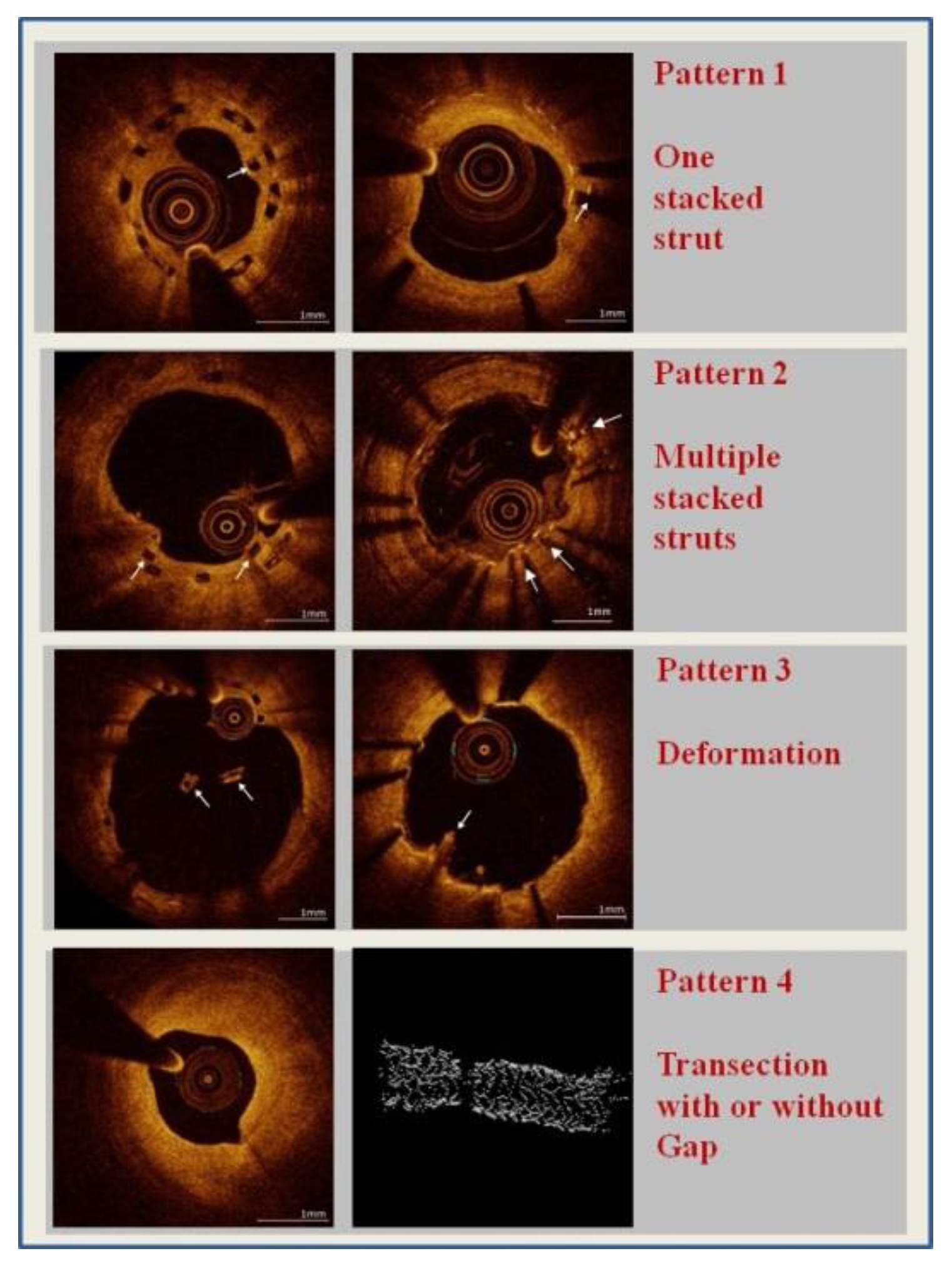
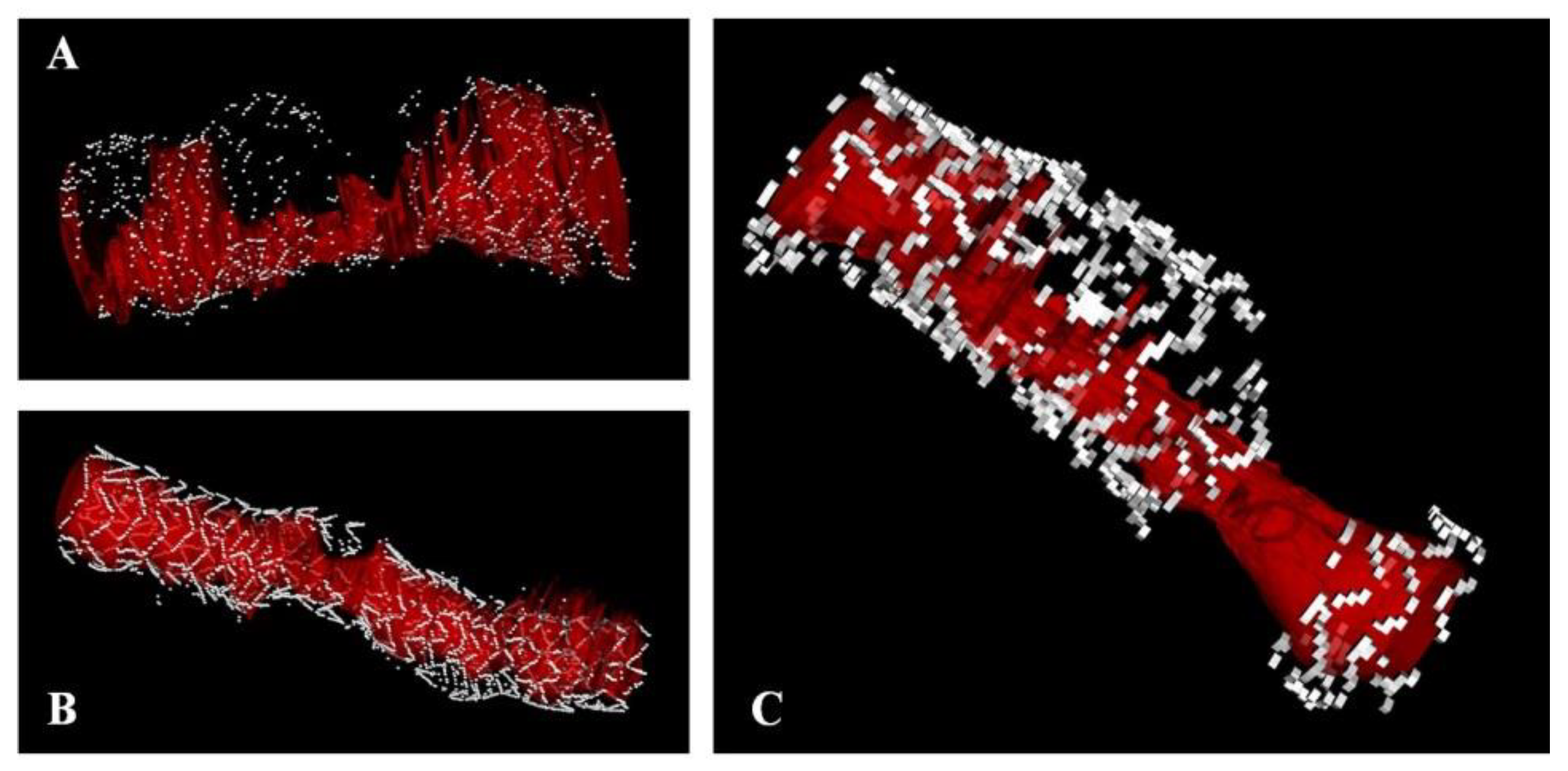
2.4. Strut Fractures
- -
- Pattern 1: one single stacked strut
- -
- Pattern 2: two or more stacked struts without deformation
- -
- Pattern 3: deformation with evidence of isolated (malapposed) struts or groups of struts not fitting the normal circular geometry of the scaffold in one or more cross sections
- -
- Pattern 4: transection with malalignment of the stent segments with or without gap (at least 2 consecutive frames without any strut) [14].
2.5. Statistical Analysis
3. Results
3.1. Prevalence of SFs in DES after Implantation, during Follow-Up and in the Presence of Device Failure
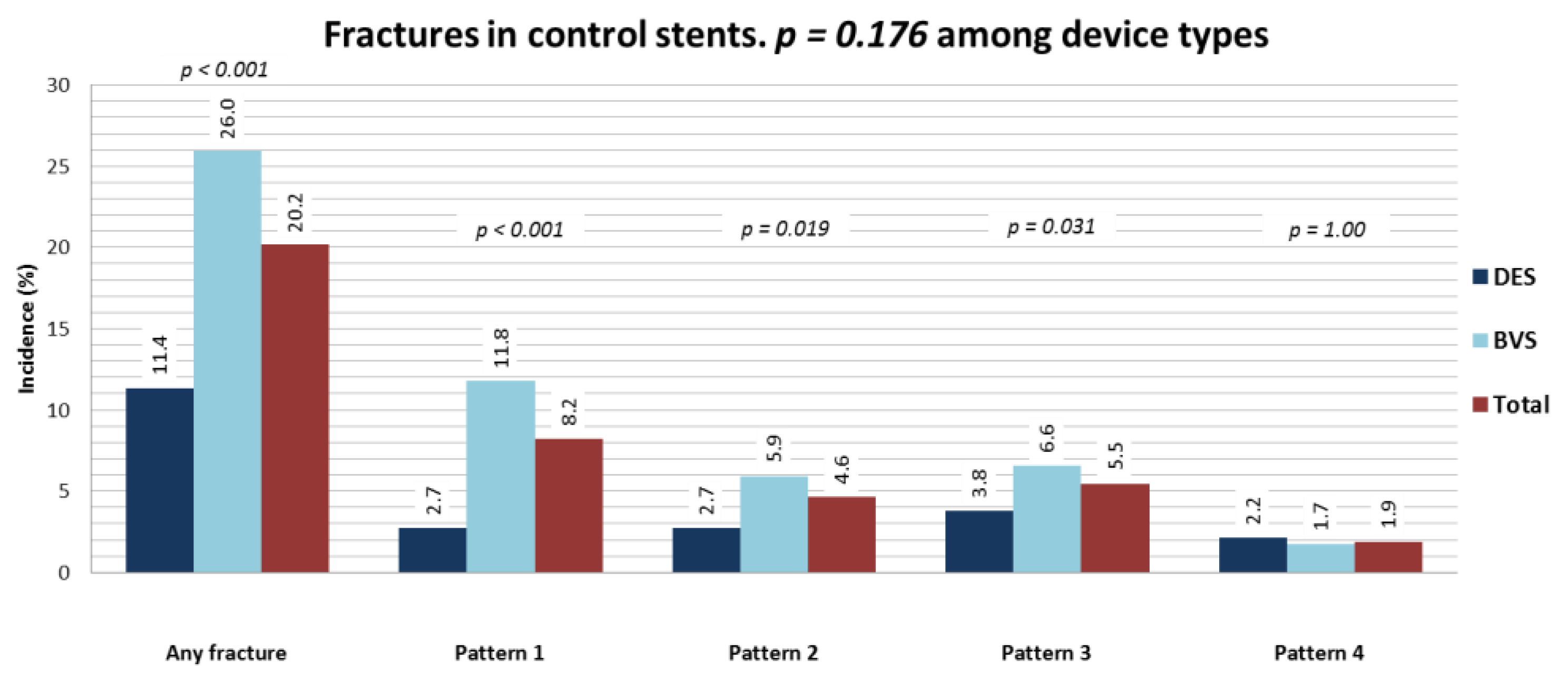
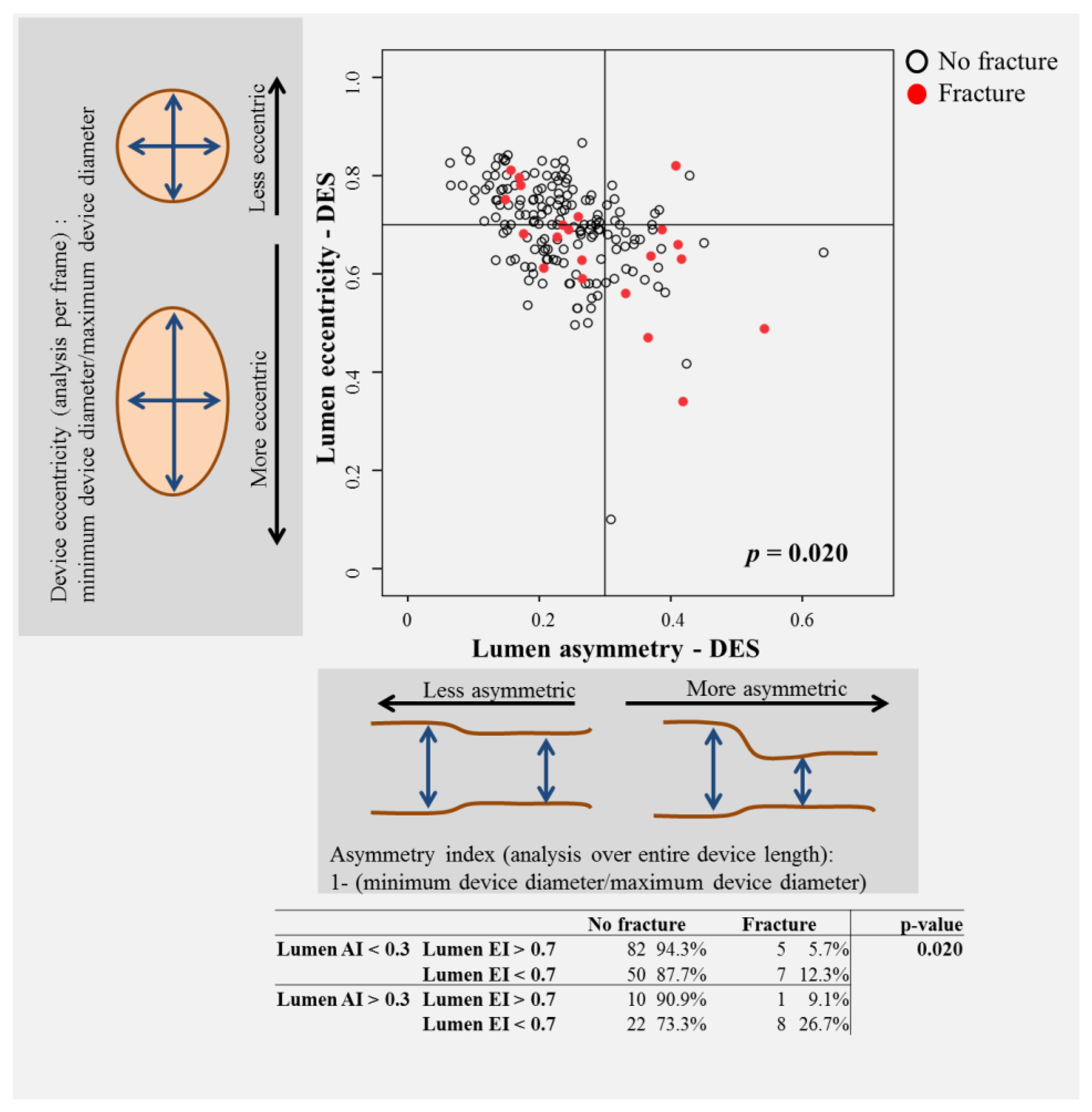
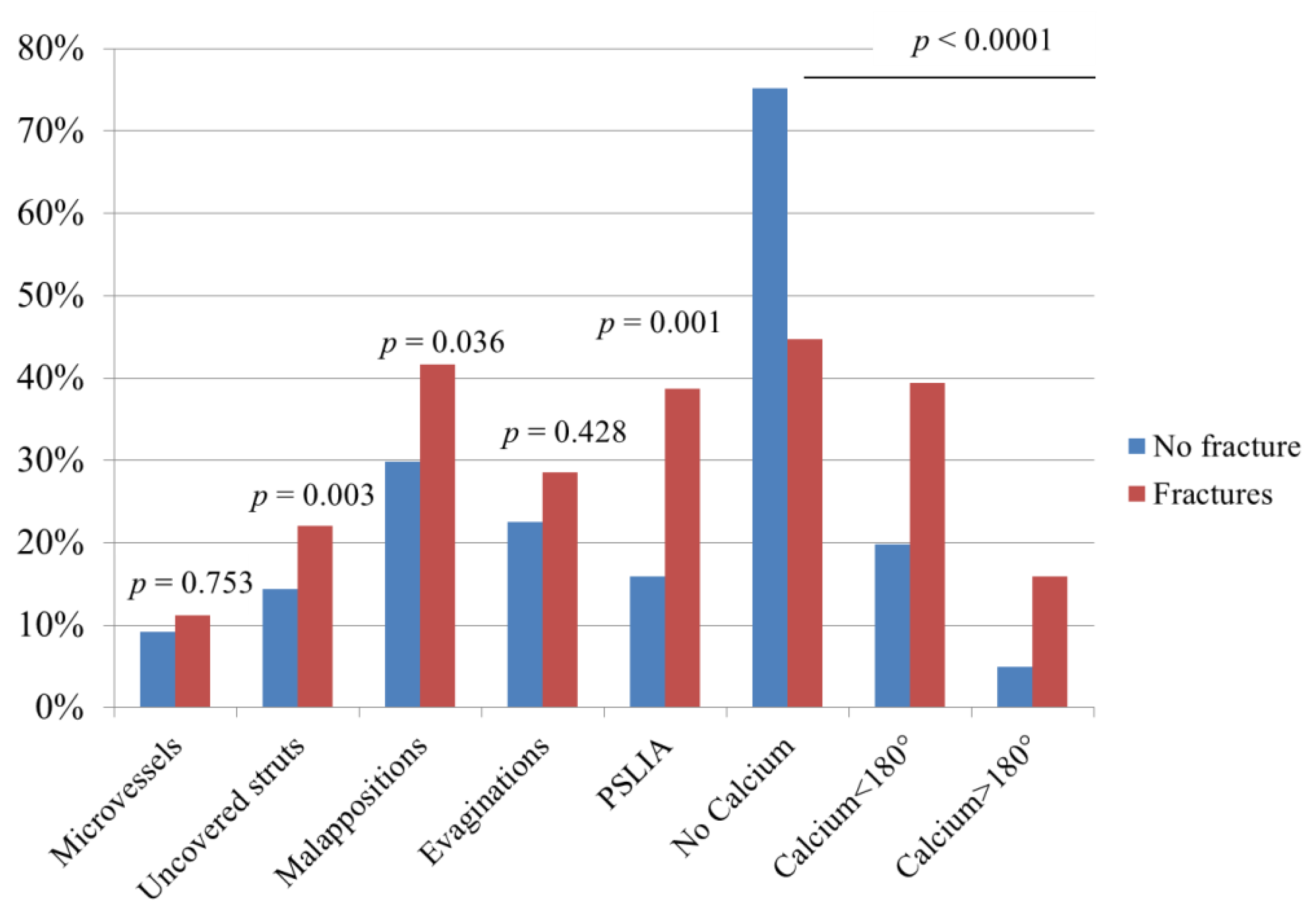
3.2. Patterns of SF and Association with OCT Characteristics
3.3. Procedural Parameters Associated with SF
3.4. Prevalence, Characteristics and Association with Procedural Parameters in BRS
3.5. The Impact of Fractures on Device Failure
4. Discussion
- (1)
- incidental findings of fractures occurred in ~8% of new-generation drug-eluting stents immediately after implantation, and this rate was as high as ~60% in the setting of device failure;
- (2)
- parameters of lesion/vessel anatomy, including bifurcation and calcific lesions were associated with fracture; increased asymmetry and eccentricity were associated with SF;
- (3)
- we propose an OCT classification based on a previously published pathological staging which allows distinguishing different degrees of SF. Using this classification, we found that the prevalence of pattern 4 (gap) SF increased by ~10 times in device failure compared to control devices;
- (4)
- fractures were associated with other OCT abnormalities, including peri-strut low-intensity areas, uncovered and/or malapposed struts (all suggestive of incomplete stent healing);
- (5)
- the presence of fractures was independently associated with device failure;
- (6)
- similar results were observed in BRS. Of note, SFs represent a step of the bioresorption of scaffolds, while they are unwanted phenomena in stents. However, the fact that SFs were associated with PSLIA, uncovered/malapposed struts and ultimately device failure in both device types supports the concept that SFs represent a risk factor for device failure in both settings.
4.1. Evidence on SF
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madhavan, M.V.; Kirtane, A.J.; Redfors, B.; Genereux, P.; Ben-Yehuda, O.; Palmerini, T.; Benedetto, U.; Biondi-Zoccai, G.; Smits, P.C.; von Birgelen, C.; et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2020, 75, 590–604. [Google Scholar] [CrossRef]
- Conway, C.; Desany, G.J.; Bailey, L.R.; Keating, J.H.; Baker, B.L.; Edelman, E.R. Fracture in drug-eluting stents increases focal intimal hyperplasia in the atherosclerosed rabbit iliac artery. Catheter. Cardiovasc. Interv. 2018, 93, 278–285. [Google Scholar] [CrossRef]
- Aoki, J.; Nakazawa, G.; Tanabe, K.; Hoye, A.; Yamamoto, H.; Nakayama, T.; Onuma, Y.; Higashikuni, Y.; Otsuki, S.; Yagishita, A.; et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter. Cardiovasc. Interv. 2007, 69, 380–386. [Google Scholar] [CrossRef]
- Kuramitsu, S.; Jinnouchi, H.; Shinozaki, T.; Hiromasa, T.; Matsumura, Y.; Yamaji, Y.; Miura, M.; Matsuda, H.; Masuda, H.; Domei, T.; et al. Incidence and Long-Term Clinical Impact of Late-Acquired Stent Fracture after Sirolimus-Eluting Stent Implantation in Narrowed Coronary Arteries. Am. J. Cardiol. 2017, 120, 55–62. [Google Scholar] [CrossRef]
- Lee, M.S.; Jurewitz, D.; Aragon, J.; Forrester, J.; Makkar, R.R.; Kar, S. Stent fracture associated with drug-eluting stents: Clinical characteristics and implications. Catheter. Cardiovasc. Interv. 2007, 69, 387–394. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.S.; Shin, D.G.; Kim, Y.J.; Hong, G.R.; Kim, W.; Shim, B.S. Frequency of stent fracture as a cause of coronary restenosis after sirolimus-eluting stent implantation. Am. J. Cardiol. 2007, 100, 627–630. [Google Scholar] [CrossRef]
- Shaikh, F.; Maddikunta, R.; Djelmami-Hani, M.; Solis, J.; Allaqaband, S.; Bajwa, T. Stent fracture, an incidental finding or a significant marker of clinical in-stent restenosis? Catheter. Cardiovasc. Interv. 2008, 71, 614–618. [Google Scholar] [CrossRef]
- Shite, J.; Matsumoto, D.; Yokoyama, M. Sirolimus-eluting stent fracture with thrombus, visualization by optical coherence tomography. Eur. Heart J. 2006, 27, 1389. [Google Scholar] [CrossRef]
- Umeda, H.; Gochi, T.; Iwase, M.; Izawa, H.; Shimizu, T.; Ishiki, R.; Inagaki, H.; Toyama, J.; Yokota, M.; Murohara, T. Frequency, predictors and outcome of stent fracture after sirolimus-eluting stent implantation. Int. J. Cardiol. 2009, 133, 321–326. [Google Scholar] [CrossRef]
- White, J.M.; Webster, M.W.; Ormiston, J.A. Strut fracture with contemporary stent platforms. Catheter. Cardiovasc. Interv. 2015, 85, 932. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.Y.; Lee, J.M.; Yoon, Y.W.; Ahn, C.M.; Kim, M.H.; Min, P.K.; Ko, Y.G.; Hong, B.K.; Choi, D.; et al. Significant association of coronary stent fracture with in-stent restenosis in sirolimus-eluting stents. Coron. Artery Dis. 2009, 20, 59–63. [Google Scholar] [CrossRef]
- Celik, T.; Iyisoy, A.; Dogru, M.T.; Isik, E. Coronary stent strut fracture after drug-eluting stent implantation: A newly recognized complication. Int. J. Cardiol. 2009, 132, 121–122. [Google Scholar] [CrossRef]
- Popma, J.J.; Tiroch, K.; Almonacid, A.; Cohen, S.; Kandzari, D.E.; Leon, M.B. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am. J. Cardiol. 2009, 103, 923–929. [Google Scholar] [CrossRef]
- Gori, T.; Jansen, T.; Weissner, M.; Foin, N.; Wenzel, P.; Schulz, E.; Cook, S.; Munzel, T. Coronary evaginations and peri-scaffold aneurysms following implantation of bioresorbable scaffolds: Incidence, outcome, and optical coherence tomography analysis of possible mechanisms. Eur. Heart J. 2016, 37, 2040–2049. [Google Scholar] [CrossRef]
- Kan, J.; Ge, Z.; Zhang, J.J.; Liu, Z.Z.; Tian, N.L.; Ye, F.; Li, S.J.; Qian, X.S.; Yang, S.; Chen, M.X.; et al. Incidence and Clinical Outcomes of Stent Fractures on the Basis of 6555 Patients and 16,482 Drug-Eluting Stents from 4 Centers. JACC Cardiovasc. Interv. 2016, 9, 1115–1123. [Google Scholar] [CrossRef]
- Nakazawa, G.; Finn, A.V.; Vorpahl, M.; Ladich, E.; Kutys, R.; Balazs, I.; Kolodgie, F.D.; Virmani, R. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J. Am. Coll. Cardiol. 2009, 54, 1924–1931. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef]
- Gori, T.; Schulz, E.; Hink, U.; Kress, M.; Weiers, N.; Weissner, M.; Jabs, A.; Wenzel, P.; Capodanno, D.; Munzel, T. Clinical, Angiographic, Functional, and Imaging Outcomes 12 Months after Implantation of Drug-Eluting Bioresorbable Vascular Scaffolds in Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2015, 8, 770–777. [Google Scholar] [CrossRef][Green Version]
- Won, H.; Shin, D.H.; Kim, B.K.; Mintz, G.S.; Kim, J.S.; Ko, Y.G.; Choi, D.; Jang, Y.; Hong, M.K. Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int. J. Cardiovasc. Imaging 2013, 29, 1255–1263. [Google Scholar] [CrossRef]
- Raber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef] [PubMed]
- Gori, T. Vascular Wall Reactions to Coronary Stents-Clinical Implications for Stent Failure. Life 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, S.; Sonoda, S.; Ando, K.; Otake, H.; Natsuaki, M.; Anai, R.; Honda, Y.; Kadota, K.; Kobayashi, Y.; Kimura, T. Drug-eluting stent thrombosis: Current and future perspectives. Cardiovasc. Interv. Ther. 2021, 36, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Buckley, L.; Duran, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzman, L.A. Restenosis, Stent Thrombosis, and Bleeding Complications: Navigating Between Scylla and Charybdis. J. Am. Coll. Cardiol. 2018, 71, 1676–1695. [Google Scholar] [CrossRef]
- Leong, D.P.; Dundon, B.K.; Puri, R.; Yeend, R.A. Very late stent fracture associated with a sirolimus-eluting stent. Heart Lung Circ. 2008, 17, 426–428. [Google Scholar] [CrossRef]
- Canan, T.; Lee, M.S. Drug-eluting stent fracture: Incidence, contributing factors, and clinical implications. Catheter. Cardiovasc. Interv. 2010, 75, 237–245. [Google Scholar] [CrossRef]
- Chakravarty, T.; White, A.J.; Buch, M.; Naik, H.; Doctor, N.; Schapira, J.; Kar, S.; Forrester, J.S.; Weiss, R.E.; Makkar, R. Meta-analysis of incidence, clinical characteristics and implications of stent fracture. Am. J. Cardiol. 2010, 106, 1075–1080. [Google Scholar] [CrossRef]
- Kuramitsu, S.; Hiromasa, T.; Enomoto, S.; Shinozaki, T.; Iwabuchi, M.; Mazaki, T.; Domei, T.; Yamaji, K.; Soga, Y.; Hyodo, M.; et al. Incidence and Clinical Impact of Stent Fracture After PROMUS Element Platinum Chromium Everolimus-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2015, 8, 1180–1188. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, I.H.; Kim, Y.J. Stent fracture and restenosis after zotarolimus-eluting stent implantation. Int. J. Cardiol. 2011, 147, e29–e31. [Google Scholar] [CrossRef]
- Park, K.W.; Park, J.J.; Chae, I.H.; Seo, J.B.; Yang, H.M.; Lee, H.Y.; Kang, H.J.; Cho, Y.S.; Yeon, T.J.; Chung, W.Y.; et al. Clinical characteristics of coronary drug-eluting stent fracture: Insights from a two-center des registry. J. Korean Med. Sci. 2011, 26, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Schomig, A.; Dibra, A.; Windecker, S.; Mehilli, J.; Suarez de Lezo, J.; Kaiser, C.; Park, S.J.; Goy, J.J.; Lee, J.H.; Di Lorenzo, E.; et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 2007, 50, 1373–1380. [Google Scholar] [CrossRef]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of Very Late Bioresorbable Scaffold Thrombosis: The INVEST Registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Souteyrand, G.; Amabile, N.; Mangin, L.; Chabin, X.; Meneveau, N.; Cayla, G.; Vanzetto, G.; Barnay, P.; Trouillet, C.; Rioufol, G.; et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: Insights from the national PESTO French registry. Eur. Heart J. 2016, 37, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
| DES-Control Patients Only | |||||
|---|---|---|---|---|---|
| No Fracture (n = 141) | Fracture (n = 19) | p-Value | |||
| Clinical Presentation (per patient) | mean/median/n | SD/IQR/% | mean/median/n | SD/IQR/% | |
| Male | 105 | 74.5% | 14 | 73.7% | 0.941 |
| Age (years) | 66.0 | 17.0 | 62.0 | 24.0 | 0.565 |
| Positive family history | 48 | 34.0% | 6 | 31.6% | 0.831 |
| Diabetes mellitus | 47 | 33.3% | 7 | 36.8% | 0.761 |
| Hypertension | 109 | 77.3% | 14 | 73.7% | 0.725 |
| Hyperlipidemia | 76 | 53.9% | 8 | 42.1% | 0.334 |
| Active smoker | 42 | 29.8% | 10 | 52.6% | 0.129 |
| Indication for stent implantation | 0.399 | ||||
| Planned | 21 | 15.9% | 2 | 10.5% | |
| Stable angina | 56 | 42.4% | 9 | 47.4% | |
| Instable angina | 15 | 11.4% | 0 | 0.0% | |
| NSTEMI | 19 | 14.4% | 5 | 26.3% | |
| STEMI | 21 | 15.9% | 3 | 15.8% | |
| LVEF | 55 | 10 | 55 | 5 | 0.903 |
| n_vessel-disease | 0.066 | ||||
| 1-vessel | 43 | 30.7% | 1 | 5.3% | |
| 2-vessel | 55 | 39.3% | 10 | 52.6% | |
| 3-vessel | 42 | 30.0% | 8 | 42.1% | |
| Antiplatelet Therapy | 0.148 | ||||
| Clopidogrel | 80 | 58% | 7 | 38% | |
| Prasugrel | 29 | 21% | 5 | 28% | |
| Ticagrelor | 30 | 22% | 6 | 33% | |
| Angiographic Characteristics (per device) | n = 164 | n = 21 | |||
| treated vessel | 0.905 | ||||
| RCA | 52 | 31.7% | 8 | 38.1% | |
| LAD | 84 | 51.2% | 9 | 42.9% | |
| LCX | 22 | 13.4% | 3 | 14.3% | |
| LM | 6 | 3.7% | 1 | 4.8% | |
| ACC/AHA classification | 0.853 | ||||
| Type A | 23 | 15.2% | 2 | 12.5% | |
| Type B1 | 34 | 22.5% | 5 | 31.3% | |
| Type B2 | 67 | 44.4% | 7 | 43.8% | |
| Type C | 27 | 17.9% | 2 | 12.5% | |
| De novo lesion | 125 | 76.2% | 3 | 14.3% | 0.328 |
| Implantation on thrombus | 18 | 11.0% | 2 | 9.5% | 0.840 |
| Implantation in CTO | 4 | 2.4% | 0 | 0.0% | 0.469 |
| Implantation with overlap | 42 | 25.6% | 6 | 28.6% | 0.771 |
| Implantation on bifurcation | 18 | 11.0% | 6 | 28.6% | 0.024 |
| Predilatation | 119 | 72.6% | 18 | 85.7% | 0.195 |
| Ballon diameter (mm) | 2.8 | 0.5 | 2.8 | 0.3 | 0.167 |
| Ballon length (mm) | 15.0 | 8.0 | 15.0 | 12.0 | 0.989 |
| Predilatation pressure (atm) | 14.6 | 3.5 | 12.8 | 4.4 | 0.418 |
| Diameter stent | 3.2 | 0.5 | 2.9 | 0.2 | 0.003 |
| Length stent (mm) | 18.0 | 13.0 | 28.0 | 15.0 | 0.429 |
| Implantation pressure (atm) | 13.8 | 2.3 | 12.8 | 1.8 | 0.248 |
| Postdilatation | 98 | 59.8% | 9 | 42.9% | 0.140 |
| Ballon diameter (mm) | 3.3 | 0.6 | 3.1 | 0.4 | 0.311 |
| Ballon length (mm) | 14.0 | 3.0 | 12.0 | 6.0 | 0.824 |
| Postdilatation pressure (atm) | 16.1 | 5.2 | 13.6 | 3.8 | 0.109 |
| OCT analysis | |||||
| avg n_struts/frame | 9.6 | 2.5 | 9.0 | 3.2 | 0.460 |
| Length OCT (mm) | 18.8 | 12.5 | 28.2 | 6.4 | 0.490 |
| Maximal Area (mm2) | |||||
| Lumen | 10.5 | 3.4 | 8.4 | 1.8 | 0.449 |
| Vessel | 10.6 | 3.0 | 9.0 | 1.7 | 0.992 |
| Stent | 9.7 | 2.9 | 8.3 | 1.7 | 0.949 |
| Maximal Diameter (mm) | |||||
| Lumen | 3.6 | 0.6 | 3.3 | 0.3 | 0.459 |
| Vessel | 3.6 | 0.5 | 3.4 | 0.3 | 0.998 |
| Stent | 3.5 | 0.5 | 3.2 | 0.3 | 0.958 |
| Minimal Area (mm2) | |||||
| Lumen | 5.9 | 2.0 | 4.4 | 1.4 | 0.017 |
| Vessel | 6.5 | 2.1 | 5.0 | 1.6 | 0.082 |
| Stent | 5.9 | 2.0 | 4.4 | 1.5 | 0.079 |
| Minimal Diameter (mm) | |||||
| Lumen | 2.7 | 0.5 | 2.3 | 0.4 | 0.013 |
| Vessel | 2.9 | 0.5 | 2.5 | 0.4 | 0.076 |
| Stent | 2.7 | 0.5 | 2.3 | 0.4 | 0.073 |
| Avg Area (mm2) | |||||
| Lumen | 7.7 | 2.2 | 6.2 | 1.5 | 0.274 |
| Vessel | 8.4 | 2.3 | 7.0 | 1.3 | 0.492 |
| Stent | 7.6 | 2.2 | 6.3 | 1.3 | 0.502 |
| Neointima | −0.1 | 0.5 | −0.08 | 0.8 | 0.010 |
| Avg Diameter (mm) | |||||
| Lumen | 3.1 | 0.5 | 2.8 | 0.3 | 0.259 |
| Vessel | 3.2 | 0.4 | 3.0 | 0.3 | 0.518 |
| Stent | 3.1 | 0.4 | 2.8 | 0.3 | 0.528 |
| Avg Stenosis (%) | |||||
| Stenosis area | 8.7 | 6.0 | 7.5 | 11.8 | 0.018 |
| Stenosis diameter | 4.5 | 2.9 | 3.8 | 6.4 | 0.018 |
| Device/artery ratio | 1.05 | 0.12 | 1.11 | 0.18 | 0.071 |
| Eccentricity and Asymmetry | |||||
| Lumen AI > 0.3 | 32 | 19.5% | 9 | 42.9% | 0.015 |
| Stent AI > 0.3 | 19 | 11.6% | 6 | 28.6% | 0.032 |
| Lumen EI < 0.7 | 72 | 43.9% | 15 | 71.4% | 0.017 |
| Stent EI < 0.7 | 35 | 21.3% | 8 | 38.1% | 0.087 |
| Maximal lumen asymmetry | 0.25 | 0.13 | 0.26 | 0.07 | 0.027 |
| Maximal stent asymmetry | 0.22 | 0.12 | 0.26 | 0.11 | 0.009 |
| Maximal lumen eccentricity | 0.70 | 0.12 | 0.69 | 0.09 | 0.089 |
| Maximal stente ccentricity | 0.75 | 0.12 | 0.74 | 0.15 | 0.322 |
| Qualitative Analysis | |||||
| Microvessels | 2 | 6.9% | 2 | 18.2% | <0.001 |
| Uncovered struts | 2 | 6.9% | 7 | 63.6% | <0.001 |
| Malappositions | 66 | 40.5% | 11 | 52.4% | 0.545 |
| Evaginations | 8 | 26.7% | 2 | 18.2% | 0.001 |
| PSLIA | 6 | 20.7% | 5 | 41.7% | 0.001 |
| No Calcium | 113 | 73.9% | 4 | 20.0% | <0.001 |
| Calcium < 180° | 32 | 20.9% | 9 | 45.0% | |
| Calcium > 180° | 8 | 5.2% | 7 | 35.0% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schochlow, K.; Weissner, M.; Blachutzik, F.; Boeder, N.F.; Tröbs, M.; Lorenz, L.; Dijkstra, J.; Münzel, T.; Achenbach, S.; Nef, H.; et al. Coronary Stent Strut Fractures: Classification, Prevalence and Clinical Associations. J. Clin. Med. 2021, 10, 1765. https://doi.org/10.3390/jcm10081765
Schochlow K, Weissner M, Blachutzik F, Boeder NF, Tröbs M, Lorenz L, Dijkstra J, Münzel T, Achenbach S, Nef H, et al. Coronary Stent Strut Fractures: Classification, Prevalence and Clinical Associations. Journal of Clinical Medicine. 2021; 10(8):1765. https://doi.org/10.3390/jcm10081765
Chicago/Turabian StyleSchochlow, Katharina, Melissa Weissner, Florian Blachutzik, Niklas F. Boeder, Monique Tröbs, Liv Lorenz, Jouke Dijkstra, Thomas Münzel, Stephan Achenbach, Holger Nef, and et al. 2021. "Coronary Stent Strut Fractures: Classification, Prevalence and Clinical Associations" Journal of Clinical Medicine 10, no. 8: 1765. https://doi.org/10.3390/jcm10081765
APA StyleSchochlow, K., Weissner, M., Blachutzik, F., Boeder, N. F., Tröbs, M., Lorenz, L., Dijkstra, J., Münzel, T., Achenbach, S., Nef, H., & Gori, T. (2021). Coronary Stent Strut Fractures: Classification, Prevalence and Clinical Associations. Journal of Clinical Medicine, 10(8), 1765. https://doi.org/10.3390/jcm10081765






