Risk Factors for Mortality in Adult COVID-19 Patients Who Develop Bloodstream Infections Mostly Caused by Antimicrobial-Resistant Organisms: Analysis at a Large Teaching Hospital in Italy
Abstract
:1. Introduction
2. Materials and Methods
Study Design, Data Collection and Definitions
3. Data Analysis
4. Results
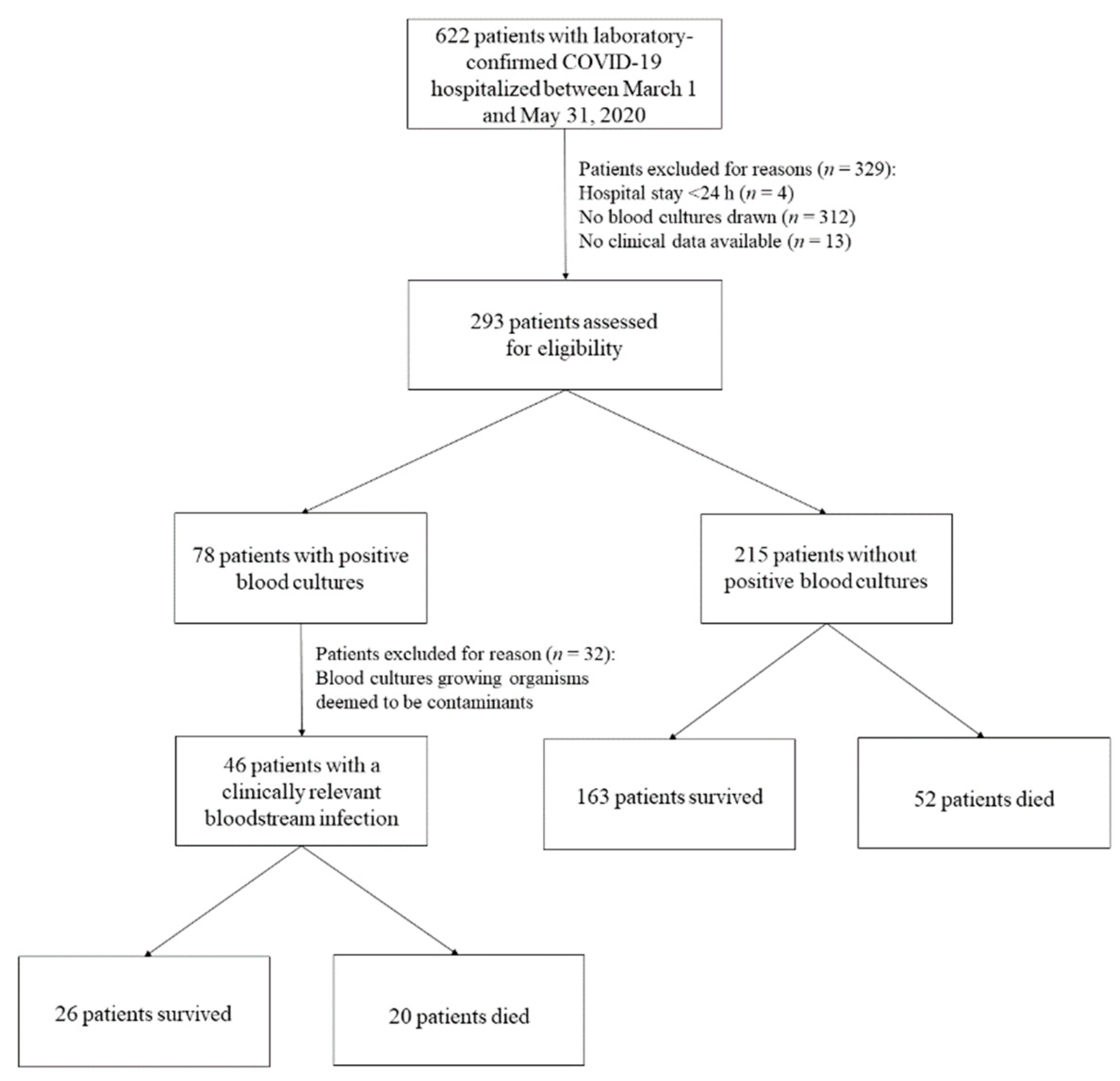
4.1. Characteristics of COVID-19 Patients Who Developed BSI
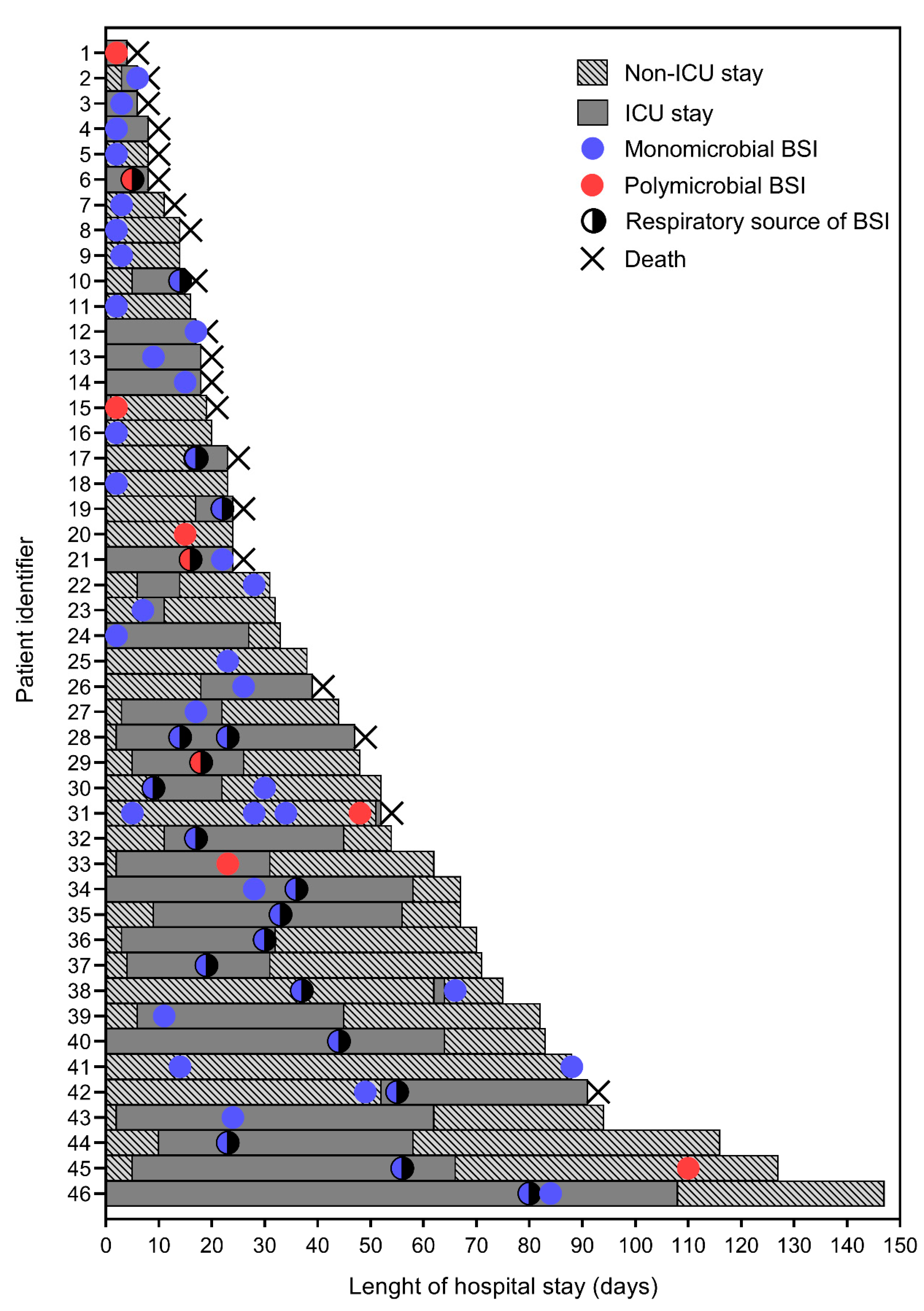
4.2. Characteristics of BSI Episodes in COVID-19 Patients
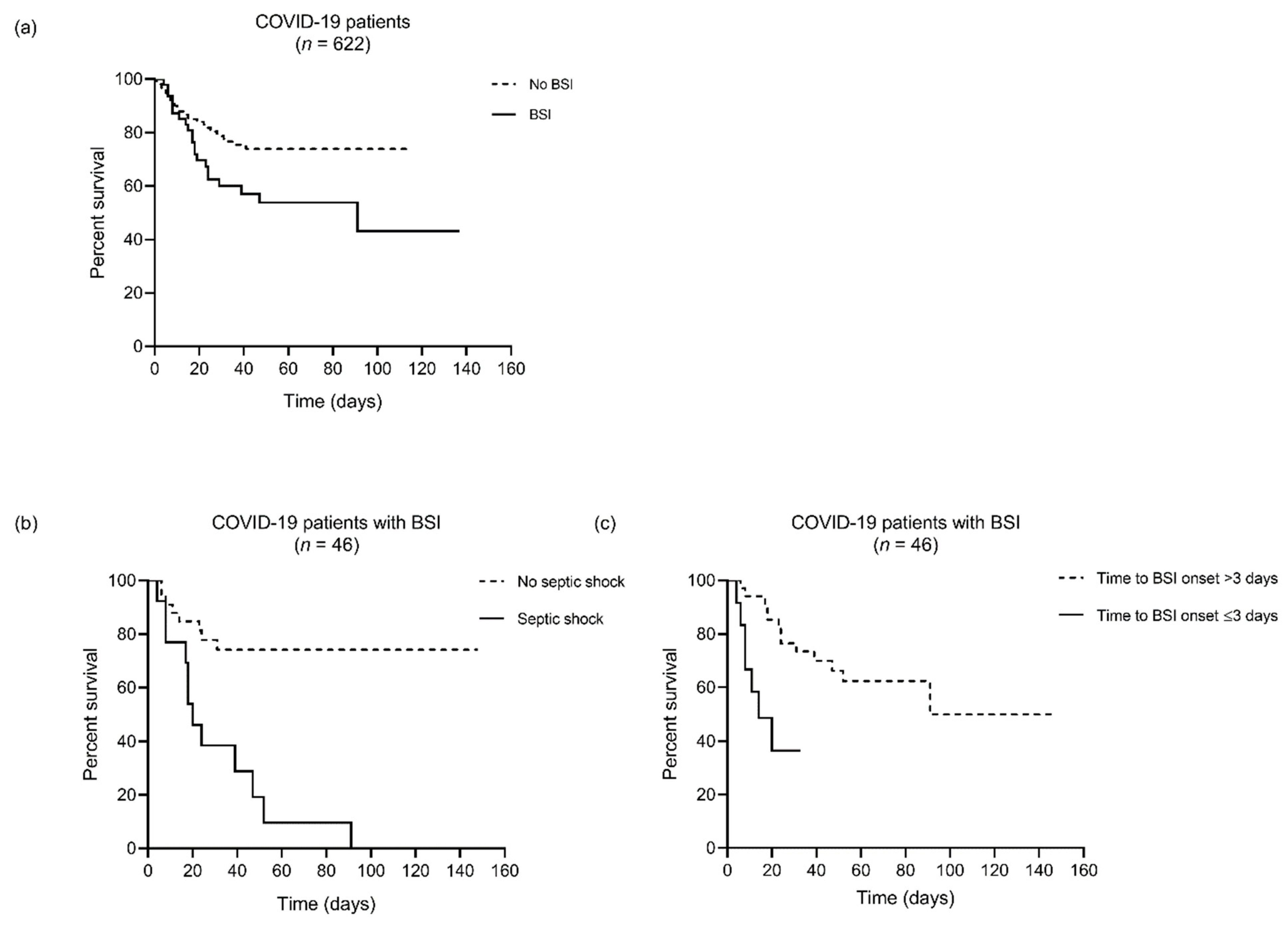
4.3. Risk Factors Associated with In-Hospital Mortality among BSI Patients
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Weekly Epidemiological Update. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update (accessed on 27 January 2021).
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. COVID-19, superinfections and antimicrobial development: What can we expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ling, Y.; Bai, T.; Xie, Y.; Huang, J.; Li, J.; Xiong, W.; Yang, D.; Chen, R.; Lu, F.; et al. COVID-19 with different severities: A multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 2020, 201, 1380–1388. [Google Scholar] [CrossRef]
- Manohar, P.; Loh, B.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. Secondary bacterial infections in patients with viral pneumonia. Front. Med. 2020, 7, 420. [Google Scholar] [CrossRef]
- Gomez-Simmonds, A.; Annavajhala, M.K.; McConville, T.H.; Dietz, D.E.; Shoucri, S.M.; Laracy, J.C.; Rozenberg, F.D.; Nelson, B.; Greendyke, W.G.; Furuya, E.Y.; et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J. Antimicrob. Chemother. 2021, 76, 380–384. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Dupper, A.C.; Malik, Y.; Gavioli, E.M.; Banga, J.; Berbel Caban, A.; Nadkarni, D.; Obla, A.; Vasa, C.V.; Mazo, D.; et al. Staphylococcus aureus bacteremia in patients infected with COVID-19: A case series. Open Forum Infect. Dis. 2020, 7, ofaa518. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 153. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Bamford, C.G. SARS-CoV-2, bacterial co-infections, and AMR: The deadly trio in COVID-19? EMBO Mol. Med. 2020, 12, e12560. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, M.; Jorth, P. The unrecognized threat of secondary bacterial infections with COVID-19. mBio 2020, 11, e01806-20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases: Interim Guidance. Available online: https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19-laboratory-2020.5-eng.pdf?sequence=1&isAllowed=y (accessed on 5 February 2021).
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 5 February 2021).
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement. CLSI Document M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination/?no_cache=1 (accessed on 5 February 2021).
- Cortazzo, V.; D’Inzeo, T.; Giordano, L.; Fiori, B.; Brigante, G.; Luzzaro, F.; Liotti, F.M.; Menchinelli, G.; Sanguinetti, M.; Spanu, T.; et al. Comparing BioFire FilmArray BCID2 and BCID panels for direct detection of bacterial pathogens and antimicrobial resistance genes from positive blood cultures. J. Clin. Microbiol. 2021, 59, e03163-20. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Torelli, R.; Vella, A.; Leone, P.M.; De Angelis, G.; De Carolis, E.; Ventura, G.; Sanguinetti, M.; Fantoni, M. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: A fatal case report. J. Fungi 2020, 6, 163. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 5 February 2021).
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare—Associated Infections and Antimicrobial Use in European Acute Care Hospitals—Protocol Version 5.3. Stockholm: ECDC. 2016. Available online: https://www.ecdc.europa.eu/en/publications-data/point-prevalence-survey-healthcare-associated-infections-and-antimicrobial-use-3 (accessed on 5 February 2021).
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: An observational study. Crit. Care Med. 2021, 49, e31–e40. [Google Scholar] [CrossRef]
- Wang, L.; Amin, A.K.; Khanna, P.; Aali, A.; McGregor, A.; Bassett, P.; Gopal Rao, G. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021, 76, 796–803. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2020, 76, 1078–1084. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Cohen, J.; Vincent, J.L.; Adhikari, N.K.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015, 15, 581–614. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Sepulveda, J.; Westblade, L.F.; Whittier, S.; Satlin, M.J.; Greendyke, W.G.; Aaron, J.G.; Zucker, J.; Dietz, D.; Sobieszczyk, M.; Choi, J.J.; et al. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J. Clin. Microbiol. 2020, 58, e00875-20. [Google Scholar] [CrossRef]
- Nori, P.; Cowman, K.; Chen, V.; Bartash, R.; Szymczak, W.; Madaline, T.; Punjabi Katiyar, C.; Jain, R.; Aldrich, M.; Weston, G.; et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2021, 42, 84–88. [Google Scholar] [CrossRef]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ren, C.; Yao, R.Q.; Feng, Y.W.; Yao, Y.M. Clinical features and development of sepsis in patients infected with SARS-CoV-2: A retrospective analysis of 150 cases outside Wuhan, China. Intensive Care Med. 2020, 46, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lin, G.L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and immune pathogenesis of viral sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clin. Microbiol. Infect. 2021, 27, 9–11. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2020. Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed on 5 February 2021).
| No. (%) of Patients with or Values of Characteristics by Groups | ||||
|---|---|---|---|---|
| Characteristics | All (n = 46) | Survivors (n = 26) | Non-Survivors (n = 20) | p Value |
| Age, years, median (IQR) | 69.5 (62.0–82.0) | 75.5 (64.0–83.0) | 69.0 (61.0–67.0) | 0.11 |
| Male | 33 (71.7) | 20 (76.9) | 13 (65.0) | 0.37 |
| Charlson comorbidities at admission | ||||
| Myocardial infarction | 7 (15.2) | 3 (11.5) | 4 (20.0) | 0.35 |
| Congestive heart failure | 4 (8.7) | 2 (7.7) | 2 (10.0) | 0.59 |
| Peripheral vascular disease | 8 (17.4) | 5 (19.2) | 3 (15.0) | 0.51 |
| Cerebrovascular disease | 3 (6.5) | 1 (3.9) | 2 (10.0) | 0.40 |
| Dementia | 11 (23.9) | 7 (26.9) | 4 (20.0) | 0.42 |
| Chronic pulmonary disease | 10 (21.7) | 5 (19.2) | 5 (25.0) | 0.45 |
| Rheumatologic disease | 3 (6.5) | 1 (3.9) | 2 (10.0) | 0.40 |
| Diabetes without complications | 7 (15.2) | 3 (11.5) | 4 (20.0) | 0.35 |
| Diabetes with chronic complications | 4 (8.7) | 2 (7.7) | 2 (10.0) | 0.59 |
| Hemiplegia or paraplegia | 3 (6.5) | 1 (3.9) | 2 (10.0) | 0.40 |
| Kidney disease | 6 (13.0) | 2 (7.7) | 4 (20.0) | 0.22 |
| Any malignancy a | 6 (13.0) | 4 (15.4) | 2 (10.0) | 0.46 |
| Charlson comorbidity index score, mean (SD) | 2.0 (1.8) | 1.8 (1.5) | 2.4 (2.0) | 0.30 |
| Hypertension | 23 (50.0) | 13 (50.0) | 10 (50.0) | 0.62 |
| COVID-19 severity status b | ||||
| Moderate | 12 (26.1) | 8 (30.8) | 4 (20.0) | 0.31 |
| Severe | 26 (56.5) | 15 (57.7) | 11 (55.0) | 0.54 |
| Critical | 8 (17.4) | 3 (11.5) | 5 (25.0) | 0.21 |
| Infection-related characteristics | ||||
| SOFA score, median (IQR) c | 6.5 (3.0–9.0) | 6.0 (2.0–9.0) | 8.0 (4.0–9.0) | 0.20 |
| C-reactive protein, mg/L, median (IQR) c | 116.1 (59.6–182.9) | 95.2 (59.0–163.7) | 147.7 (100.3–212.7) | 0.11 |
| Procalcitonin, ng/mL, median (IQR) c | 0.4 (0.2–1.6) | 0.2 (0.2–0.8) | 0.6 (0.3–8.0) | 0.02 |
| Interleukin 6, pg/mL, median (IQR) c | 133.0 (40.0–487.2) | 108.9 (32.2–442.8) | 216.1 (81.1–652.0) | 0.32 |
| Septic shock | 13 (28.3) | 1 (3.9) | 12 (60.0) | <0.001 |
| Respiratory source of infection | 19 (41.3) | 12 (46.2) | 7 (35.0) | 0.32 |
| Recurrent infection | 5 (10.9) | 4 (15.4) | 1 (5.0) | 0.26 |
| Antimicrobial-resistant infection | 24 (52.2) | 13 (50.0) | 11 (55.0) | 0.77 |
| ICU-acquired infection | 26 (56.5) | 14 (53.9) | 12 (60.0) | 0.45 |
| Time to infection onset, days, median (IQR) c | 15.2 (2.7–23.4) | 18.1 (9.4–27.7) | 8.5 (1.8–17.3) | 0.02 |
| Inappropriate empirical antimicrobial therapy c,d | 22 (47.8) | 14 (53.9) | 8 (40.0) | 0.26 |
| ICU admission | 36 (78.3) | 19 (73.1) | 17 (85.0) | 0.27 |
| ICU stay, days, median (IQR) | 9 (1–27) | 20 (0–39) | 8 (2–18) | 0.03 |
| Hospital stay, days, median (IQR) | 33 (18–70) | 58 (32–82) | 18 (8–28) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posteraro, B.; De Angelis, G.; Menchinelli, G.; D’Inzeo, T.; Fiori, B.; De Maio, F.; Cortazzo, V.; Sanguinetti, M.; Spanu, T. Risk Factors for Mortality in Adult COVID-19 Patients Who Develop Bloodstream Infections Mostly Caused by Antimicrobial-Resistant Organisms: Analysis at a Large Teaching Hospital in Italy. J. Clin. Med. 2021, 10, 1752. https://doi.org/10.3390/jcm10081752
Posteraro B, De Angelis G, Menchinelli G, D’Inzeo T, Fiori B, De Maio F, Cortazzo V, Sanguinetti M, Spanu T. Risk Factors for Mortality in Adult COVID-19 Patients Who Develop Bloodstream Infections Mostly Caused by Antimicrobial-Resistant Organisms: Analysis at a Large Teaching Hospital in Italy. Journal of Clinical Medicine. 2021; 10(8):1752. https://doi.org/10.3390/jcm10081752
Chicago/Turabian StylePosteraro, Brunella, Giulia De Angelis, Giulia Menchinelli, Tiziana D’Inzeo, Barbara Fiori, Flavio De Maio, Venere Cortazzo, Maurizio Sanguinetti, and Teresa Spanu. 2021. "Risk Factors for Mortality in Adult COVID-19 Patients Who Develop Bloodstream Infections Mostly Caused by Antimicrobial-Resistant Organisms: Analysis at a Large Teaching Hospital in Italy" Journal of Clinical Medicine 10, no. 8: 1752. https://doi.org/10.3390/jcm10081752
APA StylePosteraro, B., De Angelis, G., Menchinelli, G., D’Inzeo, T., Fiori, B., De Maio, F., Cortazzo, V., Sanguinetti, M., & Spanu, T. (2021). Risk Factors for Mortality in Adult COVID-19 Patients Who Develop Bloodstream Infections Mostly Caused by Antimicrobial-Resistant Organisms: Analysis at a Large Teaching Hospital in Italy. Journal of Clinical Medicine, 10(8), 1752. https://doi.org/10.3390/jcm10081752








