FDG-PET/CT and Para-Aortic Staging in Endometrial Cancer. A French Multicentric Study
Abstract
1. Introduction
2. Materials and Methods
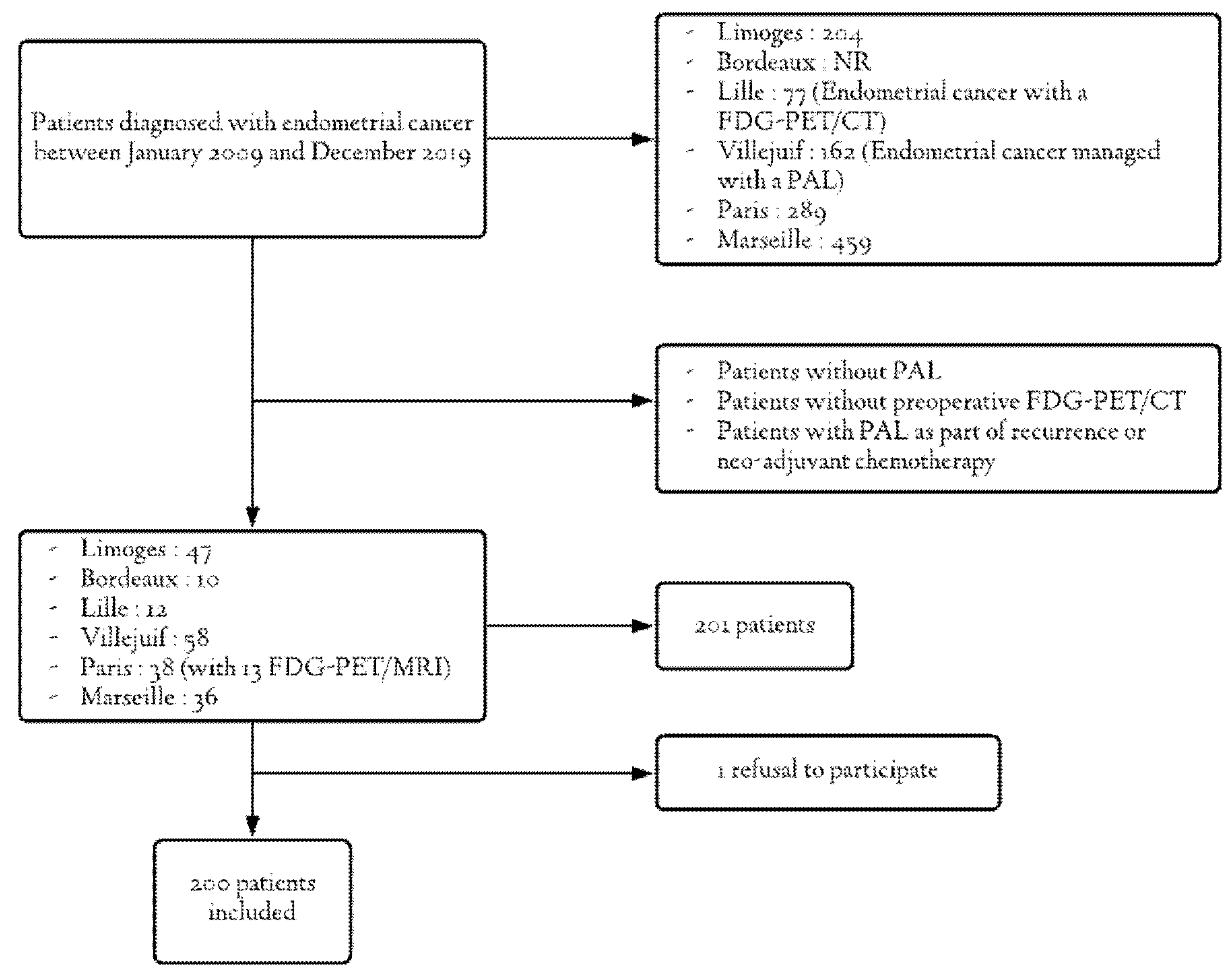
2.1. Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection
- Patient characteristics: date of birth, date of diagnosis, BMI and ASA score prior to lymph node surgery.
- Baseline data: initial histology, initial grade, MRI results of para-aortic involvement (size of pelvic and para-aortic nodes), preoperative FDG-PET/CT results (maximum SUV of tumor and pelvic and para-aortic nodes and their size) and stage.
- Initial indication for PAL: at the same time as hysterectomy, after hysterectomy results or after a positive pelvic lymph node finding (lymphadenectomy or sentinel node (SN)).
- Node surgery: date and approach (laparoscopy, laparotomy and/or robotic surgery).
- Post-operative anatomopathological results: peritoneal cytology, definitive histology, grade, presence of emboli, presence of hormonal receptors, tumor size, presence of pelvic and/or para-aortic lymph node metastases and their maximum size, final FIGO stage.
2.5. Aims of the Study
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Main Objective
3.3. FDG-PET/CT Performance According to Histological Type
3.4. FDG-PET/CT Performance According to Its Date
3.5. Comparison of FDG-PET/CT and MRI Performances
4. Discussion
4.1. Overall Performance
4.2. FDG-PET/CT Performance According to Histological Type
4.3. FDG-PET/CT Performance According to Its Date
4.4. Comparison with MRI
4.5. Strengths of the Study
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defossez, G.; Le Guyader-Peyrou, S.; Uhry, Z.; Grosclaude, P.; Colonna, M.; Dantony, E.; Delafosse, P.; Molinié, F.; Woronof, A.-S.; Bouvier, A.-M.; et al. Estimations Nationales de L’incidence et de la Mortalité par Cancer en France Métropolitaine Entre 1990 et 2018; Tumeurs solides; Santé Publique France: Paris, France, 2019; Volume 1.
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Otteanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Koskas, M.; Bassot, K.; Graesslin, O.; Aristizabal, P.; Barranger, E.; Clavel-Chapelon, F.; Haddad, B.; Luton, D.; Darai, E.; Rouzier, R. Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol. Oncol. 2013, 129, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.; Philippe, A.-C.; Bourdel, N.; Rabischong, B.; Canis, M.; Le Bouedec, G.; Mulliez, A.; Dauplat, J.; Pomel, C. Les lymphadénectomies dans le cancer de l’endomètre, bilan après 4ans de pratique, doit-on poursuivre? Bull. Cancer 2015, 102, 428–435. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Bryant, A.; Dickinson, H.O.; Kehoe, S.; Morrison, J. Lymphadenectomy for the Management of Endometrial Cancer. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Rédacteur; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Querleu, D.; Darai, E.; Lecuru, F.; Rafii, A.; Chereau, E.; Collinet, P.; Crochet, P.; Marret, H.; Mery, E.; Thomas, L.; et al. Prise en charge primaire des cancers de l’endomètre: Recommandations SFOG-CNGOF. Gynécol. Obstét. Fertil. Sénol. 2017, 45, 715–725. [Google Scholar]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Gouy, S.; Morice, P.; Narducci, F.; Uzan, C.; Gilmore, J.; Kolesnikov-Gauthier, H.; Querleu, D.; Haie-Meder, C.; Leblanc, E. Nodal-staging surgery for locally advanced cervical cancer in the era of PET. Lancet Oncol. 2012, 13, e212–e220. [Google Scholar] [CrossRef]
- Legros, M.; Margueritte, F.; Tardieu, A.; Deluche, E.; Mbou, V.B.; Lacorre, A.; Ceuca, A.; Aubard, Y.; Monteil, J.; Sallee, C.; et al. Para-aortic Lymph Node Invasion in High-risk Endometrial Cancer: Performance of 18 FDG FDG-PET/CT. Anticancer Res. 2019, 39, 619–625. [Google Scholar] [CrossRef]
- Bian, L.; Wang, M.; Gong, J.; Liu, H.; Wang, N.; Wen, N.; Fan, W.; Xu, B.; Wand, M.; Ye, M.; et al. Comparison of integrated PET/MRI with PET/CT in evaluation of endometrial cancer: A retrospective analysis of 81 cases. PeerJ 2019, 7, e7081. [Google Scholar] [CrossRef]
- Kitajima, K.; Suenaga, Y.; Ueno, Y.; Kanda, T.; Maeda, T.; Takahashi, S.; Ebina, Y.; Miyahara, Y.; Yamada, H.; Sugimura, K. Value of fusion of PET and MRI for staging of endometrial cancer: Comparison with 18F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur. J. Radiol. 2013, 82, 1672–1676. [Google Scholar] [CrossRef]
- Chao, A.; Chang, T.-C.; Ng, K.-K.; Hsueh, S.; Huang, H.-J.; Chou, H.-H.; Tsai, C.-S.; Yen, T.-C.; Wu, T.-I.; Lai, C.-H. 18F-FDG PET in the management of endometrial cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 36–44. [Google Scholar] [CrossRef]
- Suzuki, R.; Miyagi, E.; Takahashi, N.; Sukegawa, A.; Suzuki, A.; Koike, I.; Sugiura, K.; Okamoto, N.; Inoue, T.; Hirahara, F. Validity of positron emission tomography using fluoro-2-deoxyglucose for the preoperative evaluation of endometrial cancer. Int. J. Gynecol. Cancer 2007, 17, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Fukasawa, I.; Inaba, N.; Kaji, Y.; Sugimura, K. Accuracy of 18 F-FDG PET/CT in Detecting Pelvic and Paraaortic Lymph Node Metastasis in Patients with Endometrial Cancer. Am. J. Roentgenol. 2008, 190, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kim, E.N.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol. Oncol. 2008, 108, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Nakamoto, Y.; Saga, T.; Higashi, T.; Hamanaka, Y.; Tatsumi, M.; Hayashida, K.; Hara, T.; Konishi, I.; Fujii, S.; et al. Clinical value of FDG-PET for preoperative evaluation of endometrial cancer. Ann. Nucl. Med. 2011, 25, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Crivellaro, C.; Signorelli, M.; Guerra, L.; De Ponti, E.; Pirovano, C.; Fruscio, R.; Elisei, F.; Montanelli, L.; Buda, A.; Messa, C. Tailoring systematic lymphadenectomy in high-risk clinical early stage endometrial cancer: The role of 18F-FDG PET/CT. Gynecol. Oncol. 2013, 130, 306–311. [Google Scholar] [CrossRef]
- Gholkar, N.; Prasad, G.; Saha, S.; Srinivasan, R.; Suri, V.; Bhattacharya, A. The accuracy of integrated [18F] fluorodeoxyglucose-positron emission tomography/computed tomography in detection of pelvic and para-aortic nodal metastasis in patients with high risk endometrial cancer. World J. Nucl. Med. 2014, 13, 170. [Google Scholar] [CrossRef]
- Mayoral, M.; Paredes, P.; Domènech, B.; Fusté, P.; Vidal-Sicart, S.; Tapias, A.; Torne, A.; Pahisa, J.; Ordi, J.; Pons, F.; et al. 18F-FDG PET/CT and sentinel lymph node biopsy in the staging of patients with cervical and endometrial cancer. Role of dual-time-point imaging. Rev. Esp. Med. Nucl. Imagen Mol. 2017, 36, 20–26. [Google Scholar] [CrossRef]
- Atri, M.; Zhang, Z.; Dehdashti, F.; Lee, S.I.; Marques, H.; Ali, S.; Koh, W.-J.; Mannel, R.S.; DiSilvestro, P.; King, S.A.; et al. Utility of PET/CT to Evaluate Retroperitoneal Lymph Node Metastasis in High-Risk Endometrial Cancer: Results of ACRIN 6671/GOG 0233 Trial. Radiology 2017, 283, 450–459. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.J.; Choi, H.J.; Song, I.H.; Sung, C.O.; Kim, H.O.; Chae, S.-Y.; Kim, Y.-T.; Nam, J.-H. The Value of Preoperative Positron Emission Tomography/Computed Tomography in Node-Negative Endometrial Cancer on Magnetic Resonance Imaging. Ann. Surg. Oncol. 2017, 24, 2303–2310. [Google Scholar] [CrossRef]
- Koskas, M.; Rouzier, R.; Amant, F. Staging for endometrial cancer: The controversy around lymphadenectomy—Can this be resolved? Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 845–857. [Google Scholar] [CrossRef]
- Onda, T.; Yoshikawa, H.; Mizutani, K.; Mishima, M.; Yokota, H.; Nagano, H.; Ozaki, Y.; Murakami, A.; Ueda, K.; Taketani, Y. Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br. J. Cancer 1997, 75, 1836–1841. [Google Scholar] [CrossRef]
- Hirahatake, K.; Hareyama, H.; Sakuragi, N.; Nishiya, M.; Makinoda, S.; Fujimoto, S. A clinical and pathologic study on para-aortic lymph node metastasis in endometrial carcinoma. J. Surg. Oncol. 1997, 65, 82–87. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Maruyama, H.; Sato, S.; Saito, Y. Indispensability of Pelvic and Paraaortic Lymphadenectomy in Endometrial Cancers. Gynecol. Oncol. 1997, 64, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Watari, H.; Kato, T.; Mitamura, T.; Hosaka, M.; Sudo, S.; Takeda, M.; Kobayashi, N.; Dong, P.; Todo, Y.; et al. Distribution of Lymph Node Metastasis Sites in Endometrial Cancer Undergoing Systematic Pelvic and Para-Aortic Lymphadenectomy: A Proposal of Optimal Lymphadenectomy for Future Clinical Trials. Ann. Surg. Oncol. 2014, 21, 2755–2761. [Google Scholar] [CrossRef]
- Ballester, M.; Dubernard, G.; Lécuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)—The final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef]
- Kakhki, V.R.D.; Shahriari, S.; Treglia, G.; Hasanzadeh, M.; Zakavi, S.R.; Yousefi, Z.; Kadkhodayan, S.; Sadeghi, R. Diagnostic Performance of Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography Imaging for Detection of Primary Lesion and Staging of Endometrial Cancer Patients: Systematic Review and Meta-Analysis of the Literature. Int. J. Gynecol. Cancer 2013, 23, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, S.L.; Jensen, L.N.; Loft, A.; Berthelsen, A.K.; Costa, J.; Tabor, A.; Qvist, I.; Hansen, M.R.; Fisker, R.; Andersen, E.S.; et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer—A multicenter prospective comparative study. Gynecol. Oncol. 2013, 128, 300–308. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, A.; Yun, M.; Kim, Y.T.; Kang, W.J. Comparison of FDG PET/CT and MRI in lymph node staging of endometrial cancer. Ann. Nucl. Med. 2016, 30, 104–113. [Google Scholar] [CrossRef]
- Fasmer, K.E.; Gulati, A.; Dybvik, J.A.; Ytre-Hauge, S.; Salvesen, Ø.; Trovik, J.; Krakstad, C.; Haldorsen, I.S. Preoperative 18F-FDG PET/CT tumor markers outperform MRI-based markers for the prediction of lymph node metastases in primary endometrial cancer. Eur. Radiol. 2020, 30, 2443–2453. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Mean ± Standard Deviation (Extremes) or n (%) (N = 200) |
|---|---|
| Age at diagnosis (years) | 62.72 ± 9.19 (27–83) |
| BMI (kg/m2) | 27.90 ± 6.31 (15.43–50.17) |
| Score ASA 3 | |
| 1 | 15 (7.50) |
| 2 | 15 (7.50) |
| 3 | 14 (7.00) |
| NR 1 | 72 (36.00) |
| Initial histological type | |
| Endometrioid | 96 (48.00) |
| Clear cells | 16 (8.00) |
| Papillary serous | 44 (22.00) |
| Carcinosarcoma | 26 (13.00) |
| Poorly differentiated | 16 (8.00) |
| In situ | 1 (0.50) |
| Hyperplasia with atypia | 1 (0.50) |
| Initial grade | |
| 1 | 25 (12.50) |
| 2 | 48 (24.00) |
| 3 | 108 (54.00) |
| NR | 19 (9.50) |
| Initial stage 2 | |
| IA | 37 (18.50) |
| IB | 34 (17.00) |
| II | 15 (7.50) |
| IIIA | 10 (5.00) |
| IIIB | 3 (1.50) |
| IIIC1 | 37 (18.50) |
| IIIC2 | 59 (29.50) |
| IVA | 2 (1.00) |
| IVB | 3 (1.50) |
| Initial indication for PAL 4 | |
| At diagnosis | 149 (74.50) |
| After hysterectomy results | 42 (21.00) |
| After positive pelvic lymph node finding | 9 (4.50) |
| Post-Operative Data | Mean ± Standard Deviation (Extremes) or n (%) (N = 200) |
|---|---|
| Peritoneal cytology | |
| Positive | 18 (9.00) |
| Negative | 92 (46.00) |
| NP 1 | 90 (45.00) |
| Final histology | |
| Endometrioid | 108 (54.00) |
| Clear cells | 19 (9.50) |
| Papillary serous | 41 (20.50) |
| Carcinosarcoma | 32 (16.00) |
| Grade | |
| 1 | 13 (6.50) |
| 2 | 52 (26.00) |
| 3 | 132 (66.00) |
| NR 2 | 3 (1.50) |
| Emboli | |
| Yes | 100 (50.00) |
| No | 85 (42.50) |
| NR | 15 (7.50) |
| Hormonal receptors | |
| Positive | 82 (41.00) |
| Negative | 63 (31.50) |
| NR | 55 (27.50) |
| Tumor size (mm) | 51.46 ± 30.59 (1; 180) |
| Number of patients with para-aortic lymph node involvement | 55 (27.50) |
| Number of PA lymph nodes removed at PAL 3 | 20.33 ± 13.56 (1; 109) |
| Number of positive lymph nodes | 4.73 ± 6.14 (1; 26) |
| Number of patients with pelvic lymph node involvement | 60/181 (33.15) |
| Number of pelvic lymph nodes removed | 15.34 ± 7.54 (2; 52) |
| Number of positives lymph nodes | 2.90 ± 2.91 (1; 21) |
| Final FIGO 4 stage: | |
| IA | 43 (21.50) |
| IB | 23 (11.50) |
| II | 23 (11.50) |
| IIIA | 17 (8.50) |
| IIIB | 7 (3.50) |
| IIIC1 | 22 (11.00) |
| IIIC2 | 46 (23.00) |
| IVA | 5 (2.50) |
| IVB | 14 (7.00) |
| Performances | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|
| FDG-PET/CT | 61.8 | 89.7 | 69.4 | 86.1 | 0.76 |
| MRI | 26.5 | 89.5 | 48.1 | 76.8 | 0.58 |
| p-value | <0.01 | 0.82 | <0.01 |
| Studies | Number of Patients | Number of PAL | Number of Positive PAL | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Chao et al., 2006 [12] | 49 | NR | 13 | 85 | 95 | 84.6 | 95.2 |
| Suzuki et al., 2007 [13] | 30 | 19 | 1 | 0 | 100 | 0 | 94.7 |
| Kitajima et al., 2008 [14] | 40 | 34 | NR | 51.7 | 99.4 | 83.3 | 97.3 |
| Park et al., 2008 [15] | 53 | 31 | 7 | 57.1 | 87.5 | 57.1 | 87.5 |
| Suga et al., 2011 [16] | 30 | 15 | 4 | 100 | 100 | 100 | 100 |
| Crivellaro et al., 2013 [17] | 76 | 15 | 6 | 85.7 | 96 | 87.5 | 96.3 |
| Gholkar et al., 2014 [18] | 20 | 13 | 1 | 100 | 66.7 | 20 | 100 |
| Mayoral et al., 2016 [19] | 13 | 12 | 3 | 33 | 88.9 | 50 | 80 |
| Atri et al., 2017 [20] | 215 | 160 | 23 | 65 | 88.0 | 48.4 | 93.8 |
| Park et al., 2017 [21] | 362 | 118 | 11 | 18.2 | 98.1 | 92.1 | 50 |
| Legros et al., 2019 [9] | 81 | 35 | 14 | 50 | 100 | 100 | 75 |
| Our study | 200 | 200 | 55 | 61.8 | 89.7 | 69.4 | 86.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallée, C.; Margueritte, F.; Gouy, S.; Tardieu, A.; Belghiti, J.; Lambaudie, E.; Collinet, P.; Guyon, F.; Legros, M.; Monteil, J.; et al. FDG-PET/CT and Para-Aortic Staging in Endometrial Cancer. A French Multicentric Study. J. Clin. Med. 2021, 10, 1746. https://doi.org/10.3390/jcm10081746
Sallée C, Margueritte F, Gouy S, Tardieu A, Belghiti J, Lambaudie E, Collinet P, Guyon F, Legros M, Monteil J, et al. FDG-PET/CT and Para-Aortic Staging in Endometrial Cancer. A French Multicentric Study. Journal of Clinical Medicine. 2021; 10(8):1746. https://doi.org/10.3390/jcm10081746
Chicago/Turabian StyleSallée, Camille, François Margueritte, Sébastien Gouy, Antoine Tardieu, Jérémie Belghiti, Eric Lambaudie, Pierre Collinet, Frédéric Guyon, Maxime Legros, Jacques Monteil, and et al. 2021. "FDG-PET/CT and Para-Aortic Staging in Endometrial Cancer. A French Multicentric Study" Journal of Clinical Medicine 10, no. 8: 1746. https://doi.org/10.3390/jcm10081746
APA StyleSallée, C., Margueritte, F., Gouy, S., Tardieu, A., Belghiti, J., Lambaudie, E., Collinet, P., Guyon, F., Legros, M., Monteil, J., & Gauthier, T. (2021). FDG-PET/CT and Para-Aortic Staging in Endometrial Cancer. A French Multicentric Study. Journal of Clinical Medicine, 10(8), 1746. https://doi.org/10.3390/jcm10081746






