Cardiac Implantable Electronic Devices in Hemodialysis and Chronic Kidney Disease Patients—An Experience-Based Narrative Review
Abstract
1. Introduction
2. CIED Evidence in CKD Patients
2.1. CIED Procedure in a CKD Patient
2.2. CIEDs in a Patient on Hemodialysis
2.3. Vascular Access in a Patient with a CIED
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saad, T.F.; Ahmed, W.; Davis, K.; Jurkovitz, C. Cardiovascular Implantable Electronic Devices in Hemodialysis Patients: Prevalence and Implications for Arteriovenous Hemodialysis Access Interventions. Semin. Dial. 2015, 28, 94–100. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Matar, L.; Smith, P.; Casey, M.; Addish, M.A.; Kelly, K.; Wood, C.; Merlino, J.; Goyal, A.; Leon, A.R.; et al. Long-term survival of implantable cardioverter defibrillator recipients with end-stage renal disease. J. Arrhythmia 2017, 33, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75, A6–A7. [Google Scholar] [CrossRef] [PubMed]
- Turakhia, M.P.; Blankestijn, P.J.; Carrero, J.-J.; Clase, C.M.; Deo, R.; Herzog, C.A.; Kasner, S.E.; Passman, R.S.; Pecoits-Filho, R.; Reinecke, H.; et al. Chronic kidney disease and arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur. Heart J. 2018, 39, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.D.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the american heart association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.; Nadkarni, G.N.; Yacoub, R.; Saha, A.; Simoes, P.; Parikh, C.R.; Coca, S.G. Representation of patients with kidney disease in trials of cardiovascular interventions: An updated systematic review. JAMA Intern. Med. 2016, 176, 121–124. [Google Scholar] [CrossRef]
- Santini, M.; Di Fusco, S.A.; Santini, A.; Magris, B.; Pignalberi, C.; Aquilani, S.; Colivicchi, F.; Gargaro, A.; Ricci, R.P. Prevalence and predictor factors of severe venous obstruction after cardiovascular electronic device implantation. EP Eur. 2016, 18, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Albertini, C.M.D.M.; Da Silva, K.R.; Filho, J.M.D.M.L.; Crevelari, E.S.; Filho, M.M.; Carnevale, F.C.; Costa, R. Usefulness of preoperative venography in patients with cardiac implantable electronic devices submitted to lead replacement or device upgrade procedures. Arq. Bras. Cardiol. 2018, 111, 686–696. [Google Scholar] [CrossRef]
- Boczar, K.; Ząbek, A.; Haberka, K.; Hardzina, M.; Dębski, M.; Rydlewska, A.; Nowosielska-Ząbek, E.; Lelakowski, J.; Małecka, B. Venous stenosis and occlusion in the presence of endocardial leads. Adv. Clin. Exp. Med. 2016, 25, 83–91. [Google Scholar] [CrossRef]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Rev. Esp. Cardiol. 2014, 67, 58. [Google Scholar] [CrossRef][Green Version]
- Herzog, C.A.; Li, S.; Weinhandl, E.D.; Strief, J.W.; Collins, A.J.; Gilbertson, D.T. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005, 68, 818–825. [Google Scholar] [CrossRef]
- Payne, T.; Waller, J.; Kheda, M.; Nahman, N.S., Jr.; Maalouf, J.; Gopal, A.; Hreibe, H. Efficacy of implantable cardioverter-defibrillators for secondary prevention of sudden cardiac death in patients with end-stage renal disease. J. Innov. Card. Rhythm Manag. 2020, 11, 4199–4208. [Google Scholar] [CrossRef]
- Wang, A.Y.; Lam, C.W.; Chan, I.H.; Wang, M.; Lui, S.F.; Sanderson, J.E. Sudden cardiac death in end-stage renal disease patients: A 5-year prospective analysis. Hypertension 2010, 56, 210–216. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2016, 18, 891–975. [Google Scholar]
- Pun, P.H.; Al-Khatib, S.M.; Han, J.Y.; Edwards, R.; Bardy, G.H.; Bigger, J.T.; Buxton, A.E.; Moss, A.J.; Lee, K.L.; Steinman, R.; et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: A meta-analysis of patient-level data from 3 randomized trials. Am. J. Kidney Dis. 2014, 64, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.H.; Hellkamp, A.S.; Sanders, G.D.; Middleton, J.P.; Hammill, S.C.; Al-Khalidi, H.R.; Curtis, L.H.; Fonarow, G.C.; Al-Khatib, S.M. Primary prevention implantable cardioverter defibrillators in end-stage kidney disease patients on dialysis: A matched cohort study. Nephrol. Dial. Transplant. 2015, 30, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Moss, A.J.; McNitt, S.; Zareba, W.; Andrews, M.L.; Hall, W.J.; Greenberg, H.; Case, R.B.; Multicenter Automatic Defibrillator Implantation Trial-II Investigators. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am. J. Cardiol. 2006, 98, 485–490. [Google Scholar] [CrossRef]
- Jukema, J.W.; Timal, R.J.; Rotmans, J.I.; Hensen, L.C.R.; Buiten, M.S.; de Bie, M.K.; Putter, H.; Zwinderman, A.H.; van Erven, L.; Krol-van Straaten, M.J.; et al. Prophylactic use of implantable cardioverter-defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation 2019, 139, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R.; Stromberg, K.; Johnson, L.C.; Wiles, B.M.; Mavrakanas, T.A.; Charytan, D.M. A systematic review of the incidence of arrhythmias in hemodialysis patients undergoing long-term monitoring with implantable loop recorders. Kidney Int. Rep. 2020, 6, 56–65. [Google Scholar] [CrossRef]
- Mehdi, B.; Kaveh, H.; Ali, V.F. Implantable cardioverter-defibrillators in patients with esrd: Complications, management, and literature review. Curr. Cardiol. Rev. 2019, 15, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.; Punnam, S.R.; Brar, S.S.; Goyal, S.K.; Gardiner, J.C.; Shah, A.J.; Thakur, R.K. Implantable defibrillators improve survival in end-stage renal disease: Results from a multicenter registry. Am. J. Nephrol. 2010, 32, 305–310. [Google Scholar] [CrossRef]
- Nakhoul, G.N.; Schold, J.D.; Arrigain, S.; Harb, S.C.; Jolly, S.; Wilkoff, B.L.; Nally, J.V., Jr.; Navaneethan, S.D. Implantable cardioverter-defibrillators in patients with CKD: A propensity-matched mortality analysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1119–1127. [Google Scholar] [CrossRef]
- Shurrab, M.; Ko, D.T.; Zayed, Y.; Navaneethan, S.D.; Yadak, N.; Yaseen, A.; Kaoutskaia, A.; Qamhia, W.; Hamdan, Z.; Haj-Yahia, S.; et al. Outcomes of ICDs and CRTs in patients with chronic kidney disease: A meta-analysis of 21,000 patients. J. Interv. Card. Electrophysiol. 2018, 53, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Höke, U.; Khidir, M.J.; van der Velde, E.T.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Cardiac resynchronization therapy in CKD stage 4 patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 1740–1748. [Google Scholar] [CrossRef]
- Friedman, D.J.; Singh, J.P.; Curtis, J.P.; Tang, W.H.W.; Bao, H.; Spatz, E.S.; Hernandez, A.F.; Patel, U.D.; Al-Khatib, S.M. Comparative effectiveness of CRT-D versus defibrillator alone in HF patients with moderate-to-severe chronic kidney disease. J. Am. Coll. Cardiol. 2015, 66, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.W.; Szeto, C.C.; Chan, J.Y.; Zhang, Q.; Chan, H.C.; Yip, G.W.; Yu, C.M. Prognostic value of renal function in patients with cardiac resynchronization therapy. Int. J. Cardiol. 2007, 122, 10–16. [Google Scholar] [CrossRef]
- Gala-Błądzińska, A.; Romanek, J.; Mazur, D.; Stepek, T.; Braun, M.; Szafarz, P.; Chlebuś, M.; Przybylski, A. Reduced albuminuria and potassemia indicate early renal repair processes after resynchronization therapy in cardiorenal syndrome type 2. Cardiol. Res. Pract. 2020, 2020, 2727108. [Google Scholar] [CrossRef]
- Johnson, E.S.; Thorp, M.L.; Platt, R.W.; Smith, D.H. Predicting the risk of dialysis and transplant among patients with CKD: A retrospective cohort study. Am. J. Kidney Dis. 2008, 52, 653–660. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Ballew, S.H.; Carrero, J.J.; Djurdjev, O.; Heerspink, H.J.L.; Ho, K.; Ito, S.; Marks, A.; Naimark, D.; et al. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. 2018, 93, 1442–1451. [Google Scholar] [CrossRef]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Elley, C.R.; et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. J. Am. Med. Assoc. 2016, 315, 164–174. [Google Scholar] [CrossRef]
- Major, R.W.; Shepherd, D.; Medcalf, J.F.; Xu, G.; Gray, L.J.; Brunskill, N.J. The Kidney Failure Risk Equation for prediction of end stage renal disease in UK primary care: An external validation and clinical impact projection cohort study. PLoS Med. 2019, 16, e1002955, Erratum in 2020, 17, e1003313. [Google Scholar] [CrossRef]
- Available online: https://qxmd.com/calculate/calculator_308/kidney-failure-risk-equation-4-variable (accessed on 13 April 2021).
- Barakat, A.F.; Wazni, O.M.; Tarakji, K.G.; Tarakji, K.G.; Callahan, T.; Nimri, N.; Saliba, W.I.; Shah, S.; Rehman, K.A.; Rickard, J.; et al. Transvenous lead extraction in chronić kidney disease and dialysis patients with infected cardiac devices. Circulation 2018, 11, e005706. [Google Scholar] [CrossRef]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Renal Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef]
- Ferreira, H.; Nunes, A.; Oliveira, A.; Beco, A.; Santos, J.; Pestana, M. Planning vascular access in peritoneal dialysis-defining high-risk patients. Perit. Dial. Int. 2018, 38, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Savelieva, I.; Dan, G.A.; Deharo, J.C.; Ferro, C.; Israel, C.W.; Lane, D.A.; La Manna, G.; Morton, J.; Mitjans, A.M.; et al. Chronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: Clinical significance and implications for decision making-a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1169–1196. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J. Surgical options for endocardial lead placement when upper veins are obstructed or nonusable. J. Interv. Card. Electrophysiol. 2004, 11, 149–154. [Google Scholar] [CrossRef]
- Asif, A.; Carrillo, R.; Garisto, J.D.; Lopera, G.; Ladino, M.; Barakat, U.; Eid, N.; Salman, L. Epicardial cardiac rhythm devices for dialysis patients: Minimizing the risk of infection and preserving central veins. Semin Dial. 2012, 25, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Zenati, M.A.; Bonanomi, G.; Chin, A.; Schwartzman, D. Left heart pacing lead implantation using subxiphoid videopericardioscopy. J. Cardiovasc. Electrophysiol. 2003, 14, 949–953. [Google Scholar] [CrossRef]
- DeRose, J.J.; Ashton, R.C.; Belsley, S.; Swistel, D.G.; Vloka, M.; Ehlert, F.; Shaw, R.; Sackner-Bernstein, J.; Hillel, Z.; Steinberg, J.S. Robotically assisted left ventricular epicardial lead implantation for biventricular pacing. J. Am. Coll. Cardiol. 2003, 41, 1414–1419. [Google Scholar] [CrossRef]
- Kempa, M.; Laskawski, G.; Budrejko, S.; Slawinski, G.; Raczak, G.; Rogowski, J. Implantation of a dual-chamber pacemaker with epicardial leads in adults using a minimally invasive subxyphoid approach. Pacing Clin. Electrophysiol. 2019, 42, 537–541. [Google Scholar] [CrossRef]
- Marini, M.; Branzoli, S.; Moggio, P.; Martin, M.; Belotti, G.; Molon, G.; Guarracini, F.; Coser, A.; Quintarelli, S.; Pederzolli, C.; et al. Epicardial left ventricular lead implantation in cardiac resynchronization therapy patients via a video-assisted thoracoscopic technique: Long-term outcome. Clin. Cardiol. 2020, 43, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Lapar, D.J.; Swenson, B.R.; Maxwell, C.D.; Girotti, M.E.; Bergin, J.D.; Kern, J.A.; Dimarco, J.P.; Mahapatra, S. Surgically placed left ventricular leads provide similar outcomes to percutaneous leads in patients with failed coronary sinus lead placement. Heart Rhythm 2010, 7, 619–625. [Google Scholar] [CrossRef]
- Amraoui, S.; Sohal, M.; Li, A.; Williams, S.; Scully, P.; Jackson, T.; Claridge, S.; Behar, J.; Ritter, P.; Barandon, L.; et al. Comparison of delayed transvenous reimplantation and immediate surgical epicardial approach in pacing-dependent patients undergoing extraction of infected permanent pacemakers. Heart Rhythm 2015, 12, 1209–1215. [Google Scholar] [CrossRef]
- Vaidya, V.R.; Sugrue, A.; Padmanabhan, D.; Killu, A.M.; Naksuk, N.; Al-Masry, A.A.; Isath, A.; Pedersen, J.; Yngsdal, L.; Ladewig, D.J.; et al. Percutaneous epicardial pacing using a novel transverse sinus device. J. Cardiovasc. Electrophysiol. 2018, 29, 1308–1316. [Google Scholar] [CrossRef]
- Killu, A.M.; Naksuk, N.; Stárek, Z.; DeSimone, C.V.; Syed, F.F.; Gaba, P.; Wolf, J.; Lehar, F.; Pesl, M.; Leinveber, P.; et al. A novel defibrillation tool: Percutaneously delivered, partially insulated epicardial defibrillation. JACC Clin. Electrophysiol. 2017, 3, 747–755. [Google Scholar] [CrossRef]
- Tomaske, M.; Gerritse, B.; Kretzers, L.; Prêtre, R.; Dodge-Khatami, A.; Rahn, M.; Bauersfeld, U. A 12-year experience of bipolar steroid-eluting epicardial pacing leads in children. Ann. Thorac. Surg. 2008, 85, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Ector, B.; Willems, R.; Heidbüchel, H.; Gewillig, M.; Mertens, L.; Meyns, B.; Daenen, W.; Ector, H. Epicardial pacing: A single centre study on 321 leads in 138 patients. Acta Cardiol. 2006, 61, 343–351. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.J.; Attenhofer Jost, C.H.; Warnes, C.A.; Hodge, D.; Hyberger, L.; Connolly, H.M.; Asirvatham, S.J.; Dearani, J.A.; Hayes, D.L.; Ammash, N.M. Epicardial versus endocardial permanent pacing in adults with congenital heart disease. J. Interv. Card. Electrophysiol. 2010, 28, 235–243. [Google Scholar] [CrossRef]
- Bracke, F.A.; Ozdemir, I.; van Gelder, B. The femoral route revisited: An alternative for pectoral pacing lead implantation. Neth. Heart J. 2010, 18, 42–44. [Google Scholar] [PubMed]
- El Ellestad, M.H.; French, J. Iliac vein approach to permanent pacemaker implantation. Pacing Clin. Electrophysiol. 1989, 12, 1030–1033. [Google Scholar] [CrossRef]
- Higgins, S.L. Biventricular ICD placement percutaneously via the iliac vein: Case reports and a review. J. Innov. Card. Rhythm Manag. 2017, 8, 2784–2789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, A.; Halleran, S.M.; Krishnan, K.; Trohman, R.G. Rescue permanent iliac vein pacing after epicardial lead failure: An unusual reversal of pacing fortune. Europace 2008, 10, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Chami, M.F.; Clementy, N.; Garweg, C.; Omar, R.; Duray, G.Z.; Gornick, C.C.; Leyva, F.; Sagi, V.; Piccini, J.P.; Soejima, K.; et al. Leadless pacemaker implantation in hemodialysis patients: Experience with the micra transcatheter pacemaker. JACC Clin. Electrophysiol. 2019, 5, 162–170. [Google Scholar] [CrossRef]
- Steinwender, C.; Khelae, S.K.; Garweg, C.; Chan, J.Y.S.; Ritter, P.; Johansen, J.B.; Sagi, V.; Epstein, L.M.; Piccini, J.P.; Pascual, M.; et al. Atrioventricular synchronous pacing using a leadless ventricular pacemaker: Results from the MARVEL 2 study. JACC Clin. Electrophysiol. 2020, 6, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018, 15, e190–e252, Erratum in: 2018, 138, e210–e271. [Google Scholar] [CrossRef]
- Pun, P.H.; Parzynski, C.S.; Friedman, D.J.; Sanders, G.; Curtis, J.P.; Al-Khatib, S.M. Trends in use and in-hospital outcomes of subcutaneous implantable cardioverter defibrillators in patients undergoing long-term dialysis. Clin. J. Am. Soc. Nephrol. 2020, 15, 1622–1630. [Google Scholar] [CrossRef]
- Dhamija, R.K.; Tan, H.; Philbin, E.; Mathew, R.O.; Sidhu, M.S.; Wang, J.; Saour, B.; Haqqie, S.S.; Beathard, G.; Yevzlin, A.S.; et al. Subcutaneous implantable cardioverter defibrillator for dialysis patients: A strategy to reduce central vein stenoses and infections. Am. J. Kidney Dis. 2015, 66, 154–158. [Google Scholar] [CrossRef]
- Chang, S.C.; Patton, K.K.; Robinson, M.R.; Poole, J.E.; Prutkin, J.M. Subcutaneous ICD screening with the Boston Scientific ZOOM programmer versus a 12-lead ECG machine. Pacing Clin. Electrophysiol. 2018, 41, 511–516. [Google Scholar] [CrossRef]
- Knops, R.E.; Olde Nordkamp, L.R.A.; Delnoy, P.H.M.; Boersma, L.V.A.; Kuschyk, J.; El-Chami, M.F.; Bonnemeier, H.; Behr, E.R.; Brouwer, T.F.; Kääb, S.; et al. Subcutaneous or transvenous defibrillator therapy. N. Engl. J. Med. 2020, 383, 526–536. [Google Scholar] [CrossRef]
- Gold, M.R.; Lambiase, P.D.; El-Chami, M.F.; Knops, R.E.; Aasbo, J.D.; Bongiorni, M.G.; Russo, A.M.; Deharo, J.C.; Burke, M.C.; Dinerman, J.; et al. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021, 143, 7–17. [Google Scholar] [CrossRef]
- Koman, E.; Gupta, A.; Subzposh, F.; Saltzman, H.; Kutalek, S.P. Outcomes of subcutaneous implantable cardioverter-defibrillator implantation in patients on hemodialysis. J. Interv. Card. Electrophysiol. 2016, 45, 219–223. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Levy, M.; Kelli, H.M.; Casey, M.; Hoskins, M.H.; Goyal, A.; Langberg, J.J.; Patel, A.; Delurgio, D.; Lloyd, M.S.; et al. Outcome of subcutaneous implantable cardioverter defibrillator implantation in patients with end-stage renal disease on dialysis. J. Cardiovasc. Electrophysiol. 2015, 26, 900–904. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/medical-devices/medical-device-recalls/boston-scientific-corporation-recalls-emblem-s-icd-subcutaneous-implantable-cardioverter (accessed on 13 April 2021).
- Available online: https://www.fda.gov/medical-devices/medical-device-recalls/boston-scientific-recalls-emblem-s-icd-subcutaneous-electrode-model-3501-due-risk-fractures (accessed on 13 April 2021).
- Auricchio, A.; Delnoy, P.P.; Regoli, F.; Seifert, M.; Markou, T.; Butter, C. First-in-man implantation of leadless ultrasound-based cardiac stimulation pacing system: Novel endocardial left ventricular resynchronization therapy in heart failure patients. Europace 2013, 15, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Sieniewicz, B.J.; Betts, T.R.; James, S.; Turley, A.; Butter, C.; Seifert, M.; Boersma, L.V.A.; Riahi, S.; Neuzil, P.; Biffi, M.; et al. Real-world experience of leadless left ventricular endocardial cardiac resynchronization therapy: A multicenter international registry of the WiSE-CRT pacing system. Heart Rhythm 2020, 17, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Montemerlo, E.; Pozzi, M.; De Ceglia, S.; Santini, F.; Piazzi, E.; Rovaris, G. First-in-man fully leadless transvenous CRT-P with a transseptal implant of WISE-CRT® system and Micra® PM. Pacing Clin. Electrophysiol. 2019, 42, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.; Jabeur, M.; Jacon, P.; Rinaldi, C.A.; Leclercq, C.; Rovaris, G.; Arnold, M.; Venier, S.; Neuzil, P.; Defaye, P. European experience with a first totally leadless cardiac resynchronization therapy pacemaker system. Europace 2020, 13, euaa342. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.H.; Abou-Zamzam, A.M., Jr.; Limm, W.; Wong, L.; Wong, L. Symptomatic subclavian vein stenosis and occlusion in hemodialysis patients with transvenous pacemakers. Ann. Vasc. Surg. 2003, 17, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.D.; McFarland, G.; Itoga, N.K.; Sorial, E.; Garcia-Toca, M. Arteriovenous fistula and graft construction in patients with implantable cardiac devices: Does side matter? Ann. Vasc. Surg. 2019, 54, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Nam, G.B.; Chang, J.W.; Kim, M.J.; Han, Y.; Kwon, T.W.; Cho, Y.P. Impact of transvenous cardiac implantable electronic devices in chronic hemodialysis patients: A single-center, observational comparative study. BMC Nephrol. 2018, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Saad, T.F.; Weiner, H.L. Venous hemodialysis catheters and cardiac implantable electronic devices: Avoiding a high-risk combination. Semin. Dial. 2017, 30, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Gooden, J.Y.; Le, K.Y.; Baddour, L.M.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Sohail, M.R. Clinical presentation and outcomes of cardiovascular implantable electronic device infections in hemodialysis patients. Am. J. Kidney Dis. 2014, 64, 104–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guha, A.; Maddox, W.R.; Colombo, R.; Nahman, N.S., Jr.; Kintziger, K.W.; Waller, J.L.; Diamond, M.; Murphy, M.; Kheda, M.; Litwin, S.E.; et al. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm 2015, 12, 2395–2401. [Google Scholar]
- Asif, A.; Salman, L.; Lopera, G.; Haqqie, S.S.; Carrillo, R. Transvenous cardiac implantable electronic devices and hemodialysis catheters: Recommendations to curtail a potentially lethal combination. Semin. Dial. 2012, 25, 582–586. [Google Scholar] [CrossRef]
- Al-Hijji, M.A.; Killu, A.M.; Yousefian, O.; Hodge, D.O.; Park, J.Y.; Hebsur, S.; El Sabbagh, A.; Pretorius, V.G.; Ackerman, M.J.; Friedman, P.A.; et al. Outcomes of lead extraction without subsequent device reimplantation. Europace 2017, 19, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
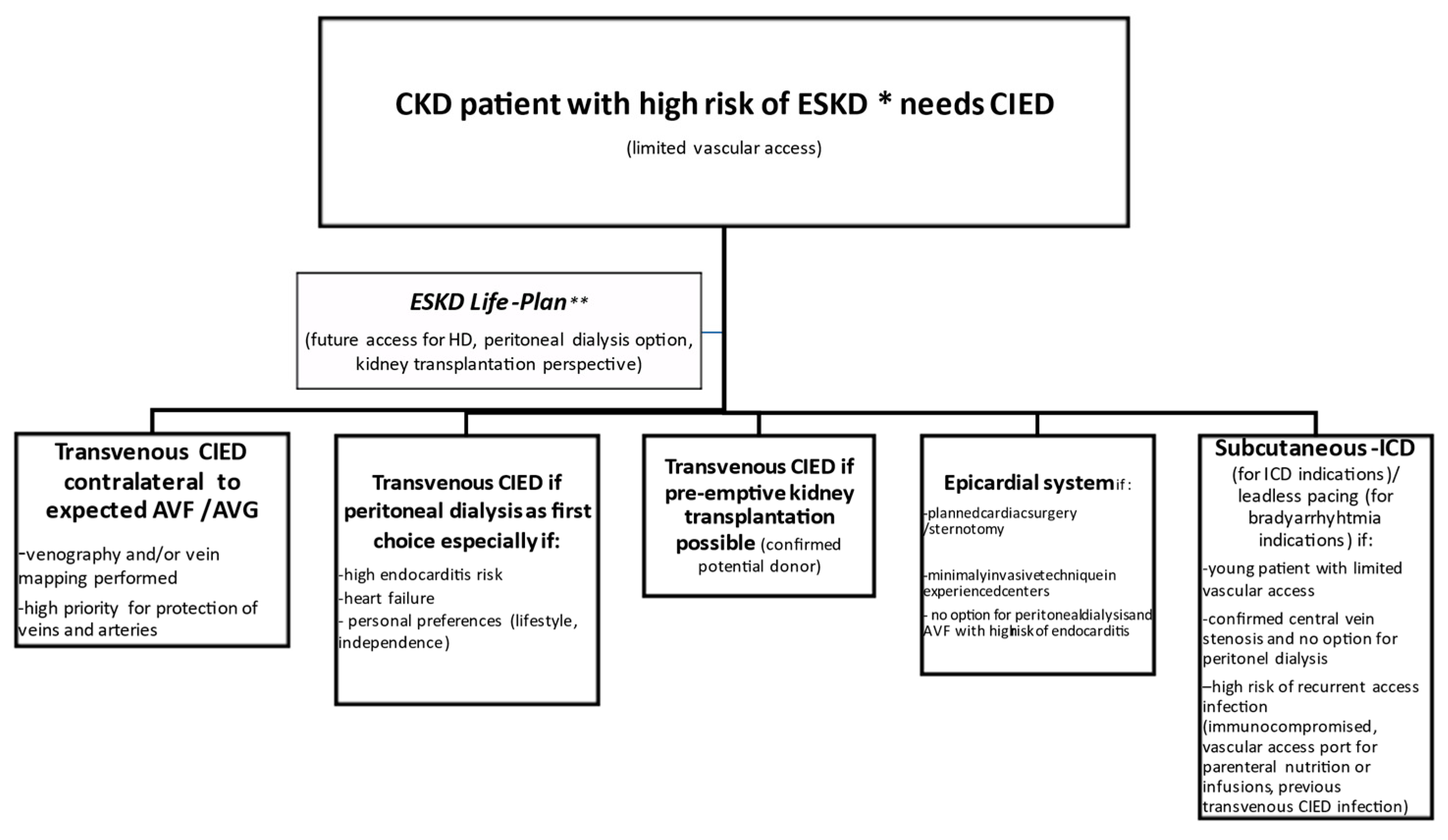
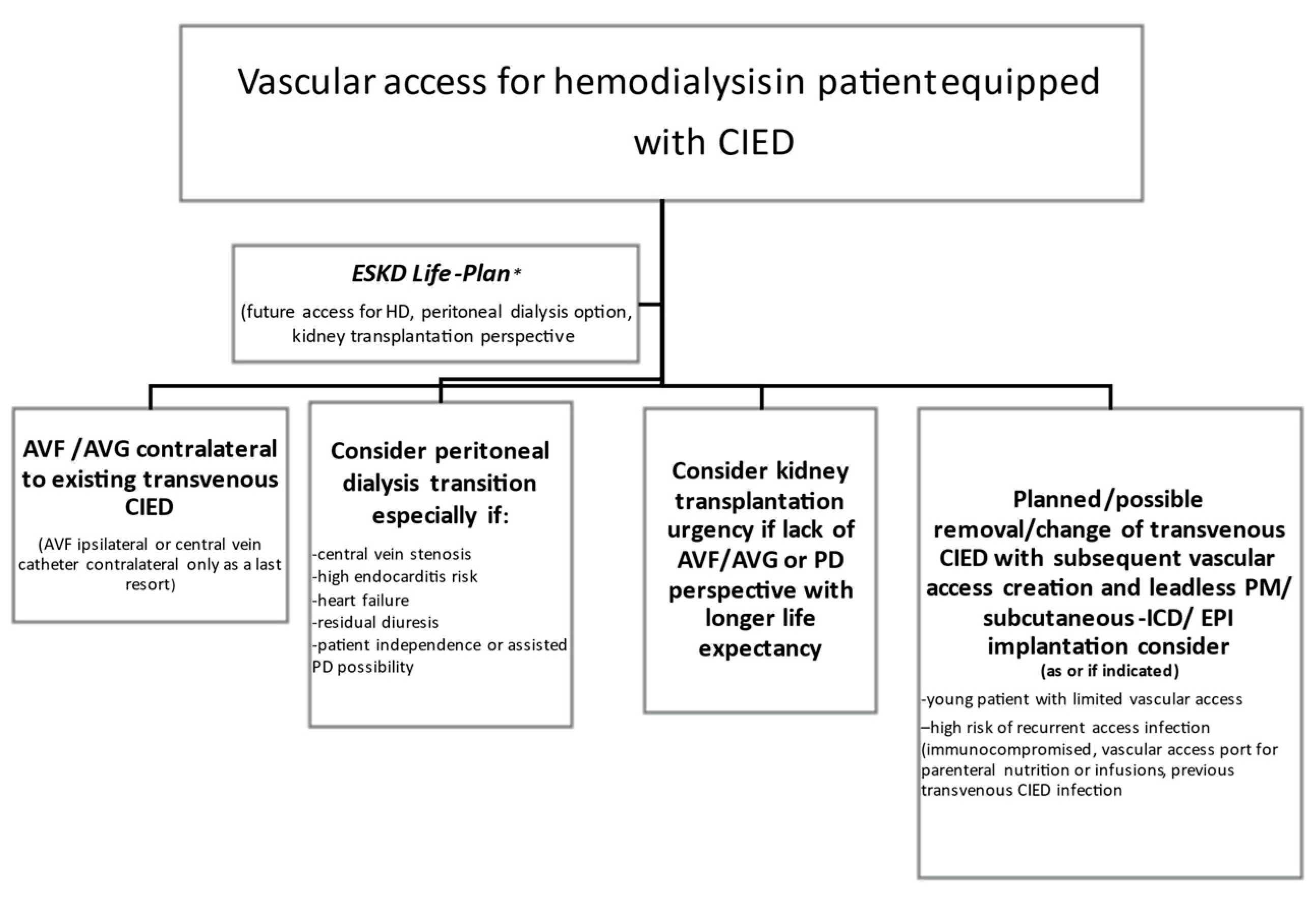
| CIED Option | Comment |
|---|---|
| Leadless pacemaker (endovascular implantation), subcutaneous ICD (S-ICD), endocardial LV pacing based on ultrasound technology | Preferred if indications are limited to pacing or high-energy therapy only; no data regarding long-term outcomes |
| Surgically implanted epicardial system | When thoracotomy/heart surgery for other reasons is considered; higher risk of lead malfunction; minimally invasive technique available |
| Low lateral thoracic or axillary implantation | In patients with limited prepectoral options (previous pocket infection); involves axillary vein puncture |
| Femoral/iliac pacing system | Usually in bilateral subclavian vein occlusion/superior vena cava syndrome; unsuitable in case of poor hygiene (diapers) or local skin infection; risk of lead fracture in walking patients—use of the iliac vein should limit the risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, K.; Kusztal, M. Cardiac Implantable Electronic Devices in Hemodialysis and Chronic Kidney Disease Patients—An Experience-Based Narrative Review. J. Clin. Med. 2021, 10, 1745. https://doi.org/10.3390/jcm10081745
Nowak K, Kusztal M. Cardiac Implantable Electronic Devices in Hemodialysis and Chronic Kidney Disease Patients—An Experience-Based Narrative Review. Journal of Clinical Medicine. 2021; 10(8):1745. https://doi.org/10.3390/jcm10081745
Chicago/Turabian StyleNowak, Krzysztof, and Mariusz Kusztal. 2021. "Cardiac Implantable Electronic Devices in Hemodialysis and Chronic Kidney Disease Patients—An Experience-Based Narrative Review" Journal of Clinical Medicine 10, no. 8: 1745. https://doi.org/10.3390/jcm10081745
APA StyleNowak, K., & Kusztal, M. (2021). Cardiac Implantable Electronic Devices in Hemodialysis and Chronic Kidney Disease Patients—An Experience-Based Narrative Review. Journal of Clinical Medicine, 10(8), 1745. https://doi.org/10.3390/jcm10081745






