Dysregulated Immunity and Immunotherapy after Sepsis
Abstract
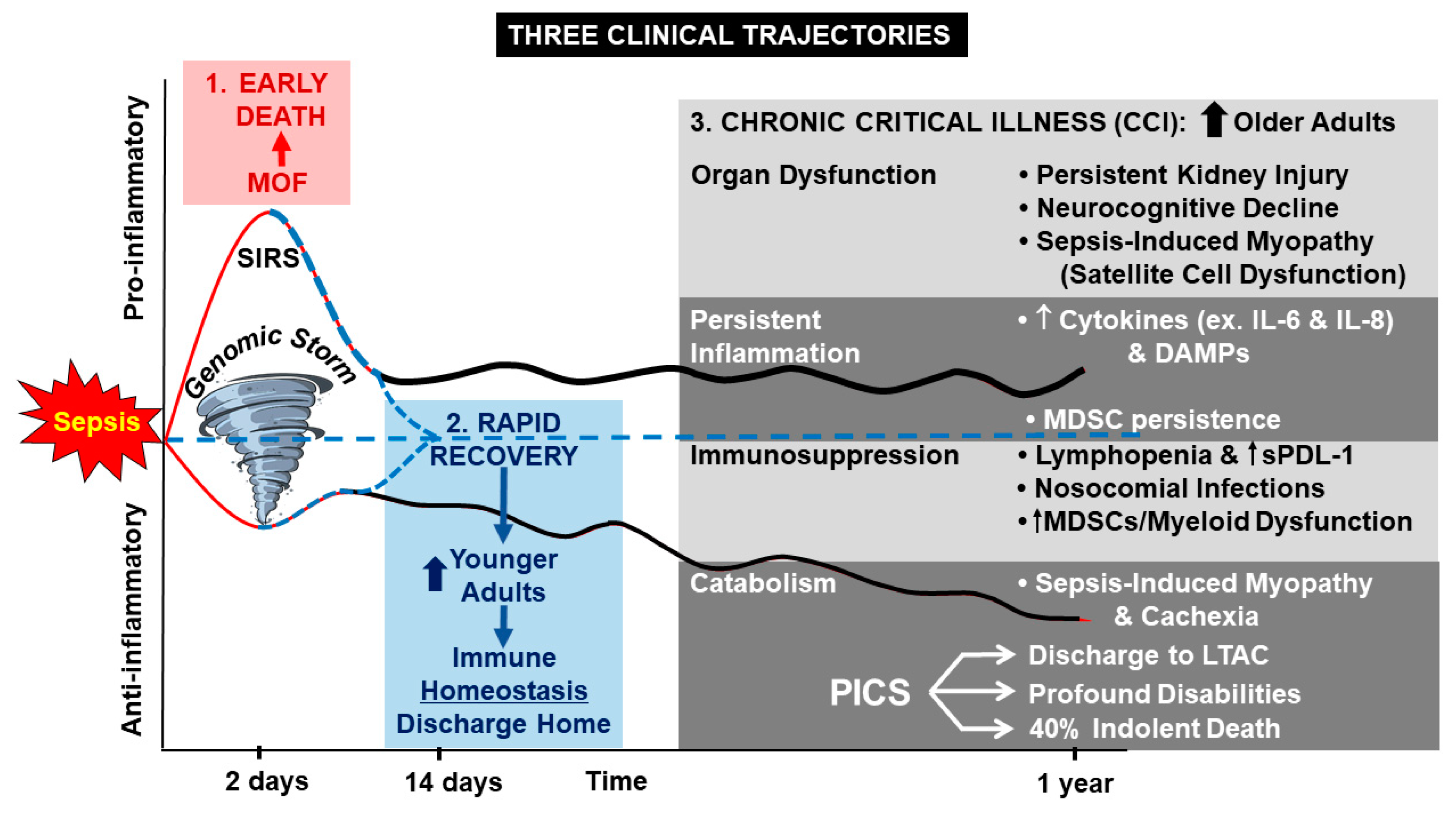
1. Introduction
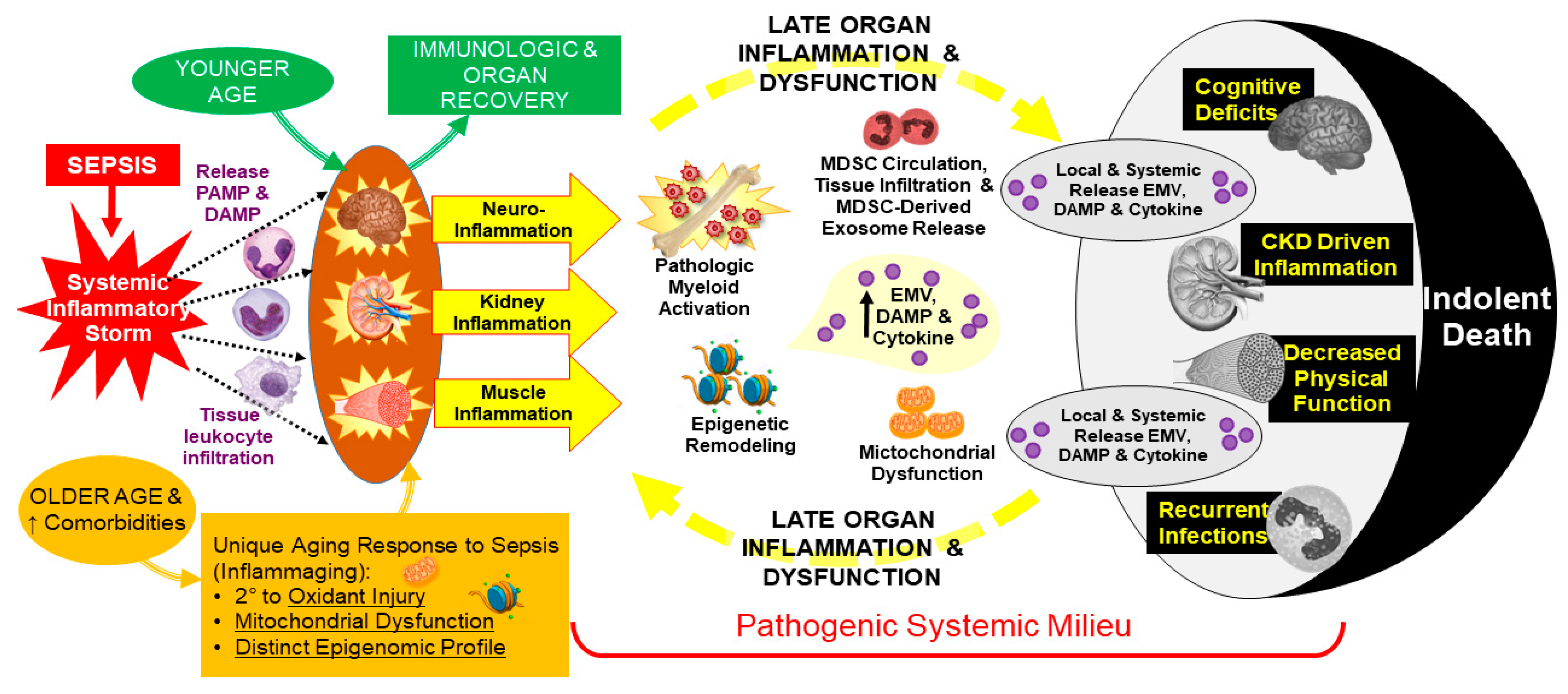
2. Persistent Inflammation
3. Persistent Immunosuppression
4. Persistent Catabolism
5. Dysregulated Myelopoiesis
6. Immunotherapy
| Intervention | Result | Ref |
|---|---|---|
| GM-CSF | Restoration of monocytic immunocompetence. Shortened time of mechanical ventilation and hospital stay. | [89] |
| G-CSF | Increased total leukocyte counts. No difference in mortality rates or complications in sepsis patients. | [90] |
| G-CSF & GM-CSF | Improved infection clearance, but no difference in mortality rates in sepsis patients. | [92] |
| IL-7 | Improved lymphocyte counts (CD4+ and CD8+ immune effector cells) in sepsis patients. | [102] |
| IL-7 | Increased CD4/CD8 T cells in HIV patients. | [104] |
| IL-7 | Increased CD4/CD8 T cells in patients with lymphopenia. | [105] |
| IFN-γ | Increased HLA-DR expression and decrease in natural killer cells in patients with sepsis. | [111] |
| IFN-γ | Decreased infection related mortality and overall mortality in trauma, but no difference in infection rates in trauma patients. | [114] |
| PD-1/PD-L1 blockade | Ex-vivo restoration of function in neutrophils, monocytes, T cells, and NK cells in whole blood from septic patients. | [122] |
| PD-1/PD-L1 blockade | In-vitro decreased T-cell apoptosis, potentiated monocytic LPS-induced TNF-α and IL-6 production from sepsis patients. | [123] |
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and trends of sepsis in us hospitals using clinical vs claims data, 2009–2014. J. Am. Med. Assoc. 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Levy, M.M.; Rhodes, A.; Phillips, G.S.; Townsend, S.R.; Schorr, C.A.; Beale, R.; Osborn, T.; Lemeshow, S.; Chiche, J.-D.; Artigas, A.; et al. surviving sepsis campaign: Association between performance metrics and outcomes in a 7.5-year study. Intensive Care Med. 2014, 40, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.A.; Moore, F.A.; Efron, P.A.; Marker, P.S.; Gabrielli, A.; Westhoff, L.S.; Lottenberg, L.; Jordan, J.; Klink, V.; Sailors, R.M.; et al. Computer versus paper system for recognition and management of sepsis in surgical in-tensive care. J. Trauma Acute Care Surg. 2014, 76, 311–317. [Google Scholar] [CrossRef]
- McKinley, B.A.; Moore, L.J.; Sucher, J.F.; Todd, S.R.; Turner, K.L.; Valdivia, A.; Sailors, R.M.; Moore, F.A. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. J. Trauma Inj. Infect. Crit. Care 2011, 70, 1153–1167. [Google Scholar] [CrossRef]
- Brakenridge, S.C.; Efron, P.A.; Cox, M.C.; Stortz, J.A.; Hawkins, R.B.; Ghita, G.; Gardner, A.; Mohr, A.M.; Anton, S.D.; Moldawer, L.L.; et al. Current epidemiology of surgical sepsis: Discordance between inpatient mortality and 1-year outcomes. Ann. Surg. 2019, 270, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; McKinley, B.A.; Turner, K.L.; Todd, S.R.; Sucher, J.F.; Valdivia, A.; Sailors, R.M.; Kao, L.S.; Moore, F.A. The epidemiology of sepsis in general surgery patients. J. Trauma: Inj. Infect. Crit. Care 2011, 70, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Gentile, L.F.; Mathia, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Hodgson, C.L.; Pilcher, D.; Bailey, M.; van Lint, A.; Chavan, S.; Bellomo, R. Timing of onset and burden of persistent critical illness in Australia and New Zealand: A retrospective, population-based, observational study. Lancet Respir. Med. 2016, 4, 566–573. [Google Scholar] [CrossRef]
- Cox, M.C.; Brakenridge, S.C.; Stortz, J.A.; Hawkins, R.B.; Darden, D.B.; Ghita, G.L.; Mohr, A.M.; Moldawer, L.L.; Efron, P.A.; Moore, F.A. Abdominal sepsis patients have a high incidence of chronic critical illness with dismal long-term outcomes. Am. J. Surg. 2020, 220, 1467–1474. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.; Brumback, B.; Cox, M.C.; Mohr, A.M.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older sepsis survivors suffer persistent disability burden and poor long-term survival. J. Am. Geriatr. Soc. 2020, 68, 1962–1969. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Cooke, C.R.; Wunsch, H.; Kahn, J.M. Population burden of long-term survivorship after severe sepsis in older Americans. J. Am. Geriatr. Soc. 2012, 60, 1070–1077. [Google Scholar] [CrossRef]
- Stortz, J.A.; Mira, J.C.; Raymond, S.L.; Loftus, T.J.; Ozrazgat-Baslanti, T.; Wang, Z.; Ghita, G.L.; Leeuwenburgh, C.; Segal, M.S.; Bihorac, A.; et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J. Trauma Acute Care Surg. 2018, 84, 342–349. [Google Scholar] [CrossRef]
- Guirgis, F.W.; Brakenridge, S.; Sutchu, S.; Khadpe, J.D.; Robinson, T.; Westenbarger, R.; Topp, S.T.; Kalynych, C.J.; Reynolds, J.; Dodani, S. The long-term burden of severe sepsis and septic shock: Sepsis recidivism and organ dysfunction. J. Trauma Acute Care Surg. 2016, 81, 525–532. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. J. Am. Med Assoc. 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical in-tensive care. J Trauma Acute Care Surg 2012, 72, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L. Parallels between cancer and infectious disease. N. Engl. J. Med. 2014, 371, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, L.; Hoepelman, R.J.; Spijkerman, R.; De Groot, M.C.H.; Van Wessem, K.J.P.; Koenderman, L.; Leenen, L.P.H.; Hietbrink, F. Persistent inflammation, immunosuppression and catabolism syndrome (PICS) after polytrauma: A rare syndrome with major consequences. J. Clin. Med. 2020, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Loftus, T.J.; Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Hollen, M.K.; Weiss, B.P.; Miller, E.S.; Bihorac, A.; Larson, S.D.; et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front. Immunol. 2018, 9, 595. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Kamel, A.Y.; Rosenthal, C.M.; Brakenridge, S.; Croft, C.A.; Moore, F.A. chronic critical illness: Application of what we know. Nutr. Clin. Pract. 2018, 33, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.D.; Moore, F.A. Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): A new phenotype of multiple organ failure. J. Adv. Nutr. Hum. Metab. 2015, 1, 10. [Google Scholar] [CrossRef]
- Fenner, B.P.; Darden, D.B.; Kelly, L.S.; Rincon, J.; Brakenridge, S.C.; Larson, S.D.; Moore, F.A.; Efron, P.A.; Moldawer, L.L. Immunological endotyping of chronic critical illness after severe sepsis. Front. Med. 2021, 7, 616694. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Ward, P.A. Sepsis-induced immune dysfunction: Can immune therapies reduce mortality? J. Clin. Investig. 2016, 126, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Siracusa, M.C.; Yap, G.S.; Gause, W.C. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol. 2016, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.B.; Stortz, J.A.; Holden, D.C.; Wang, Z.; Raymond, S.L.; Cox, M.C.; Efron, P.A.; Brakenridge, S.C.; Moore, F.A.; Moldawer, L.L. Persistently increased cell-free DNA concentrations only modestly contribute to outcome and host response in sepsis survivors with chronic critical illness. Surgery 2020, 167, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like recep-tors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in Inflammation and Cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef]
- Bruns, A.M.; Pollpeter, D.; Hadizadeh, N.; Myong, S.; Marko, J.F.; Horvath, C.M. ATP hydrolysis enhances RNA recognition and antiviral signal transduction by the innate immune sensor, laboratory of genetics and physiology 2 (LGP2). J. Biol. Chem. 2013, 288, 938–946. [Google Scholar] [CrossRef]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Kingeter, L.M.; Lin, X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflam-matory responses. Cell Mol. Immunol. 2012, 9, 105–112. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nat. Cell Biol. 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Yoo, Y.; Son, M.; Lee, J.; Jeong, G.-B.; Park, Y.M.; Salekdeh, G.H.; Lee, B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017, 177, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, A.; Saleh, M. The inflammasomes: Molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Front. Microbiol. 2011, 2, 15. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nat. Cell Biol. 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Walton, A.H.; Muenzer, J.T.; Rasche, D.; Boomer, J.S.; Sato, B.; Brownstein, B.H.; Pachot, A.; Brooks, T.L.; Deych, E.; Shannon, W.D.; et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE 2014, 9, e98819. [Google Scholar] [CrossRef]
- Fleshner, M.; Crane, C.R. Exosomes, DAMPs and miRNA: Features of stress physiology and immune ho-meostasis. Trends Immunol. 2017, 38, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-W.; Kim, S.-J.; Cho, H.-I.; Lee, S.-M. DAMPs activating innate immune responses in sepsis. Ageing Res. Rev. 2015, 24, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Maslanik, T.; Mahaffey, L.; Tannura, K.; Beninson, L.; Greenwood, B.N.; Fleshner, M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav. Immun. 2013, 28, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav. Immun. 2013, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ren, J.; Wu, J.; Li, G.; Wu, X.; Liu, S.; Li, J.; Gu, G.; Wang, G. Elevated levels of plasma mitochondrial DNA are associated with clinical outcome in in-tra-abdominal infections caused by severe trauma. Surg. Infect. 2017, 18, 610–618. [Google Scholar] [CrossRef]
- Timmermans, K.; Kox, M.; Scheffer, G.J.; Pickkers, P. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock 2016, 45, 607–612. [Google Scholar] [CrossRef]
- Ingels, C.; Derese, I.; Wouters, P.J.; Van den Berghe, G.; Vanhorebeek, I. Soluble RAGE and the RAGE ligands HMGB1 and S100A12 in critical illness: Impact of glycemic control with insulin and relation with clinical outcome. Shock 2015, 43, 109–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Achouiti, A.; Föll, D.; Vogl, T.; van Till, J.W.; Laterre, P.-F.; Dugernier, T.; Wittebole, X.; Boermeester, M.A.; Roth, J.; van der Poll, T.; et al. S100A12 and soluble receptor for advanced glycation end products levels during human severe sepsis. Shock 2013, 40, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front. Immunol. 2018, 9, 2499. [Google Scholar] [CrossRef]
- Huet, O.; Dupic, L.; Harrois, A.; Duranteau, J. Oxidative stress and endothelial dysfunction during sepsis. Front. Biosci. 2011, 16, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.L.; Murphy, J.T. Reactive oxygen species mediate endotoxin-induced human dermal endothelial NF-kappaB activation. J. Surg. Res. 2003, 111, 120–126. [Google Scholar] [CrossRef]
- Singer, M. Metabolic failure. Crit. Care Med. 2005, 33, S539–S542. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Barter, J.; Kumar, A.; Stortz, J.A.; Hollen, M.; Nacionales, D.; Efron, P.A.; Moldawer, L.L.; Foster, T.C. Age and sex influence the hippocampal response and recovery following sepsis. Mol. Neurobiol. 2019, 56, 8557–8572. [Google Scholar] [CrossRef]
- Widmann, C.N.; Heneka, M.T. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014, 13, 630–636. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M.; Hampson, P. Immunesenescence and inflammaging: A contributory factor in the poor outcome of the geriatric trauma patient. Ageing Res. Rev. 2015, 24, 349–357. [Google Scholar] [CrossRef]
- Stortz, J.A.; Murphy, T.J.; Raymond, S.L.; Mira, J.C.; Ungaro, R.; Dirain, M.L.; Nacionales, D.C.; Loftus, T.J.; Wang, Z.; Ozrazgat-Baslanti, T.; et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock 2018, 49, 249–258. [Google Scholar] [CrossRef]
- Drewry, A.M.; Ablordeppey, E.A.; Murray, E.T.; Beiter, E.R.; Walton, A.H.; Hall, M.W.; Hotchkiss, R.S. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: A prospective observational study. Crit. Care 2016, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Landelle, C.; Lepape, A.; Voirin, N.; Tognet, E.; Venet, F.; Bohé, J.; Vanhems, P.; Monneret, G. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010, 36, 1859–1866. [Google Scholar] [CrossRef]
- Munoz, C.; Carlet, J.; Fitting, C.; Misset, B.; Blériot, J.P.; Cavaillon, J.M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Investig. 1991, 88, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Chaudry, I.H. Immune dysfunction in murine polymicrobial sepsis: Mediators, macrophages, lymphocytes and apoptosis. Shock 1996, 6, 27–38. [Google Scholar] [CrossRef]
- Jensen, I.J.; Sjaastad, F.V.; Griffith, T.S.; Badovinac, V.P. Sepsis-induced T cell immunoparalysis: The ins and outs of impaired T cell immunity. J. Immunol. 2018, 200, 1543–1553. [Google Scholar]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Wherry, E.J.; Ha, S.-J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunology 2007, 27, 670–684. [Google Scholar] [CrossRef]
- Loss, S.H.; Nunes, D.S.L.; Franzosi, O.S.; Salazar, G.S.; Teixeira, C.; Vieira, S.R.R. Chronic critical illness: Are we saving patients or creating victims? Rev. Bras. Ter. Intensive 2017, 29, 87–95. [Google Scholar] [CrossRef]
- Gardner, A.K.; Ghita, G.L.; Wang, Z.; Ozrazgat-Baslanti, T.; Raymond, S.L.; Mankowski, R.T.; Brumback, B.A.; Efron, P.A.; Bihorac, A.; Moore, F.A.; et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit. Care Med. 2019, 47, 566–573. [Google Scholar] [CrossRef]
- Unroe, M.; Kahn, J.M.; Carson, S.S.; Govert, J.A.; Martinu, T.; Sathy, S.J.; Clay, A.S.; Chia, J.; Gray, A.; Tulsky, J.A.; et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study. Ann. Intern. Med. 2010, 153, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Martinu, T.; Sathy, S.J.; Clay, A.S.; Chia, J.; Gray, A.L.; Olsen, M.K.; Govert, J.A.; Carson, S.S.; Tulsky, J.A. Expectations and outcomes of prolonged mechanical ventilation. Crit. Care Med. 2009, 37, 2888–2894. [Google Scholar] [CrossRef]
- Delano, M.J.; Moldawer, L.L. The origins of cachexia in acute and chronic inflammatory diseases. Nutr. Clin. Pract. 2006, 21, 68–81. [Google Scholar] [CrossRef]
- Monk, D.N.; Plank, L.D.; Franch-Arcas, G.; Finn, P.J.; Streat, S.J.; Hill, G.L. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann. Surg. 1996, 223, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Brakenridge, S.C.; Moore, F.A.; Mercier, N.R.; Cox, M.; Wu, Q.; Moldawer, L.L.; Mohr, A.M.; Efron, P.A.; Smith, R.S. Persistently elevated glucagon-like peptide-1 levels among critically Ill surgical patients after sepsis and development of chronic critical illness and dismal long-term outcomes. J. Am. Coll. Surg. 2019, 229, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and in-flammation during aging and muscle wasting disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Carlson, D.; Sun, Y.; Ma, L.; Wolf, S.E.; Minei, J.P.; Zang, Q.S. Mitochondrial ROS induces cardiac inflammation via a pathway through mtdna damage in a pneumonia-related sepsis model. PLoS ONE 2015, 10, e0139416. [Google Scholar] [CrossRef]
- Gong, Y.; Zou, L.; Feng, Y.; Li, D.; Cai, J.; Chen, D.; Chao, W. Importance of Toll-like receptor 2 in mitochondrial dysfunction during polymicrobial sepsis. Anesthesiology 2014, 121, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Mathias, B.; Delmas, A.L.; Ozrazgat-Baslanti, T.; Vanzant, E.L.; Szpila, B.E.; Mohr, A.M.; Moore, F.A.; Brakenridge, S.C.; Brumback, B.A.; Moldawer, L.L.; et al. Human myeloid-derived suppressor cells are associated with chronic immune suppres-sion after severe sepsis/septic shock. Ann. Surg. 2017, 265, 827–834. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Delano, M.J.; Kelly-Scumpia, K.M.; Moreno, C.; Scumpia, P.O.; LaFace, D.M.; Heyworth, P.G.; Efron, P.A.; Moldawer, L.L. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 2011, 17, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Scumpia, P.O.; Weinstein, J.S.; Coco, D.; Nagaraj, S.; Kelly-Scumpia, K.M.; O’Malley, K.A.; Wynn, J.L.; Antonenko, S.; Al-Quran, S.Z.; et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007, 204, 1463–1474. [Google Scholar] [CrossRef]
- Kelly, L.S.; Darden, D.B.; Fenner, B.P.; Efron, P.A.; Mohr, A.M. The hematopoietic stem/progenitor cell response to hemorrhage, injury and sepsis. Shock 2020. [Google Scholar] [CrossRef]
- Nacionales, D.C.; Szpila, B.; Ungaro, R.; Lopez, M.C.; Zhang, J.; Gentile, L.F.; Cuenca, A.L.; Vanzant, E.; Mathias, B.; Jyot, J.; et al. A detailed characterization of the dysfunctional immunity and abnormal myelo-poiesis induced by severe shock and trauma in the aged. J. Immunol. 2015, 195, 2396–2407. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Barreda, D.R.; Hanington, P.C.; Belosevic, M. Regulation of myeloid development and function by colony stimulating factors. Dev. Comp. Immunol. 2004, 28, 509–554. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Youn, J.-I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Guérin, E.; Orabona, M.; Raquil, M.A.; Giraudeau, B.; Bellier, R.; Gibot, S.; Bene, M.C.; Lacombe, F.; Droin, N.; Solary, E. Circulating immature granulocytes with T-cell killing functions predict sepsis deteriora-tion. Crit. Care. Med. 2014, 42, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Janols, H.; Bergenfelz, C.; Allaoui, R.; A Larsson, A.-K.; Rydén, L.; Björnsson, S.; Janciauskiene, S.; Wullt, M.; Bredberg, A.; Leandersson, K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J. Leukoc. Biol. 2014, 96, 685–693. [Google Scholar] [CrossRef]
- Hollen, M.K.; Stortz, J.A.; Darden, D.; Dirain, M.L.; Nacionales, D.C.; Hawkins, R.B.; Cox, M.C.; Lopez, M.-C.; Rincon, J.C.; Ungaro, R.; et al. Myeloid-derived suppressor cell function and epigenetic expression evolves over time after surgical sepsis. Crit. Care 2019, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Mohr, A.M.; Moldawer, L.L. Dysregulated myelopoiesis and hematopoietic function following acute physiologic insult. Curr. Opin. Hematol. 2018, 25, 37–43. [Google Scholar] [CrossRef]
- Darcy, C.J.; Minigo, G.; A Piera, K.; Davis, J.S.; McNeil, Y.R.; Chen, Y.; Volkheimer, A.D.; Weinberg, J.B.; Anstey, N.M.; Woodberry, T. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit. Care 2014, 18, R163. [Google Scholar] [CrossRef]
- Meisel, C.; Schefold, J.C.; Pschowski, R.; Baumann, T.; Hetzger, K.; Gregor, J.; Weber-Carstens, S.; Hasper, D.; Keh, D.; Zuckermann, H.; et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immuno-suppression: A double-blind, randomized, placebo-controlled multicenter trial. Am. J. Respir. Crit. Care Med. 2009, 180, 640–648. [Google Scholar] [CrossRef]
- Root, R.K.; Lodato, R.F.; Patrick, W.; Cade, J.F.; Fotheringham, N.; Milwee, S.; Vincent, J.L.; Torres, A.; Rello, J.; Nelson, S.; et al. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hos-pitalized with pneumonia and severe sepsis. Crit. Care. Med. 2003, 31, 367–373. [Google Scholar] [CrossRef]
- Nelson, S.; Belknap, S.M.; Carlson, R.W.; Dale, D.; DeBoisblanc, B.; Farkas, S.; Fotheringham, N.; Ho, H.; Marrie, T.; Movahhed, H.; et al. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hos-pitalized patients with community-acquired pneumonia. CAP Study Group. J. Infect. Dis. 1998, 178, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Wang, F.; Zhu, J.; Li, J.; Deng, X. Granulocyte-colony stimulating factor (G-CSF) and granulo-cyte-macrophage colony stimulating factor (GM-CSF) for sepsis: A meta-analysis. Crit. Care 2011, 15, R58. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Ambler, M.; Mahalingasivam, V.; Jones, A.; Rowan, K.; Rubenfeld, G.D. Evidence for a causal link between sepsis and long-term mortality: A systematic re-view of epidemiologic studies. Crit. Care 2016, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.D.; Eberlein, M.; Leung, J.; Needham, D.M.; Pronovost, P.J.; Sevransky, J.E. Long-term mortality and quality of life in sepsis: A systematic review. Crit. Care Med. 2010, 38, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sportès, C.; Hakim, F.T.; Memon, S.A.; Zhang, H.; Chua, K.S.; Brown, M.R.; Fleisher, T.A.; Krumlauf, M.C.; Babb, R.R.; Chow, C.K.; et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferen-tial expansion of naive T cell subsets. J. Exp. Med. 2008, 205, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Foray, A.-P.; Villars-Méchin, A.; Malcus, C.; Poitevin-Later, F.; Lepape, A.; Monneret, G.; Sawant, D.V.; Sehra, S.; Nguyen, E.T.; et al. IL-7 restores lymphocyte functions in septic patients. J. Immunol. 2012, 189, 5073–5081. [Google Scholar] [CrossRef]
- Mackall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef]
- Unsinger, J.; McGlynn, M.; Kasten, K.R.; Hoekzema, A.S.; Watanabe, E.; Muenzer, J.T.; McDonough, J.S.; Tschoep, J.; Ferguson, T.A.; McDunn, J.E.; et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sep-sis. J. Immunol. 2010, 184, 3768–3779. [Google Scholar] [CrossRef]
- Unsinger, J.; Burnham, C.-A.D.; McDonough, J.; Morre, M.; Prakash, P.S.; Caldwell, C.C.; Dunne, W.M.; Hotchkiss, R.S. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 2012, 206, 606–616. [Google Scholar] [CrossRef]
- Venet, F.; Filipe-Santos, O.; Lepape, A.; Malcus, C.; Poitevin-Later, F.; Grives, A.; Plantier, N.; Pasqual, N.; Monneret, G. Decreased T-cell repertoire diversity in sepsis: A preliminary study. Crit. Care Med. 2013, 41, 111–119. [Google Scholar] [CrossRef]
- Francois, B.; Jeannet, R.; Daix, T.; Walton, A.H.; Shotwell, M.S.; Unsinger, J.; Monneret, G.; Rimmelé, T.; Blood, T.; Morre, M.; et al. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Demaret, J.; Dupont, G.; Venet, F.; Friggeri, A.; Lepape, A.; Rimmele, T.; Morel, J.; Monneret, G. STAT5 phosphorylation in T cell subsets from septic patients in response to recombinant human interleukin-7: A pilot study. J. Leukoc. Biol. 2015, 97, 791–796. [Google Scholar] [CrossRef]
- Levy, Y.; Sereti, I.; Tambussi, G.; Routy, J.P.; Lelièvre, J.D.; Delfraissy, J.F.; Molina, J.M.; A Fischl, M.; Goujard, C.; Rodriguez, B.G.; et al. Effects of recombinant human interleukin 7 on t-cell recovery and thymic output in hiv-infected patients receiving antiretroviral therapy: Results of a phase i/iia randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 2012, 55, 291–300. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Sportès, C.; Ahmadzadeh, M.; Fry, T.J.; Ngo, L.T.; Schwarz, S.L.; Stetler-Stevenson, M.; Morton, K.E.; Mavroukakis, S.A.; Morre, M.; et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a rela-tive decrease of CD4+ T-regulatory cells. J. Immunother. 2006, 29, 313–319. [Google Scholar] [CrossRef]
- Scumpia, P.O.; Delano, M.J.; Kelly, K.M.; O’Malley, K.A.; Efron, P.A.; McAuliffe, P.F.; Brusko, T.; Ungaro, R.; Barker, T.; Wynn, J.L.; et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J. Immunol. 2006, 177, 7943–7949. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff, R.M.; Foster, G.R.; Stark, G.R. Interferons at age 50: Past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; Shuherk-Shaffer, J.; Hotchkiss, R.S.; Green, J.M. A prospective analysis of lymphocyte pheno-type and function over the course of acute sepsis. Crit. Care 2012, 16, R112. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. J. Am. Med. Assoc. 2011, 306, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Burnham, C.-A.; Compton, S.M.; Rasche, D.P.; Mazuski, R.; SMcDonough, J.; Unsinger, J.; Korman, A.J.; Green, J.M.; Hotchkiss, R.S. Blockade ofthe negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit. Care 2013, 17, R85. [Google Scholar] [CrossRef]
- Payen, D.; Faivre, V.; Miatello, J.; Leentjens, J.; Brumpt, C.; Tissières, P.; Dupuis, C.; Pickkers, P.; Lukaszewicz, A.C. Multicentric experience with interferon gamma therapy in sepsis induced immunosuppres-sion. A case series. BMC Infect. Dis. 2019, 19, 931. [Google Scholar] [CrossRef] [PubMed]
- Döcke, W.D.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.D.; Kox, W. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat. Med. 1997, 3, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Kox, W.J.; Bone, R.C.; Krausch, D.; Döcke, W.D.; Kox, S.N.; Wauer, H.; Egerer, K.; Querner, S.; Aadullah, K.; von Baehr, R.; et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syn-drome. A new approach: Proof of principle. Arch. Intern. Med. 1997, 157, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.J.; Jurkovich, G.J.; Maier, R.V.; Clemmer, T.P.; Struve, S.N.; Weigelt, J.A.; Stanford, G.G.; Herr, D.L.; Champion, H.R.; Lewis, F.R.; et al. Effect of interferon gamma on infection-related death in patients with severe injuries. Arch. Surg. 1994, 129, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.G.; Gentile, L.F.; Lopez, M.C.; Ungaro, R.; Liu, H.; Xiao, W.; Seok, J.; Mindrinos, M.N.; Ang, D.; Baslanti, T.O.; et al. Development of a genomic metric that can be rapidly used to predict clinical outcome in severely injured trauma patients. Crit. Care Med. 2013, 41, 1175–1185. [Google Scholar] [CrossRef]
- Xiao, W.; Mindrinos, M.N.; Seok, J.; Cuschieri, J.; Cuenca, A.G.; Gao, H.; Hayden, D.L.; Hennessy, L.; Moore, E.E.; Minei, J.P.; et al. A genomic storm in critically injured humans. J. Exp. Med. 2011, 208, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.K.; Bohannon, J.K.; Sherwood, E.R. Immunotherapy: A promising approach to reverse sep-sis-induced immunosuppression. Pharmacol. Res. 2016, 111, 688–702. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti–PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef] [PubMed]
- Guignant, C.; Lepape, A.; Huang, X.; Kherouf, H.; Denis, L.; Poitevin, F.; Malcus, C.; Chéron, A.; Allaouchiche, B.; Gueyffier, F.; et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit. Care 2011, 15, R99. [Google Scholar] [CrossRef]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Iwai, Y.; Terawaki, S.; Ikegawa, M.; Okazaki, T.; Honjo, T. PD-1 Inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 2003, 198, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Patera, A.C.; Drewry, A.M.; Chang, K.; Beiter, E.R.; Osborne, D.; Hotchkiss, R.S. Frontline science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J. Leukoc. Biol. 2016, 100, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Lou, J.; Zhou, Y.; Bo, L.; Zhu, J.; Zhu, K.; Wan, X.; Cai, Z.; Deng, X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on mono-cytes in septic shock patients. Crit. Care 2011, 15, R70. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nat. Cell Biol. 2005, 439, 682–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Lou, J.; Li, J.; Bo, L.; Zhu, K.; Wan, X.; Deng, X.; Cai, Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apopto-sis and reversing monocyte dysfunction. Crit. Care 2010, 14, R220. [Google Scholar] [CrossRef]
- Brahmamdam, P.; Inoue, S.; Unsinger, J.; Chang, K.C.; McDunn, J.E.; Hotchkiss, R.S. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 2010, 88, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Poe, S.L.; Arora, M.; Oriss, T.B.; Yarlagadda, M.; Isse, K.; Khare, A.; Levy, D.E.; Lee, J.S.; Mallampalli, R.K.; Chan, Y.R.; et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2013, 6, 189–199. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Théroude, C.; Roger, T. Myeloid-derived suppressor cells in sepsis. Front. Immunol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Law, A.M.; Valdes-Mora, F.; Gallego-Ortega, D. Derived suppressor cells as a therapeutic tar-get for cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef]
- Noel, G.; Wang, Q.; Osterburg, A.; Schwemberger, S.; James, L.; Haar, L.; Giacalone, N.; Thomas, I.; Ogle, C. A Ribonucleotide reductase inhibitor reverses burn-induced inflammatory defects. Shock 2010, 34, 535–544. [Google Scholar] [CrossRef]
- Sander, L.E.; Sackett, S.D.; Dierssen, U.; Beraza, N.; Linke, R.P.; Müller, M.; Blander, J.M.; Tacke, F.; Trautwein, C. Hepatic acute-phase proteins control innate immune responses during infection by pro-moting myeloid-derived suppressor cell function. J. Exp. Med. 2012, 207, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darden, D.B.; Kelly, L.S.; Fenner, B.P.; Moldawer, L.L.; Mohr, A.M.; Efron, P.A. Dysregulated Immunity and Immunotherapy after Sepsis. J. Clin. Med. 2021, 10, 1742. https://doi.org/10.3390/jcm10081742
Darden DB, Kelly LS, Fenner BP, Moldawer LL, Mohr AM, Efron PA. Dysregulated Immunity and Immunotherapy after Sepsis. Journal of Clinical Medicine. 2021; 10(8):1742. https://doi.org/10.3390/jcm10081742
Chicago/Turabian StyleDarden, Dijoia B., Lauren S. Kelly, Brittany P. Fenner, Lyle L. Moldawer, Alicia M. Mohr, and Philip A. Efron. 2021. "Dysregulated Immunity and Immunotherapy after Sepsis" Journal of Clinical Medicine 10, no. 8: 1742. https://doi.org/10.3390/jcm10081742
APA StyleDarden, D. B., Kelly, L. S., Fenner, B. P., Moldawer, L. L., Mohr, A. M., & Efron, P. A. (2021). Dysregulated Immunity and Immunotherapy after Sepsis. Journal of Clinical Medicine, 10(8), 1742. https://doi.org/10.3390/jcm10081742






