Increased Creatine Kinase May Predict A Worse COVID-19 Outcome
Abstract
:1. Introduction
2. Patients and Methods
3. Results
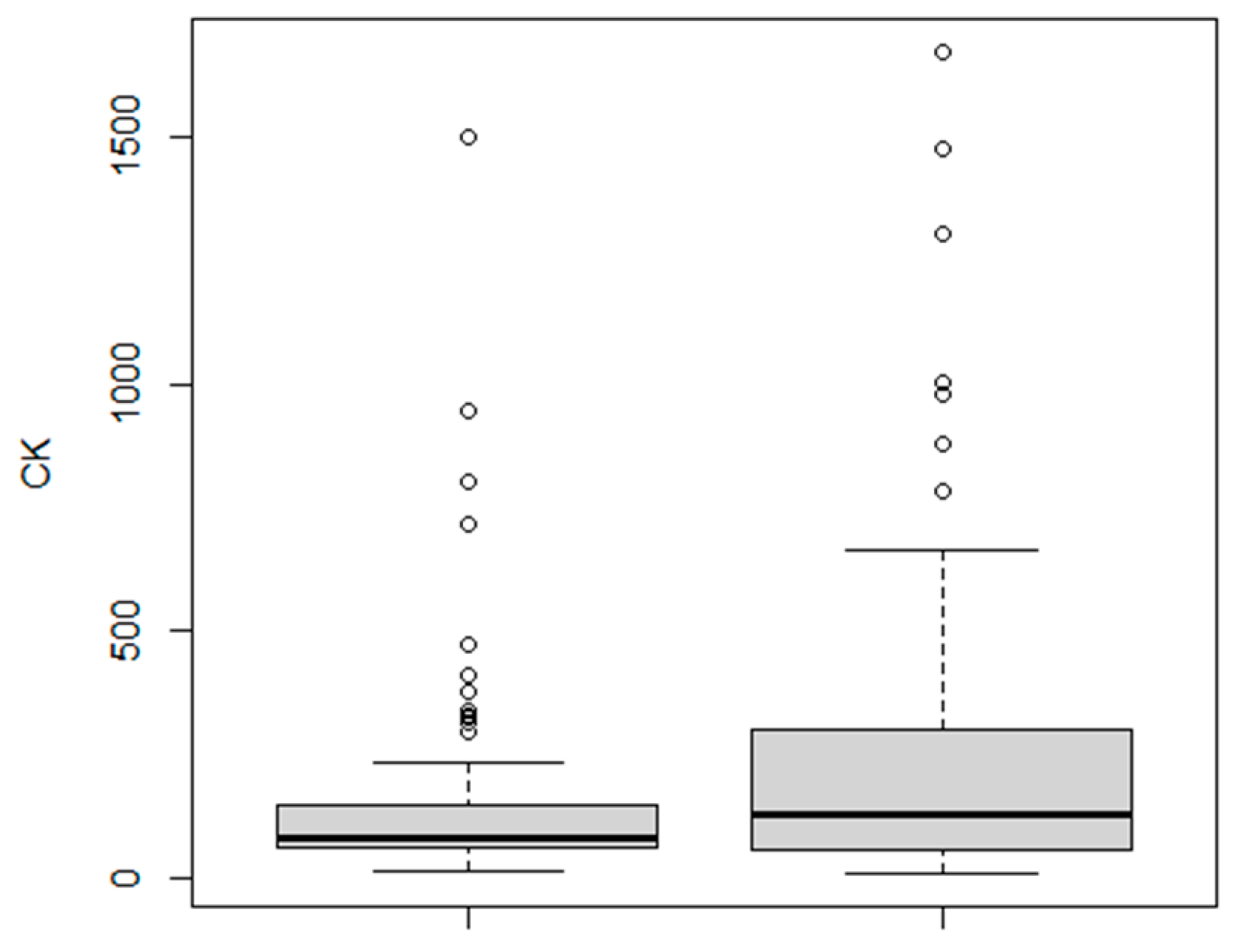
3.1. CK Levels
3.2. Age
3.3. Gender
3.4. Body Mass Index
3.5. Comorbidities
3.6. Combined Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orsucci, D.; Ienco, E.C.; Nocita, G.; Napolitano, A.; Vista, M. Neurological features of COVID-19 and their treatment: A review. Drugs Context 2020, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orsucci, D. Is creatine kinase associated with outcome in COVID-19? Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Hu, L.; Ming, Q.; Wei, X.-J.; Zhang, Z.-Y.; Chen, L.-D.; Wang, M.-H.; Yao, W.-Z.; Huang, Q.-F.; Ye, Z.-Q.; et al. Risk factors for mortality of coronavirus disease-2019 (COVID-19) patients in two centers of Hubei province, China: A retrospective analysis. PLoS ONE 2021, 16, e0246030. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, W.-S.; Guan, W.; Hong, Z.; Gao, J.; Gao, G.; Wu, G.; Qin, Y.-Y. Risk factors and predictors associated with the severity of COVID-19 in China: A systematic review, meta-analysis, and meta-regression. J. Thorac. Dis. 2020, 12, 7429–7441. [Google Scholar] [CrossRef] [PubMed]
- Mudatsir, M.; Fajar, J.K.; Wulandari, L.; Soegiarto, G.; Ilmawan, M.; Purnamasari, Y.; Mahdi, B.A.; Jayanto, G.D.; Suhendra, S.; Setianingsih, Y.A. Predictors of COVID-19 severity: A systematic review and meta-analysis. F1000Research 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, S.; Bernal, J.; Bachiller-Corral, J. Rhabdomyolysis as the main manifestation of coronavirus disease 2019. Rheumatology 2020, 59, 2174–2176. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, S.G.; Nylen, E.S.; Khosla, R. Rhabdomyolysis in patients hospitalized with COVID-19 infection: Five case series. J. Investig. Med. High Impact Case Rep. 2020, 8. [Google Scholar] [CrossRef]
- Geng, Y.; Ma, Q.; Du, Y.-S.; Peng, N.; Yang, T.; Zhang, S.-Y.; Wu, F.-F.; Lin, H.-L.; Su, L. Rhabdomyolysis is associated with in-hospital mortality in patients with COVID-19. Shock 2021. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, M.R.; Di Noia, S.; Morciano, C.; Conte, D. The impact of SARS-CoV-2 on skeletal muscles. Acta Myol. 2020, 39, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.E.; Uner, M.; Priemer, D.S.; Rosenberg, A.; Chen, L. Muscle biopsy findings in a case of SARS-CoV-2-associated muscle injury. J. Neuropathol. Exp. Neurol. 2021, 80, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Pitscheider, L.; Karolyi, M.; Burkert, F.R.; Helbok, R.; Wanschitz, J.V.; Horlings, C.; Pawelka, E.; Omid, S.; Traugott, M.; Seitz, T.; et al. Muscle involvement in SARS-CoV-2 infection. Eur. J. Neurol. 2020, 2020. [Google Scholar] [CrossRef]
- Dolhnikoff, M.; Ferranti, J.F.; Monteiro, R.A.D.A.; Duarte-Neto, A.N.; Gomes-Gouvêa, M.S.; Degaspare, N.V.; Delgado, A.F.; Fiorita, C.M.; Leal, G.N.; Rodrigues, R.M.; et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc. Health 2020, 4, 790–794. [Google Scholar] [CrossRef]
- Casey, P.; Ang, Y.; Sultan, J. COVID-19-induced sarcopenia and physical deconditioning may require reassessment of surgical risk for patients with cancer. World J. Surg. Oncol. 2021, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
| Estimate | Standard Error | z-Value | Odds Ratio | p (>|z|) | |
|---|---|---|---|---|---|
| (Intercept) | −4.1139269 | 1.0706395 | −3.842 | 0.0163 | 0.0001 |
| Age | 0.0349671 | 0.0143924 | 2.430 | 1.0400 | 0.02 |
| Sex | 0.7941428 | 0.3663038 | 2.168 | 2.2100 | 0.03 |
| IHD | 0.1554229 | 0.4968806 | 0.313 | 1.1700 | n.s. |
| CKD | 2.1506017 | 0.8105475 | 2.653 | 8.5900 | 0.008 |
| Hypertension | 0.5875029 | 0.3612523 | 1.626 | 1.8000 | n.s. |
| CK (U/L) | 0.0013924 | 0.0007198 | 1.934 | 1.0000 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsucci, D.; Trezzi, M.; Anichini, R.; Blanc, P.; Barontini, L.; Biagini, C.; Capitanini, A.; Comeglio, M.; Corsini, P.; Gemignani, F.; et al. Increased Creatine Kinase May Predict A Worse COVID-19 Outcome. J. Clin. Med. 2021, 10, 1734. https://doi.org/10.3390/jcm10081734
Orsucci D, Trezzi M, Anichini R, Blanc P, Barontini L, Biagini C, Capitanini A, Comeglio M, Corsini P, Gemignani F, et al. Increased Creatine Kinase May Predict A Worse COVID-19 Outcome. Journal of Clinical Medicine. 2021; 10(8):1734. https://doi.org/10.3390/jcm10081734
Chicago/Turabian StyleOrsucci, Daniele, Michele Trezzi, Roberto Anichini, Pierluigi Blanc, Leandro Barontini, Carlo Biagini, Alessandro Capitanini, Marco Comeglio, Paulo Corsini, Federico Gemignani, and et al. 2021. "Increased Creatine Kinase May Predict A Worse COVID-19 Outcome" Journal of Clinical Medicine 10, no. 8: 1734. https://doi.org/10.3390/jcm10081734
APA StyleOrsucci, D., Trezzi, M., Anichini, R., Blanc, P., Barontini, L., Biagini, C., Capitanini, A., Comeglio, M., Corsini, P., Gemignani, F., Giannecchini, R., Giusti, M., Lombardi, M., Marrucci, E., Natali, A., Nenci, G., Vannucci, F., & Volpi, G. (2021). Increased Creatine Kinase May Predict A Worse COVID-19 Outcome. Journal of Clinical Medicine, 10(8), 1734. https://doi.org/10.3390/jcm10081734








