Abnormal Liver Function Tests and Long-Term Outcomes in Patients Discharged after Acute Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
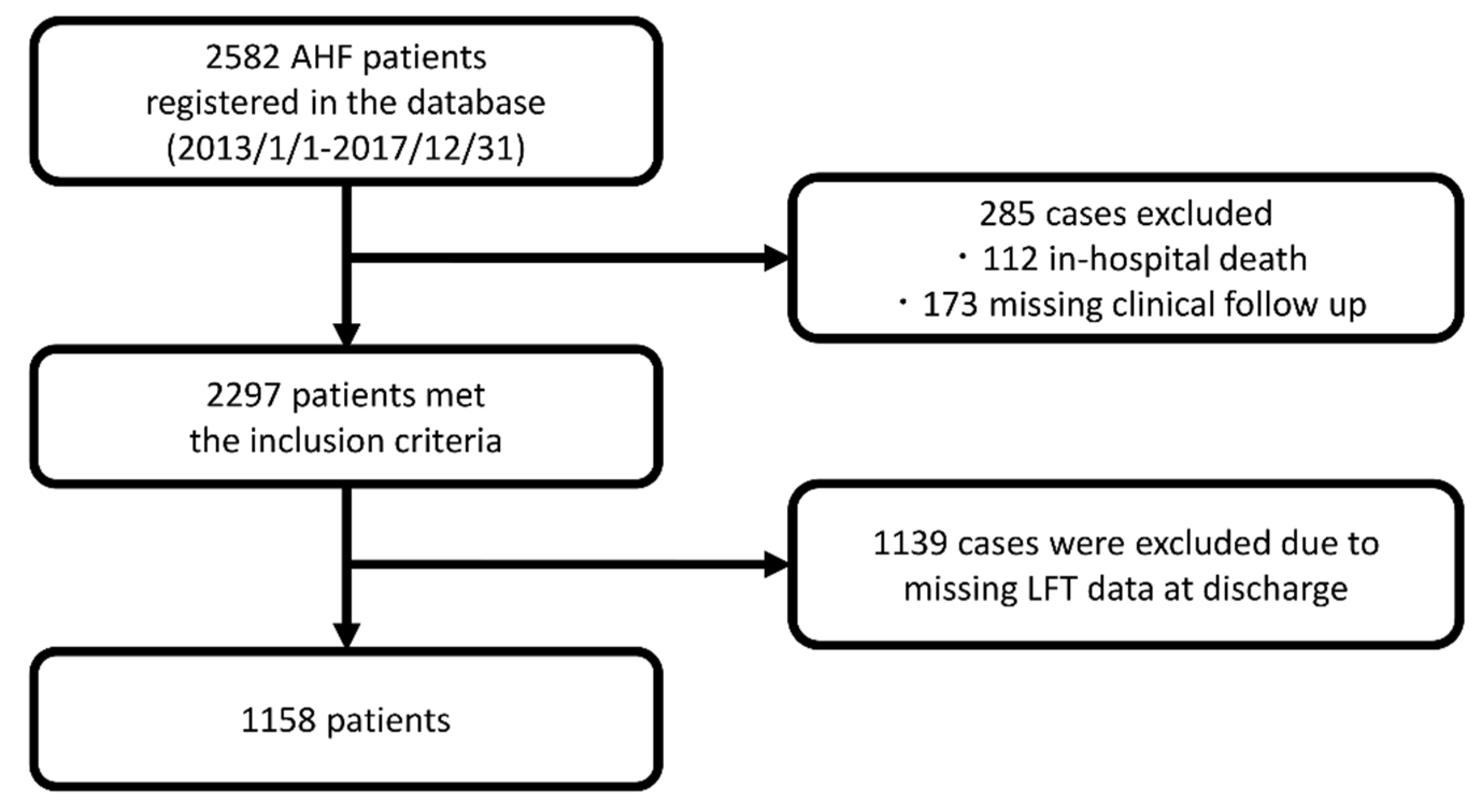
2.2. Patients
2.3. Definition of Abnormal Liver Function Tests (LFTs)
2.4. Definitions and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
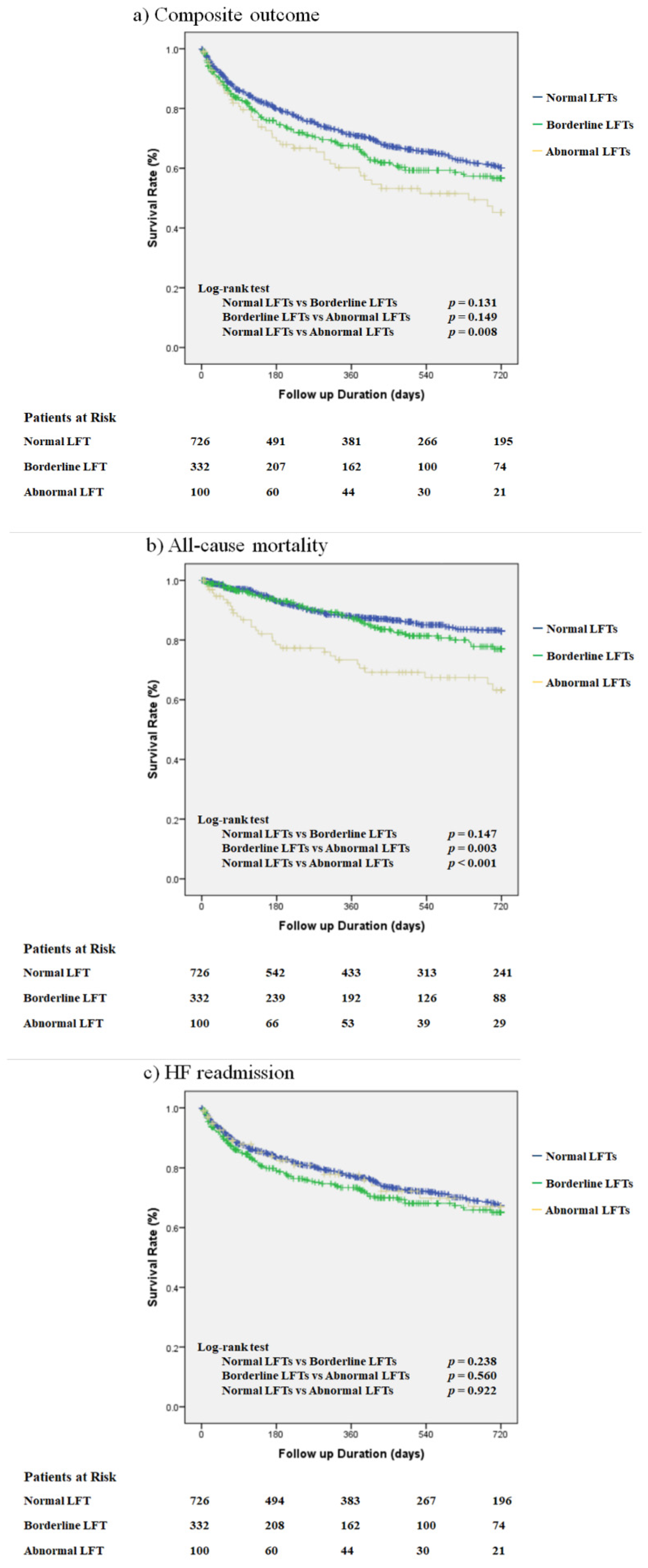
3.2. Unadjusted Outcomes
3.3. Multivariate Adjusted Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Vaduganathan, M.; Huffman, M.D.; Khan, S.; Kwasny, M.J.; Fought, A.J.; Maggioni, A.P.; Swedberg, K.; Konstam, M.A.; Zannad, F.; et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: An analysis of the EVEREST trial. Eur. J. Heart Fail. 2012, 14, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, M.; Schabath, M.B.; Guglin, M. Longitudinal trends, hemodynamic profiles, and prognostic value of abnormal liver function tests in patients with acute decompensated heart failure: An analysis of the ESCAPE trial. J. Card. Fail. 2014, 20, 476–484. [Google Scholar] [CrossRef]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef]
- Samsky, M.D.; Dunning, A.; DeVore, A.D.; Schulte, P.J.; Starling, R.C.; Tang, W.H.; Armstrong, P.W.; Ezekowitz, J.A.; Butler, J.; McMurray, J.J.; et al. Liver function tests in patients with acute heart failure and associated outcomes: Insights from ASCEND-HF. Eur. J. Heart Fail. 2016, 18, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Abe, T.; Mizuno, A.; Goda, A.; Izumi, Y.; Yagawa, M.; Akita, K.; Sawano, M.; Inohara, T.; et al. Validation of the Get With The Guideline-Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B-type natriuretic peptide level. Am. Heart J. 2016, 171, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Nagai, T.; Goda, A.; Mizuno, A.; Nagatomo, Y.; Sujino, Y.; Fukuoka, R.; Sawano, M.; Kohno, T.; et al. Validation and Recalibration of Seattle Heart Failure Model in Japanese Acute Heart Failure Patients. J. Card. Fail. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.; Anderson, K.M.; Kannel, W.B.; Grossman, W.; Levy, D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993, 88, 107–115. [Google Scholar] [CrossRef]
- Bart, B.A.; Shaw, L.K.; McCants, C.B., Jr.; Fortin, D.F.; Lee, K.L.; Califf, R.M.; O’Connor, C.M. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 1997, 30, 1002–1008. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kohsaka, S.; Sato, N.; Takano, T.; Kitai, T.; Yoshikawa, T.; Matsue, Y. 9-Year Trend in the Management of Acute Heart Failure in Japan: A Report From the National Consortium of Acute Heart Failure Registries. J. Am. Heart Assoc. 2018, 7, e008687. [Google Scholar] [CrossRef]
- Smilde, T.D.; van Veldhuisen, D.J.; Navis, G.; Voors, A.A.; Hillege, H.L. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 2006, 114, 1572–1580. [Google Scholar] [CrossRef]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Pocock, S.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; Granger, C.B.; et al. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur. J. Heart Fail. 2009, 11, 170–177. [Google Scholar] [CrossRef]
- Okada, A.; Sugano, Y.; Nagai, T.; Honda, Y.; Iwakami, N.; Nakano, H.; Takashio, S.; Honda, S.; Asaumi, Y.; Aiba, T.; et al. Usefulness of the Direct and/or Total Bilirubin to Predict Adverse Outcomes in Patients With Acute Decompensated Heart Failure. Am. J. Cardiol. 2017, 119, 2035–2041. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Edwards, C.; Cotter, G.; Davison, B.A.; Damman, K.; Teerlink, J.R.; Metra, M.; Felker, G.M.; Ponikowski, P.; Unemori, E.; et al. Liver function, in-hospital, and post-discharge clinical outcome in patients with acute heart failure-results from the relaxin for the treatment of patients with acute heart failure study. J. Card. Fail. 2014, 20, 407–413. [Google Scholar] [CrossRef]
- Bosma, P.J.; Chowdhury, J.R.; Bakker, C.; Gantla, S.; de Boer, A.; Oostra, B.A.; Lindhout, D.; Tytgat, G.N.; Jansen, P.L.; Oude Elferink, R.P.; et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N. Engl. J. Med. 1995, 333, 1171–1175. [Google Scholar] [CrossRef]
- Bjornsson, E.S.; Bergmann, O.M.; Bjornsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef] [PubMed]
- Sgro, C.; Clinard, F.; Ouazir, K.; Chanay, H.; Allard, C.; Guilleminet, C.; Lenoir, C.; Lemoine, A.; Hillon, P. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology 2002, 36, 451–455. [Google Scholar] [CrossRef]
- Moller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Zymlinski, R.; Biegus, J.; Sokolski, M.; Siwolowski, P.; Nawrocka-Millward, S.; Todd, J.; Jankowska, E.A.; Banasiak, W.; Cotter, G.; Cleland, J.G.; et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 2018, 20, 1011–1018. [Google Scholar] [CrossRef]
- Kim, M.S.; Kato, T.S.; Farr, M.; Wu, C.; Givens, R.C.; Collado, E.; Mancini, D.M.; Schulze, P.C. Hepatic dysfunction in ambulatory patients with heart failure: Application of the MELD scoring system for outcome prediction. J. Am. Coll. Cardiol. 2013, 61, 2253–2261. [Google Scholar] [CrossRef]
- Poelzl, G.; Ess, M.; Mussner-Seeber, C.; Pachinger, O.; Frick, M.; Ulmer, H. Liver dysfunction in chronic heart failure: Prevalence, characteristics and prognostic significance. Eur. J. Clin. Investig. 2012, 42, 153–163. [Google Scholar] [CrossRef]
- Cerlinskaite, K.; Hollinger, A.; Mebazaa, A.; Cinotti, R. Finding the balance between costs and quality in heart failure: A global challenge. Eur. J. Heart Fail. 2018, 20, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Nagai, T.; Kohsaka, S.; Goda, A.; Nagatomo, Y.; Mizuno, A.; Kohno, T.; Rigby, A.; Fukuda, K.; Yoshikawa, T.; et al. Outcome of hospitalised heart failure in Japan and the United Kingdom stratified by plasma N-terminal pro-B-type natriuretic peptide. Clin. Res. Cardiol. 2018, 107, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Padda, M.S.; Sanchez, M.; Akhtar, A.J.; Boyer, J.L. Drug-induced cholestasis. Hepatology 2011, 53, 1377–1387. [Google Scholar] [CrossRef]
- Beath, S.V.; Kelly, D.A. Total Parenteral Nutrition-Induced Cholestasis: Prevention and Management. Clin. Liver Dis. 2016, 20, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Mulvey, G.K.; Stauffer, B.; Patlolla, V.; Bernheim, S.M.; Keenan, P.S.; Krumholz, H.M. Statistical models and patient predictors of readmission for heart failure: A systematic review. Arch. Intern. Med. 2008, 168, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Kansagara, D.; Englander, H.; Salanitro, A.; Kagen, D.; Theobald, C.; Freeman, M.; Kripalani, S. Risk prediction models for hospital readmission: A systematic review. JAMA 2011, 306, 1688–1698. [Google Scholar] [CrossRef]


| Variables | Normal LFTs n = 726 | Borderline LFTs n = 332 | Abnormal LFTs n = 100 | p-Value |
|---|---|---|---|---|
| Age, years | 78 (67–84) | 76 (65–82) | 72 (62–80) | 0.003 |
| Male, % | 56.1 | 59.6 | 71.0 | 0.015 |
| BMI, kg/m2 | 22.9 (20.4–26.0) | 23.1 (20.6–26.0) | 23.1 (20.7–26.0) | 0.56 |
| SBP at discharge, mmHg | 112 (100–125) | 106 (98–120) | 107 (98–120) | <0.001 |
| HR at discharge, bpm | 70 (62–79) | 72 (64–80) | 74 (66–84) | 0.014 |
| NYHA class at discharge | 0.55 | |||
| Class I, % | 29.3 | 29.6 | 24.0 | |
| Class II, % | 58.3 | 58.3 | 58.0 | |
| Class III, % | 11.3 | 10.3 | 17.0 | |
| Class IV, % | 1.1 | 1.8 | 1.0 | |
| LVEF, % | 45.0 (32.0–59.0) | 45.0 (31.0–56.0) | 47.0 (30.0–58.0) | 0.61 |
| Ischemic etiology, % | 31.5 | 24.1 | 20.0 | 0.007 |
| Comorbidities | ||||
| Prior admissions for HF, % | 25.5 | 25.9 | 30.0 | 0.63 |
| Hypertension, % | 66.4 | 65.1 | 61.0 | 0.55 |
| Hyperlipidemia, % | 39.9 | 39.6 | 35.0 | 0.64 |
| Diabetes, % | 32.6 | 31.9 | 28.0 | 0.65 |
| Atrial fibrillation, % | 44.8 | 53.0 | 51.0 | 0.035 |
| Stroke, % | 14.3 | 17.2 | 16.0 | 0.48 |
| COPD, % | 5.1 | 2.1 | 7.0 | 0.037 |
| Hemodialysis, % | 2.6 | 1.2 | 1.0 | 0.24 |
| Laboratory findings at admission | ||||
| Hemoglobin, mg/dL | 11.8 (10.3–13.6) | 12.5 (10.8–14.1) | 12.6 (10.4–14.1) | 0.005 |
| Sodium, mEq/L | 140 (137–142) | 140 (137–142) | 139 (135–141) | 0.016 |
| Cr, mg/dL | 1.4 ± 1.5 | 1.2 ± 0.7 | 1.4 ± 1.1 | 0.035 |
| BUN, mg/dL | 21.2 (16.6–30.1) | 22.1 (16.1–31.5) | 23.8 (17.3–33.7) | 0.042 |
| eGFR, mL/min/1.73 m2 | 49.8 ± 24.0 | 50.9 ± 20.2 | 51.5 ± 23.3 | 0.65 |
| TB, mg/dL | 0.8 (0.6–1.1) | 1.0 (0.7–1.5) | 1.5 (0.8–2.0) | <0.001 |
| AST, IU/L | 31.0 (23.0–46.5) | 36.0 (26.0–55.0) | 42.0 (30.0–74.0) | 0.64 |
| ALT, IU/L | 21.0 (13.0–37.0) | 27.0 (17.0–47.0) | 32.0 (19.3–67.3) | 0.23 |
| ALP, IU/L | 245 (196–298) | 297 (207–412) | 389 (273–528) | <0.001 |
| Albumin, g/dL | 3.6 (3.3–3.9) | 3.6 (3.3–3.9) | 3.5 (3.3–3.8) | 0.13 |
| BNP, pg/mL | 751 (419–1370) | 573 (365–1010) | 992 (489–1880) | 0.001 |
| Laboratory findings at discharge | ||||
| Hemoglobin, mg/dL | 11.7 (10.3–13.1) | 12.4 (10.6–14.0) | 12.3 (10.3–14.0) | <0.001 |
| Sodium, mEq/L | 139 (137–141) | 139 (137–141) | 138 (135–140) | <0.001 |
| Cr, mg/dL | 1.4 ± 1.5 | 1.2 ± 1.0 | 1.2 ± 0.9 | 0.49 |
| BUN, mg/dL | 21.5 (16.0–30.3) | 22.3 (16.4–30.8) | 25.1 (17.4–33.5) | 0.051 |
| eGFR, mL/min/1.73 m2 | 49.7 ± 22.9 | 52.7 ± 22.1 | 55.5 ± 27.1 | 0.027 |
| TB, mg/dL | 0.6 (0.5–0.9) | 0.8 (0.6–1.2) | 1.2 (0.7–1.7) | <0.001 |
| AST, IU/L | 21.0 (17.0–26.0) | 27.0 (21.0–39.0) | 44.0 (31.3–55.0) | <0.001 |
| ALT, IU/L | 15.0 (11.0–22.0) | 23.0 (15.0–41.0) | 42.5 (25.0–67.5) | <0.001 |
| ALP, IU/L | 214 (175–253) | 287 (205–382) | 407 (336–543) | <0.001 |
| Albumin, g/dL | 3.5 (3.2–3.8) | 3.6 (3.2–3.9) | 3.4 (3.1–3.8) | 0.18 |
| BNP, pg/mL | 271 (137–522) | 239 (104–447) | 389 (247–663) | 0.023 |
| In-hospital treatment | ||||
| Diuretic infusion, % | 69.3 | 73.5 | 68.0 | 0.33 |
| Vasodilator, % | 60.9 | 57.8 | 47.0 | 0.028 |
| Catecholamine, % | 16.4 | 17.5 | 23.0 | 0.27 |
| NPPV, % | 24.7 | 18.4 | 20.0 | 0.061 |
| Intubation, % | 3.4 | 3.6 | 6.0 | 0.45 |
| Prescription at discharge | ||||
| Diuretics, % | 73.3 | 77.1 | 78.0 | 0.31 |
| RAS inhibitor, % | 59.6 | 59.6 | 45.0 | 0.018 |
| MRA, % | 34.2 | 36.7 | 39.0 | 0.52 |
| Beta blocker, % | 77.4 | 80.4 | 71.0 | 0.13 |
| OAC, % | 53.2 | 62.3 | 71.0 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyama, H.; Shiraishi, Y.; Kohsaka, S.; Goda, A.; Nishihata, Y.; Nagatomo, Y.; Takei, M.; Fukuda, K.; Kohno, T.; Yoshikawa, T. Abnormal Liver Function Tests and Long-Term Outcomes in Patients Discharged after Acute Heart Failure. J. Clin. Med. 2021, 10, 1730. https://doi.org/10.3390/jcm10081730
Miyama H, Shiraishi Y, Kohsaka S, Goda A, Nishihata Y, Nagatomo Y, Takei M, Fukuda K, Kohno T, Yoshikawa T. Abnormal Liver Function Tests and Long-Term Outcomes in Patients Discharged after Acute Heart Failure. Journal of Clinical Medicine. 2021; 10(8):1730. https://doi.org/10.3390/jcm10081730
Chicago/Turabian StyleMiyama, Hiroshi, Yasuyuki Shiraishi, Shun Kohsaka, Ayumi Goda, Yosuke Nishihata, Yuji Nagatomo, Makoto Takei, Keiichi Fukuda, Takashi Kohno, and Tsutomu Yoshikawa. 2021. "Abnormal Liver Function Tests and Long-Term Outcomes in Patients Discharged after Acute Heart Failure" Journal of Clinical Medicine 10, no. 8: 1730. https://doi.org/10.3390/jcm10081730
APA StyleMiyama, H., Shiraishi, Y., Kohsaka, S., Goda, A., Nishihata, Y., Nagatomo, Y., Takei, M., Fukuda, K., Kohno, T., & Yoshikawa, T. (2021). Abnormal Liver Function Tests and Long-Term Outcomes in Patients Discharged after Acute Heart Failure. Journal of Clinical Medicine, 10(8), 1730. https://doi.org/10.3390/jcm10081730







