CSF Dynamics for Shunt Prognostication and Revision in Normal Pressure Hydrocephalus
Abstract
1. Background
1.1. Rationale and Significance of the Review
1.2. Hydrocephalus: Clinical and Radiological Perspective
1.3. Importance of Investigating NPH: Where a CSF Flows; Role of Reversal Flow & CSF Dynamics
2. Infusion Test Methodology and Parameters
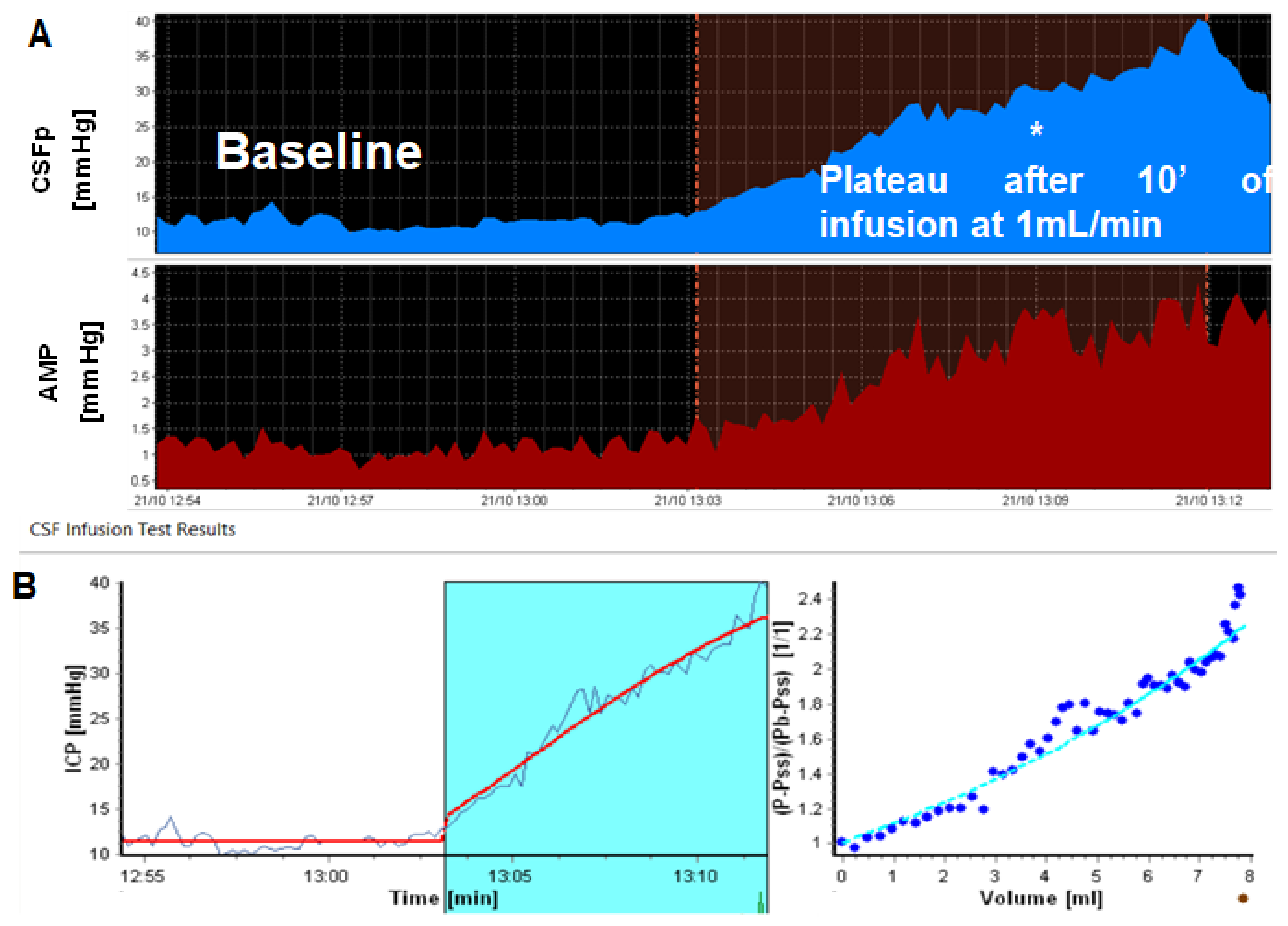
Principles of Bedside CSF Infusion Tests
3. CSF Dynamics: Interpretation of Findings Versus Relationship to Outcome after Shunting
3.1. Steady-State ICP (Baseline)
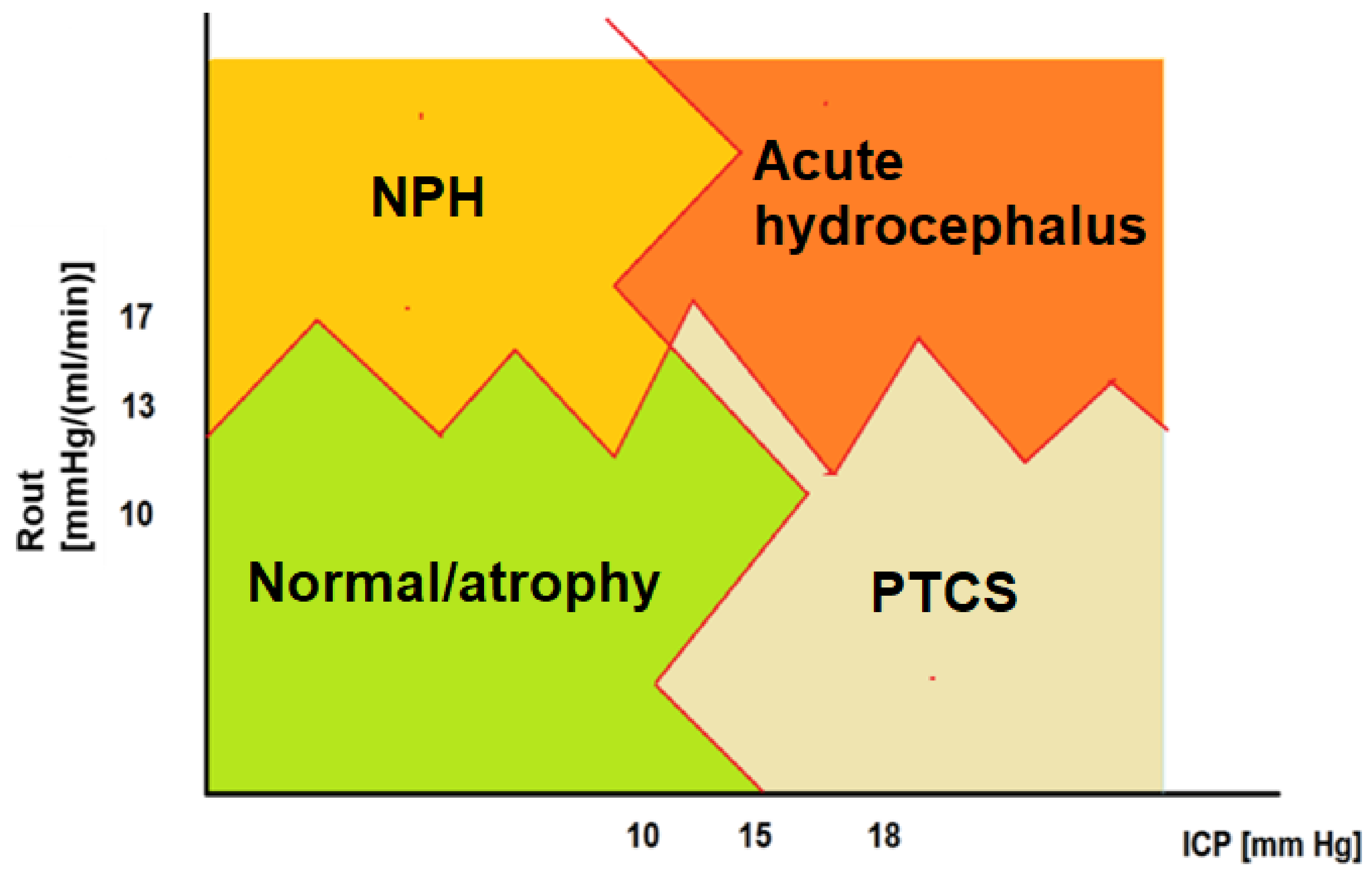
3.2. Resistance to CSF Outflow: Calculation, Normal Ranges and Thresholds
3.3. Resistance to CSF Outflow versus Outcome after Shunting
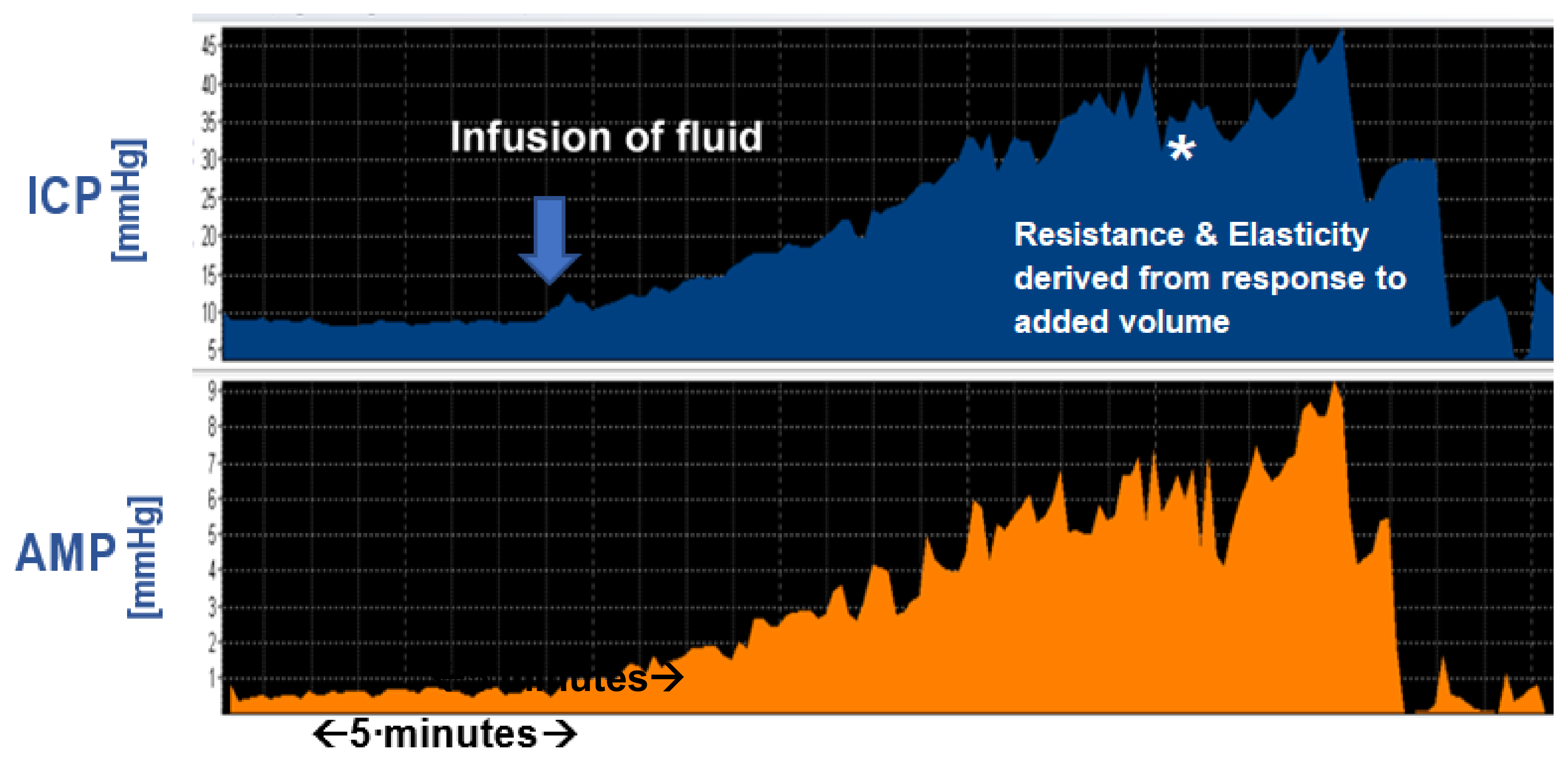
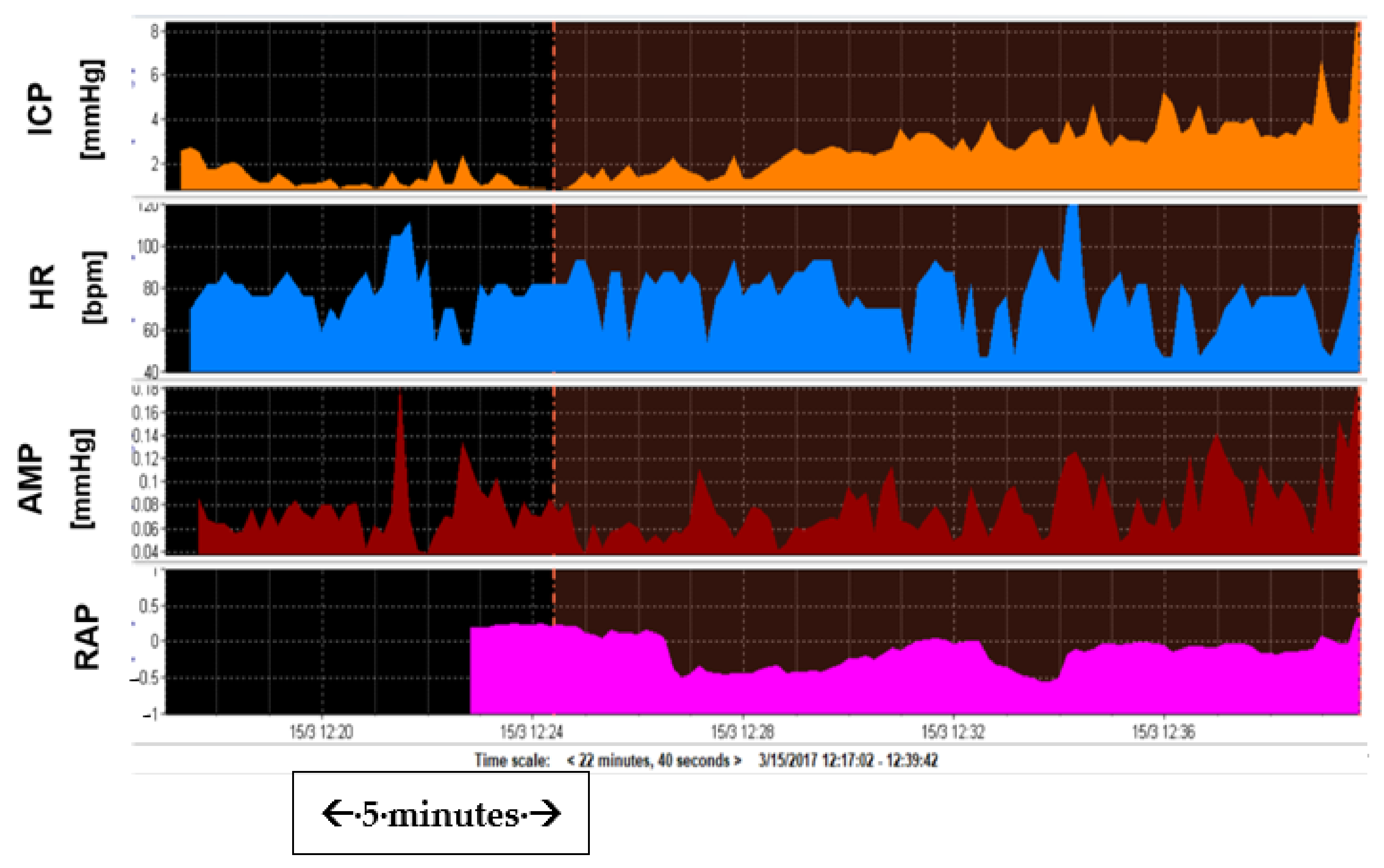
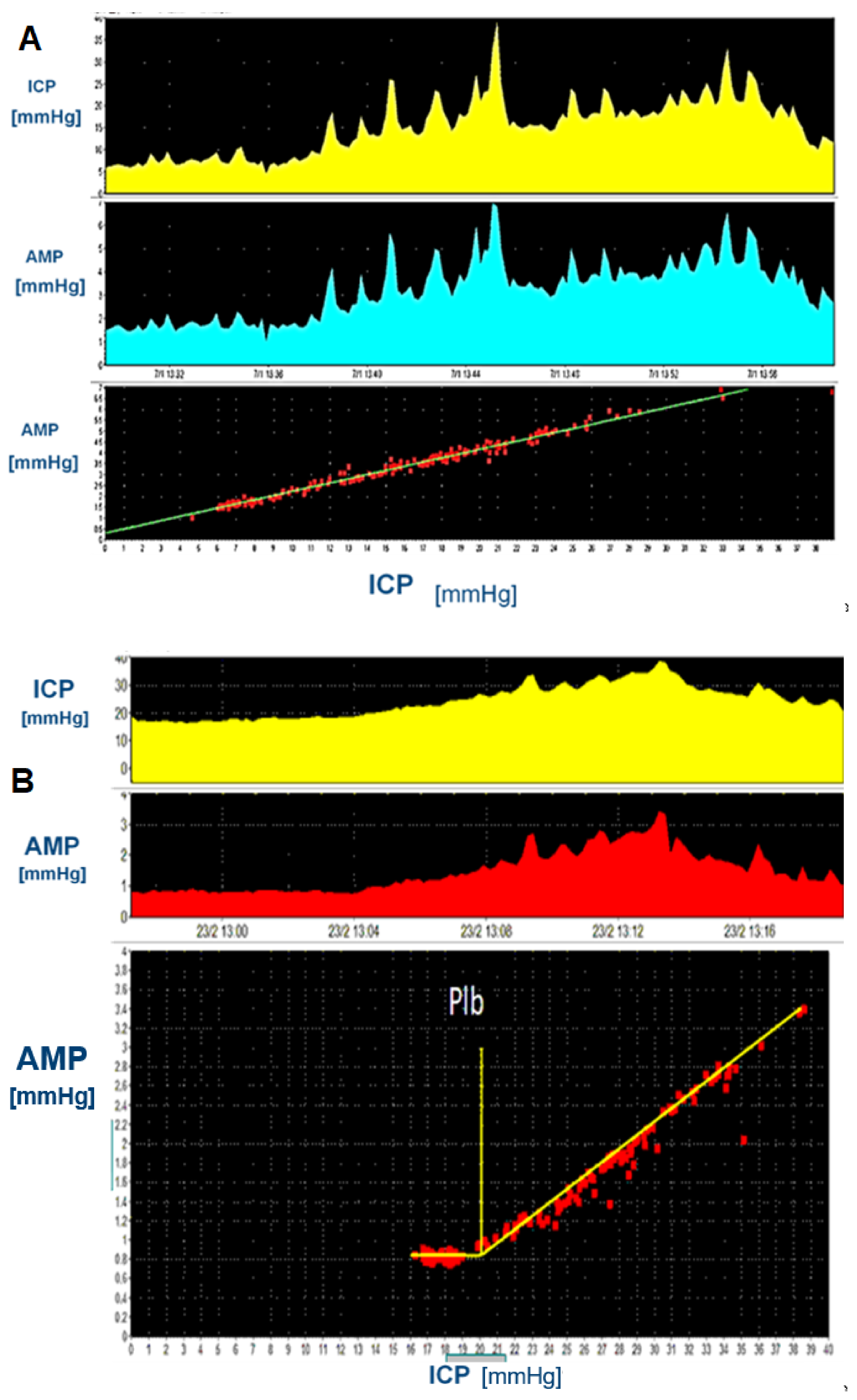
3.4. Amplitude and Amplitude-Pressure Relationship
3.5. RAP and Elasticity
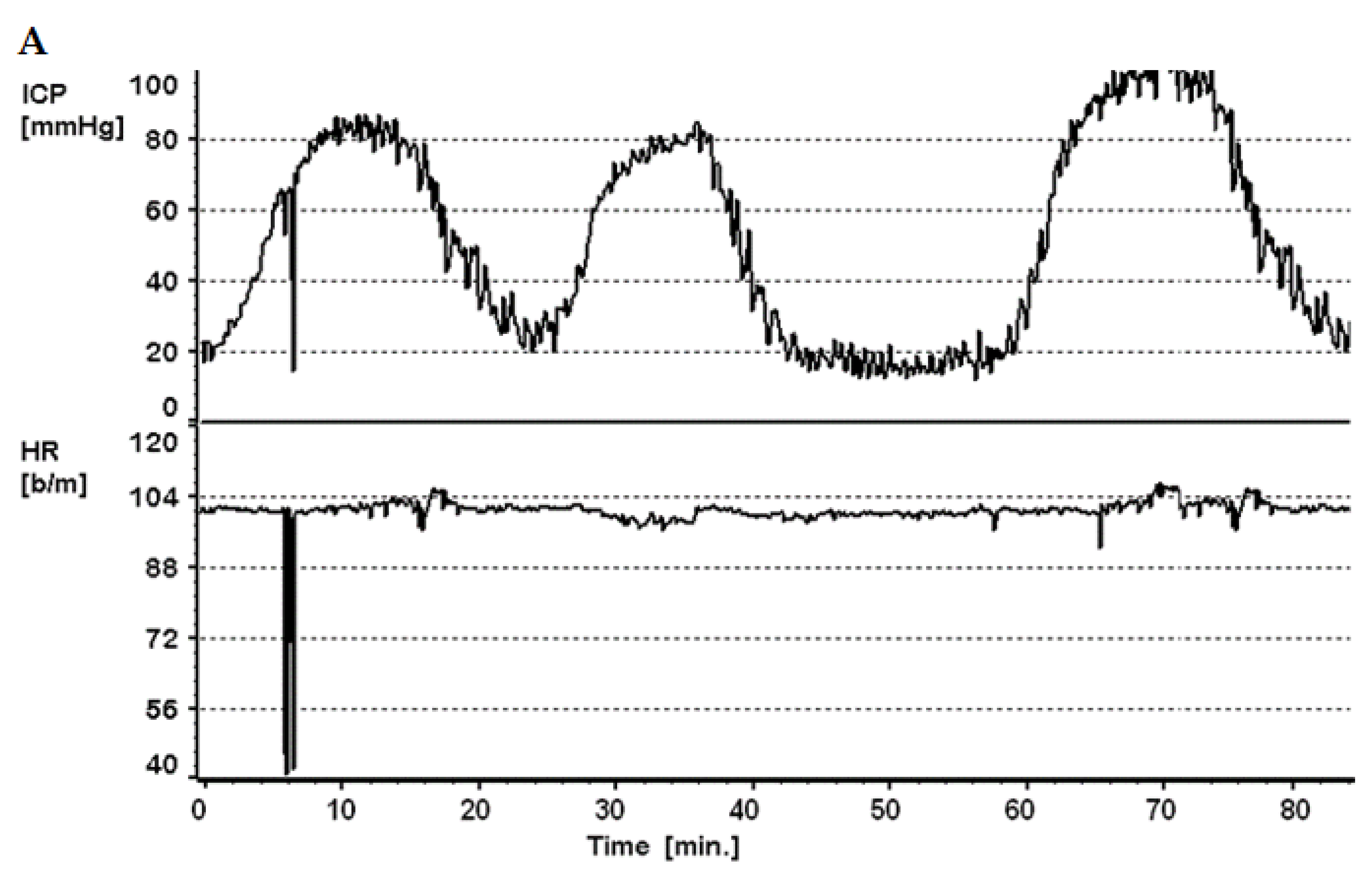
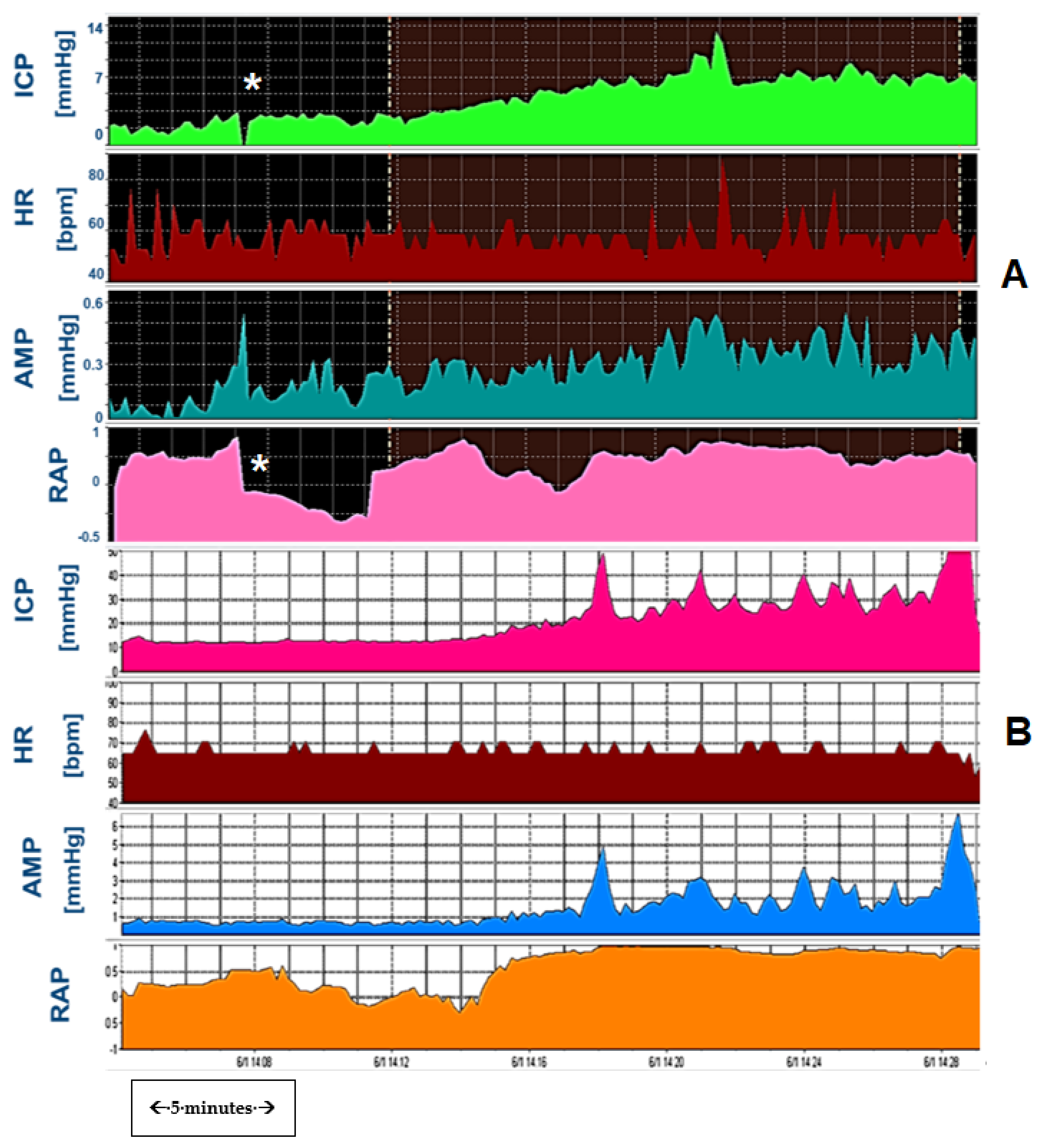
3.6. Slow Waves of Intracranial Pressure
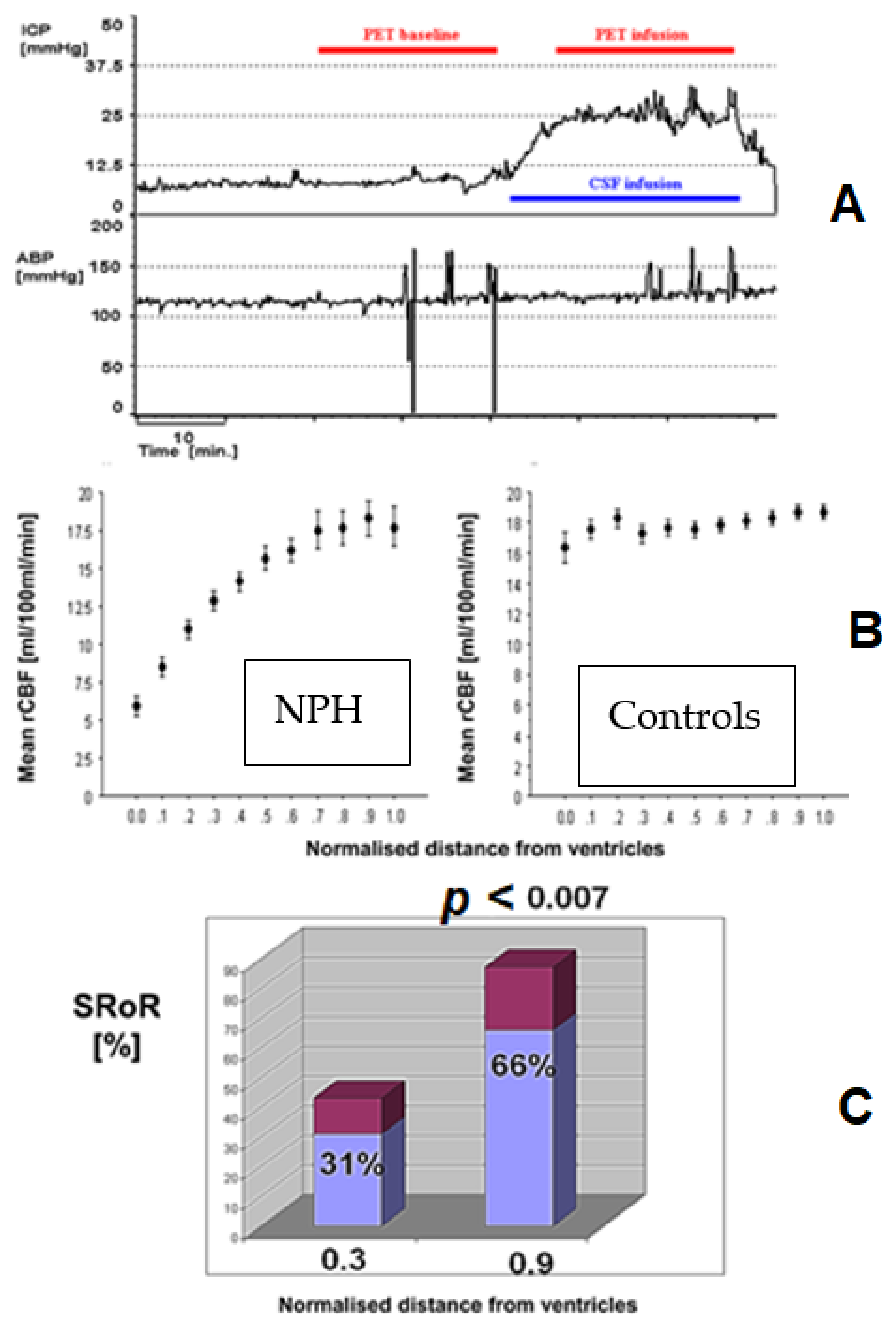
3.7. Cerebral Blood Flow and Autoregulation in Hydrocephalus
3.7.1. Slow Waves and Vasomotor Reactivity
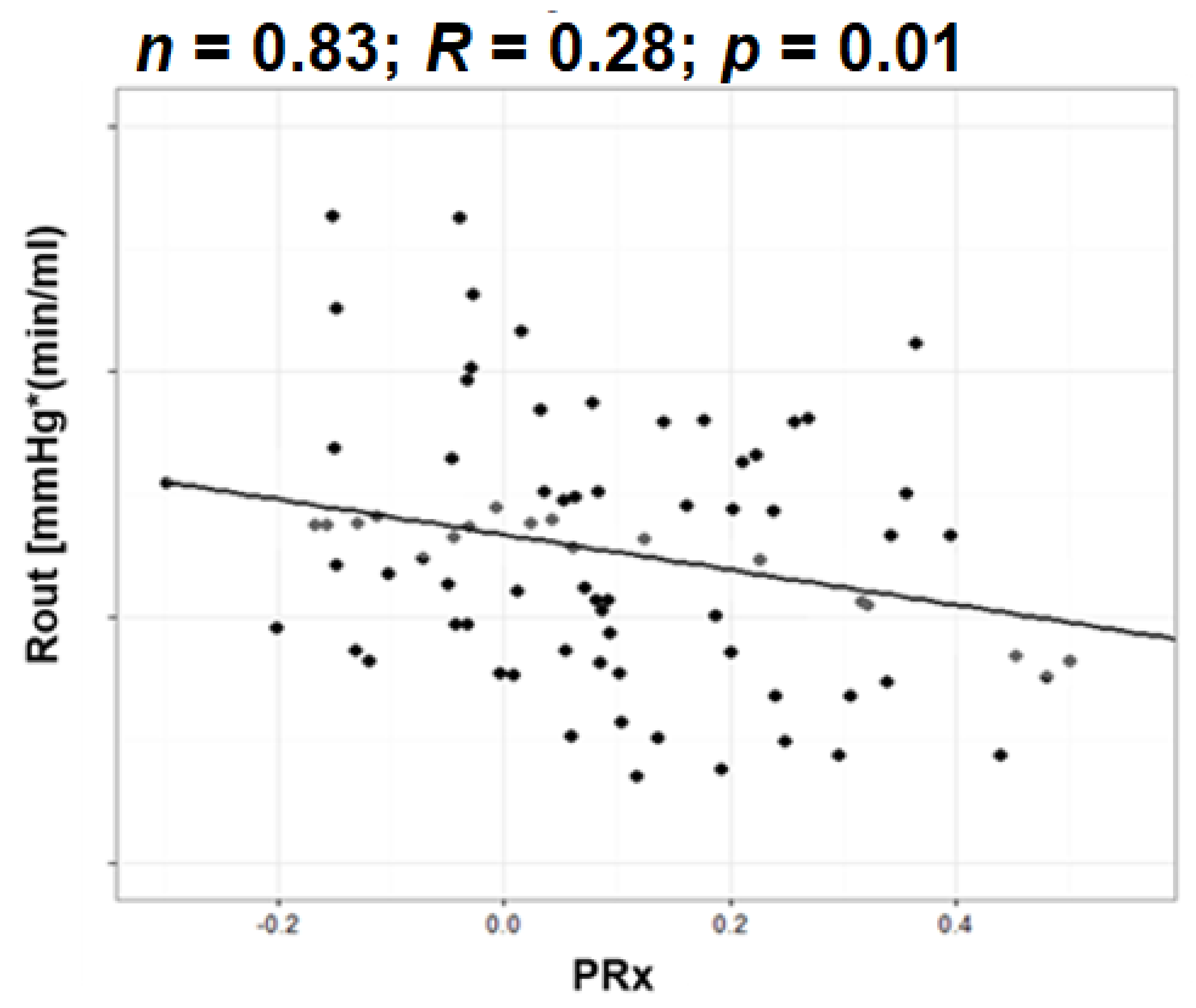
3.7.2. Resistance to CSF Outflow and Autoregulation
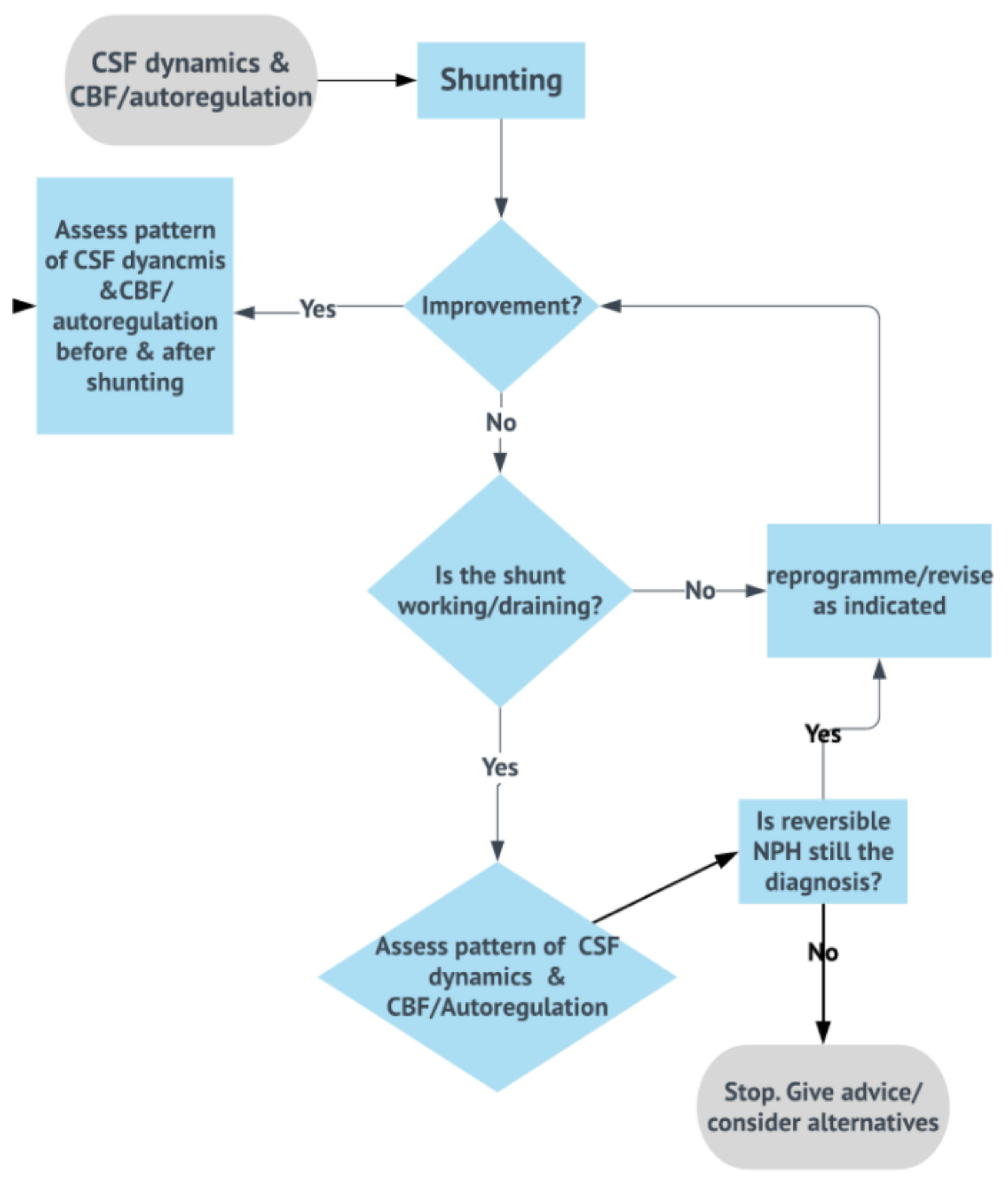
3.8. Shunt Testing In Vivo
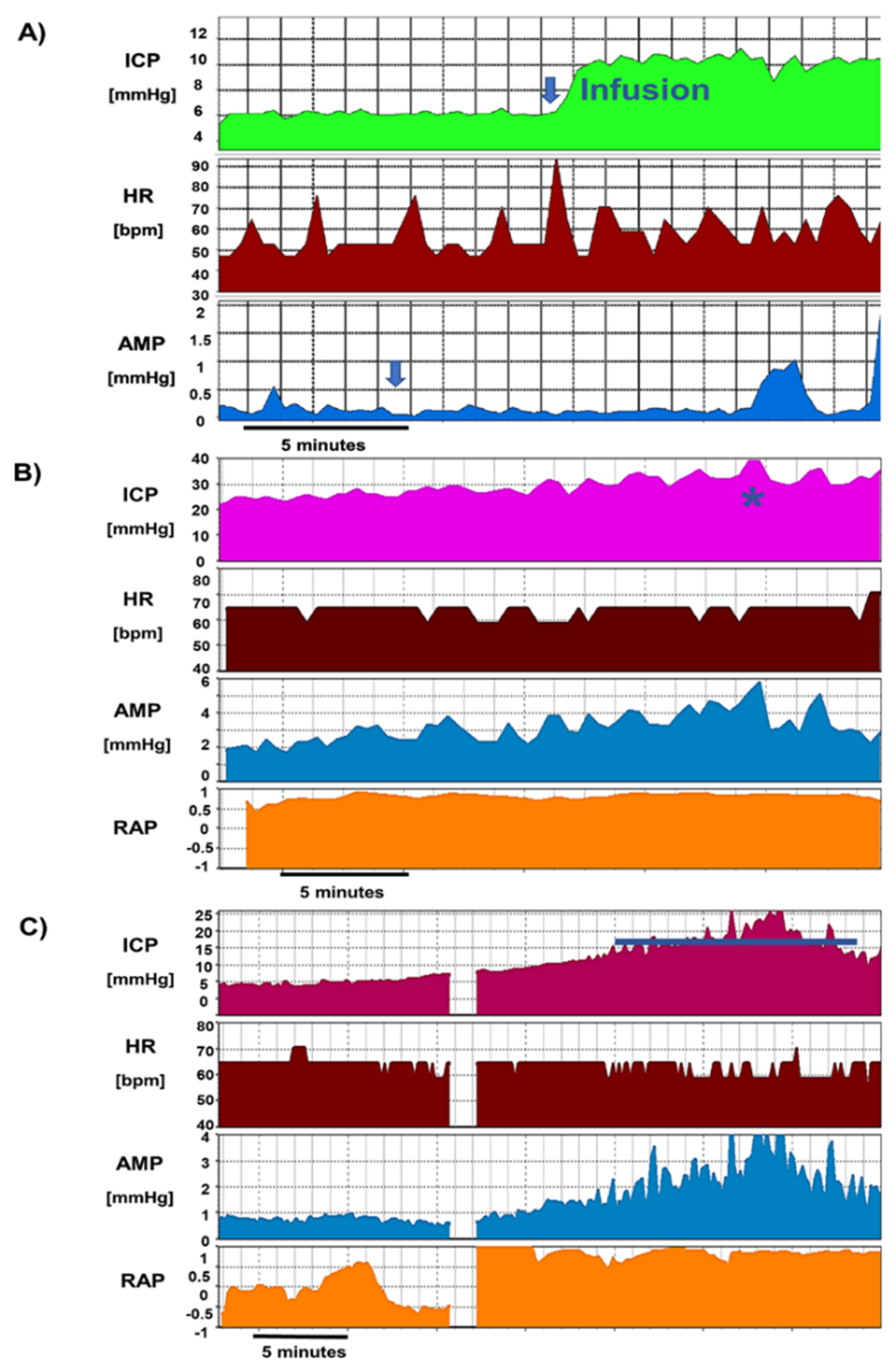
- Pulsatile ICP, within the expected limits for the shunt operating pressure, normal resistance, and critical pressure (inherent property of the shunt) not exceeded during infusion: no revision, the shunt is patent and draining well.
- Non-pulsatile “pressure” at baseline, steep jump in pressure after start of infusion, and no signs of pulse waveform recovery during infusion: proximal obstruction. If distal catheter is patent (seen by stabilization of pressure after infusion), it can proceed to revise only the proximal catheter.
- Clearly raised, pulsatile baseline pressure: distal obstruction (or significantly increased pressure on the drainage site, e.g., in raised intra-abdominal pressure). Ventricular catheter patent can revise only the distal end (or lower valve setting if as above increased intra-abdominal pressure is suspected).
- Normal opening pressure, with significant rise in ICP during infusion to values clearly exceeds shunt critical pressure; raised Rout: distal obstruction (Figure 12).
- ICPp slightly exceeding critical pressure; Rout mildly raised: mild underdrainage, consider adjusting the setting and monitor, which may require revision later.
3.9. Objective Testing of Cerebrospinal Fluid Dynamics: The New Era
3.9.1. Resistance to Cerebrospinal Fluid Outflow Thresholds
3.9.2. Cerebrospinal Fluid Dynamics versus Outcome
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marmarou, A. A Theoretical Model and Experimental Evaluation of the Cerebrospinal Fluid System. College of Engineering, Drexel University. 1973. Available online: https://books.google.co.uk/books?id=DrIaHQAACAAJ (accessed on 10 March 2021).
- Katzman, R.; Hussey, F. A Simple Constant-Infusion Manometric Test for Measurement of CSF Absorption. I. Rationale and Method. Neurology 1970, 20, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Pickard, J.; Weerakkody, R.A.; Czosnyka, M.; Schuhmann, M.U.; Schmidt, E.; Keong, N.; Santarius, T.; Pickard, J.D.; Czosnyka, Z. Clinical assessment of cerebrospinal fluid dynamics in hydrocephalus. Guide to interpretation based on observational study. Acta Neurol. Scand. 2011, 124, 85–98. [Google Scholar]
- Czosnyka, M.; Laniewski, W.; Batorski, L.; Zaworski, W. Analysis of Intracranial Pressure Waveform during Infusion Test. Acta Neurochir. 1988, 93, 140–145. [Google Scholar] [CrossRef]
- Tans, J.T.J.; Poortvliet, D.C.J. CSF outflow resistance and pressure-volume index determined by steady-state and bolus infusions. Clin. Neurol. Neurosurg. 1985, 87, 159–165. [Google Scholar] [CrossRef]
- Beck, J.; Fung, C.; Ulrich, C.T.; Fiechter, M.; Fichtner, J.; Mattle, H.P.; Mono, M.L.; Meier, N.; Mordasini, P.; Z’Graggen, W.J.; et al. Cerebrospinal fluid outflow resistance as a diagnostic marker of spontaneous cerebrospinal fluid leakage. J. Neurosurg. Spine 2017, 27, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lalou, A.D.; Czosnyka, M.; Donnelly, J.; Pickard, J.D.; Nabbanja, E.; Keong, N.C.; Garnett, M.; Czosnykal, Z.H.; Sci, F.M. Cerebral autoregulation, cerebrospinal fluid outflow resistance, and outcome following cerebrospinal fluid diversion in normal pressure hydrocephalus. J. Neurosurg. 2018, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Fremming, A.D.; Sorteberg, A. Lack of relationship between resistance to cerebrospinal fluid outflow and intracranial pressure in normal pressure hydrocephalus. Acta Neurol. Scand. 2003, 108, 381–388. [Google Scholar] [CrossRef]
- Børgesen, S.E.; Albeck, M.J.; Gjerris, F.; Czosnyka, M.; Laniewski, P. Computerized infusion test compared to steady pressure constant infusion test in measurement of resistance to CSF outflow. Acta Neurochir. 1992, 119, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.J.W.; Tans, J.T.J.; Delwel, E.J.; Egeler-Peerdeman, S.M.; Hanlo, P.W.; Wurzer, H.A.L.; Avezaat, C.J.J.; De Jong, D.A.L.; Gooskens, R.H.J.M.; Hermans, J. Dutch normal-pressure hydrocephalus study: Prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J. Neurosurg. 1997, 87, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Wikkelsø, C.; Hellström, P.; Klinge, P.M.; Tans, J.T.J.; on behalf of the European iNPH Multicentre Study Group. The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF Tap Test in patients with idiopathic normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2012, 84, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Bergsneider, M.; Klinge, P.; Relkin, N.; Black, P.M.L. INPH guidelines, part III: The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 2005, 57. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Yoon, U.; Lee, J.M.; Lee, H.W. Idiopathic normal-pressure hydrocephalus, cortical thinning, and the cerebrospinal fluid tap test. J. Neurol. Sci. 2013, 334, 55–62. [Google Scholar] [CrossRef]
- Malm, J.; Graff-Radford, N.R.; Ishikawa, M.; Kristensen, B.; Leinonen, V.; Mori, E.; Owler, B.K.; Tullberg, M.; Williams, M.A.; Relkin, N.R. Influence of comorbidities in idiopathic normal pressure hydrocephalus—Research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 2013, 10, 1–4. [Google Scholar] [CrossRef]
- Nigim, F.; Critchlow, J.F.; Schneider, B.E.; Chen, C.; Kasper, E.M. Shunting for hydrocephalus: Analysis of techniques and failure patterns. J. Surg. Res. 2014, 191, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.C.; Mazzola, C.A.; Auguste, K.I.; Klimo, P., Jr. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines. Part 5: Effect of valve type on cerebrospinal fluid shunt efficacy. J. Neurosurg. Pediatr. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Grand, W.; Leonardo, J.; Chamczuk, A.J.; Korus, A.J. Endoscopic third ventriculostomy in 250 adults with hydrocephalus: Patient selection, outcomes, and complications. Neurosurgery 2016, 78, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Hultin, L. Postoperative changes in SPECT-rCBF in hydrocephalus. In Intracranial Pressure and Brain Monitoring XII; Springer: Vienna, Austria, 2005; pp. 169–172. [Google Scholar] [CrossRef]
- Engel, D.C.; Adib, S.D.; Schuhmann, M.U.; Brendle, C. Paradigm-shift: Radiological changes in the asymptomatic iNPH-patient to be: An observational study. Fluids Barriers CNS 2018, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ryska, P.; Slezak, O.; Eklund, A.; Malm, J.; Salzer, J.; Zizka, J. Radiological markers of idiopathic normal pressure hydrocephalus: Relative comparison of their diagnostic performance. J. Neurol. Sci. 2020, 408, 116581. [Google Scholar] [CrossRef] [PubMed]
- Kojoukhova, M.; Koivisto, A.M.; Korhonen, R.; Remes, A.M.; Vanninen, R.; Soininen, H.; Jääskeläinen, J.E.; Sutela, A.; Leinonen, V. Feasibility of radiological markers in idiopathic normal pressure hydrocephalus. Acta Neurochir. 2015, 157, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Goeser, C.D.; McLeary, M.S.; Young, L.W. Diagnostic imaging of ventriculoperitoneal shunt malfunctions and complications. Radiographics 1998, 18, 635–651. [Google Scholar] [CrossRef]
- Kang, K.; Hwang, S.K.; Lee, H.W. Shunt-responsive idiopathic normal pressure hydrocephalus patient with delayed improvement after tap test. J. Korean Neurosurg. Soc. 2013, 54. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Jusue-Torres, I.; Lu, J.; Robison, J.; Patel, M.A.; Crain, B.; Carson, K.A.; Hoffberger, J.; Batra, S.; Sankey, E.; et al. Alzheimer’s disease pathology and shunt surgery outcome in normal pressure hydrocephalus. PLoS ONE 2017, 12, e0182288. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.D.; Teasdale, G.; Matheson, M.; Lindsay, K.; Galbraith, S.; Wyper, D.; MacPhetrson, P. Intraventricular Pressure Waves—The Best Predictive Test for Shunting in Normal Pressure Hydrocephalus. In Intracranial Pressure IV; Springer: Berlin/Heidelberg, Germany, 1980; pp. 498–500. [Google Scholar]
- Tullberg, M.; Petersen, J.; Hellstrom, P.; Wikkelso, C.; Lundgren-Nilsson, Å. Shunt surgery in idiopathic normal pressure hydrocephalus is cost-effective-a cost utility analysis. Acta Neurochir. 2018, 160, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Kuwana, N.; Ito, S.; Ikegami, T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol. Med. Chir. 1999, 39, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Fulop, S.C.; Dashti, S.R.; Robinson, S.; Cohen, A.R. Rethinking the indications for the ventriculoperitoneal shunt tap. J. Neurosurg. Pediatr. 2008, 1, 435–438. [Google Scholar] [CrossRef]
- Reddy, G.K.; Bollam, P.; Caldito, G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014, 81, 404–410. [Google Scholar] [CrossRef]
- Tans, J.T.J.; Boon, A.J.W. How to select patients with normal pressure hydrocephalus for shunting. Acta Neurochir. 2002, 81, 3–5. [Google Scholar]
- Gasslander, J.; Sundström, N.; Eklund, A.; Koskinen, L.-O.D.; Malm, J. Risk factors for developing subdural hematoma: A registry-based study in 1457 patients with shunted idiopathic normal pressure hydrocephalus. J. Neurosurg. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Birkeland, P.; Lauritsen, J.; Poulsen, F.R. Subdural haematoma complicating shunting for normal pressure hydrocephalus in the setting of concomitant antiplatelet medication—A report of 11 cases. Br. J. Neurosurg. 2016, 30, 567–570. [Google Scholar] [CrossRef]
- Lemcke, J.; Meier, U.; Müller, C.; Fritsch, M.J.; Kehler, U.; Langer, N.; Kiefer, M.; Eymann, R.; Schuhmann, M.U.; Speil, A.; et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: A pragmatic, randomised, open label, multicentre trial (SVASONA). J. Neurol. Neurosurg. Psychiatry 2013, 84, 850–857. [Google Scholar] [CrossRef]
- Thompson, S.D.; Shand Smith, J.D.; Khan, A.A.; Luoma, A.M.V.; Toma, A.K.; Watkins, L.D. Shunting of the over 80s in normal pressure hydrocephalus. Acta Neurochir. 2017, 88, 490–994. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, S.; Kruschat, T.; Nitzsche, N.; Deinsberger, W. Gravitational shunt units may cause under-drainage in bedridden patients. Acta Neurochir. 2009, 151, 217–221. [Google Scholar] [CrossRef]
- Fountain, D.; Kolias, A.; Laing, R.; Hutchinson, P. The financial outcome of traumatic brain injury: A single centre study. Br. J. Neurosurg. 2016, 8697, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sæhle, T.; Farahmand, D.; Eide, P.K.; Tisell, M.; Wikkelsø, C. A randomized controlled dual-center trial on shunt complications in idiopathic normal-pressure hydrocephalus treated with gradually reduced or “fixed” pressure valve settings. J. Neurosurg. 2014, 121, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Petrella, G.; Czosnyka, M.; Keong, N.; Pickard, J.D.; Czosnyka, Z. How does CSF dynamics change after shunting? Acta Neurol. Scand. 2008, 118, 182–188. [Google Scholar] [CrossRef]
- Lalou, A.; Czosnyka, M.; Garnett, M.R.; Nabbanja, E.; Petrella, G.; Hutchinson, P.J.; Pickard, J.D.; Czosnyka, Z. Shunt infusion studies: Impact on patient outcome, including health economics. Acta Neurochir. 2020, 162, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Petrella, G.; Czosnyka, M.; Smielewski, P.; Allin, D.; Guazzo, E.P.; Pickard, J.D.; Czosnyka, Z.H. In vivo assessment of hydrocephalus shunt. Acta Neurol. Scand. 2009, 120, 317–323. [Google Scholar] [CrossRef]
- Chari, A.; Czosnyka, M.; Richards, H.K.; Pickard, J.D.; Czosnyka, Z.H. Hydrocephalus shunt technology: 20 years of experience from the Cambridge Shunt Evaluation Laboratory. J. Neurosurg. 2014, 120, 697–707. [Google Scholar] [CrossRef]
- Eide, K.; Due-Tønnessen, B.; Helseth, E.; Lundar, T. Assessment of intracranial pressure volume relationships in childhood: The lumbar infusion test versus intracranial pressure monitoring. Child’s Nerv. Syst. 2001, 17, 382–390. [Google Scholar] [CrossRef]
- Dias, S.F.; Lalou, A.; Spang, R.; Haas-Lude, K.; Garnett, M.; Fernandez, H.; Czosnyka, M.; Schuhmann, M.U.; Czosnyka, Z. Value of computerized shunt infusion study in assessment of pediatric hydrocephalus shunt function—A two center cross-sectional study. Child’s Nerv. Syst. 2019, 36, 59–71. [Google Scholar] [CrossRef]
- Sundström, N.; Andersson, K.; Marmarou, A.; Malm, J.; Eklund, A. Comparison between 3 infusion methods to measure cerebrospinal fluid outflow conductance: Clinical article. J. Neurosurg. 2010, 113, 1294–1303. [Google Scholar] [CrossRef]
- Eisenträger, A.; Sobey, I.; Czosnyka, M. Parameter estimations for the cerebrospinal fluid infusion test. Math. Med. Biol. 2013, 30, 157–174. [Google Scholar] [CrossRef]
- Andersson, K.; Manchester, I.R.; Laurell, K.; Cesarini, K.G.; Malm, J.; Eklund, A. Measurement of CSF dynamics with oscillating pressure infusion. Acta Neurol. Scand. 2013, 128, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, B.; Sundbärg, G.; Rehncrona, S. Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2002, 73, 721–726. [Google Scholar] [CrossRef]
- Swallow, D.M.A.A.; Fellner, N.; Varsos, G.V.; Czosnyka, M.; Smielewski, P.; Pickard, J.D.; Czosnyka, Z. Repeatability of cerebrospinal fluid constant rate infusion study. Acta Neurol. Scand. 2014, 130, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bech-Azeddine, R.; Gjerris, F.; Waldemar, G.; Czosnyka, M.; Juhler, M. Intraventricular or lumbar infusion test in adult communicating hydrocephalus? Practical consequences and clinical outcome of shunt operation. Acta Neurochir. 2005, 147, 1027–1036. [Google Scholar] [CrossRef]
- Kasprowicz, M.; Czosnyka, Z.; Czosnyka, M.; Momjian, S.; Juniewicz, H.; Pickard, J.D. Slight elevation of baseline intracranial pressure after fluid infusion into CSF space in patients with hydrocephalus. Neurol. Res. 2004, 26, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, J. CSF hydrodynamic studies in man. 1. Method of constant pressure CSF infusion. J. Neurol. Neurosurg. Psychiatry 1977, 40, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Juniewicz, H.; Kasprowicz, M.; Czosnyka, M.; Czosnyka, Z.; Gizewski, S.; Dzik, M.; Pickard, J.D. Analysis of intracranial pressure during and after the infusion test in patients with communicating hydrocephalus. Physiol. Meas. 2005, 26, 1039–1048. [Google Scholar] [CrossRef]
- Czosnyka, M.; Maksymowicz, W.; Batorski, L.; Koszewski WCzosnyka, Z. Comparison between Classic-Differential and Automatic Shunt Functioning on the basis of infusion tests. Acta Neurochir. 1990, 106, 1–8. [Google Scholar] [CrossRef]
- Qvarlander, S.; Malm, J.; Eklund, A. CSF dynamic analysis of a predictive pulsatility-based infusion test for normal pressure hydrocephalus. Med. Biol. Eng. Comput. 2014, 52, 75–85. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Poza, J.; Santamarta, D.; Abásolo, D.; Barrio, P.; Hornero, R. Spectral analysis of intracranial pressure signals recorded during infusion studies in patients with hydrocephalus. Med. Eng. Phys. 2013, 35, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Bech, R.A.; Bøgeskov, L.; Børgesen, S.E.; Juhler, M. Indications for shunt insertion or III ventriculostomy in hydrocephalic children, guided by lumbar and intraventricular infusion tests. Child’s Nerv. Syst. 1999, 15, 213–217. [Google Scholar] [CrossRef]
- Nabbanja, E.; Pickard, J.D.; Lalou, A.D.; Czosnyka, Z.H. Use of CSF infusion studies to unblock occluded hydrocephalus ventricular shunt catheters: A preliminary report of two patients. BMJ Case Rep. 2018. [Google Scholar] [CrossRef]
- Czosnyka, Z.; Czosnyka, M.; Lavinio, A.; Keong, N.; Pickard, J.D. Clinical testing of CSF circulation. Eur. J. Anaesthesiol. 2008, 25, 142–145. [Google Scholar] [CrossRef]
- Czosnyka, M.; Whitehouse, H.; Smielewski, P.; Simac, S.; Pickard, J.D. Testing of cerebrospinal compensatory reserve in shunted and non-shunted patients: A guide to interpretation based on an observationl study. J. Neurol. Neurosurg. Psychiatry 1996, 60, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, Z.; Boogaard, F.V.D.; Momjian, S.; Gelling, L.; Pickard, J.D. The relationship between CSF circulation and cerebrovascular pressure-reactivity in normal pressure hydrocephalus. Acta Neurochir. Suppl. 2005, 95, 207–211. [Google Scholar]
- Momjian, S.; Czosnyka, Z.; Czosnyka, M.; Pickard, J.D. Link between vasogenic waves of intracranial pressure and cerebrospinal fluid outflow resistance in normal pressure hydrocephalus. Br. J. Neurosurg. 2004, 18, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.A.; Czosnyka, Z.; Momjian, S.; Czosnyka, M.; Bech, R.A.; Pickardl, J.D. Intracranial baroreflex yielding an early Cushing response in human. In Intracranial Pressure and Brain Monitoring XII; Springer: Vienna, Austria, 2005; pp. 253–256. [Google Scholar]
- Momjian, S.; Owler, B.K.; Czosnyka, Z.; Czosnyka, M.; Pena, A.; Pickard, J.D. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004, 127, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Owler, B.K.; Momjian, S.; Czosnyka, Z.; Czosnyka, M.; Péna, A.; Harris, N.G.; Smielewski, P.; Fryer, T.D.; Donovan, T.; Coles, J.; et al. Normal pressure hydrocephalus and cerebral blood flow: A PET study of baseline values. J. Cereb. Blood Flow Metab. 2004, 24, 17–23. [Google Scholar] [CrossRef]
- Owler, B.K.; Pena, A.; Momjian, S.; Czosnyka, Z.; Czosnyka, M.; Harris, N.G.; Smielewski, P.; Fryer, T.; Donvan, T.; Carpenter, A.; et al. Changes in Cerebral Blood Flow During Cerebrospinal Fluid Pressure Manipulation in Patients with Normal Pressure Hydrocephalus: A Methodological Study. J Cereb. Blood Flow Metab. 2004, 24, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Smielewski, P.; Timofeev, I.; Lavinio, A.; Guazzo, E.; Hutchinson, P.; Pickard, J.D. Intracranial Pressure: More Than a number. Neurosurg. Focus 2007, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lalou, A.D.; McTaggart, J.S.; Czosnyka, Z.H.; Garnett, M.R.; Krishnakumar, D. Czosnyka MCerebrospinal fluid dynamics, i.n.pediatric pseudotumor cerebri syndrome. Child’s Nerv. Syst. 2020, 36, 73–86. [Google Scholar] [CrossRef]
- Radolovich, D.K.; Czosnyka, M.; Timofeev, I.; Lavinio, A.; Kim, D.J.; Jaeger, M.; Hutchinson, P.; Gupta, A.; Pickard, J.D.; Smielewski, P. Transient changes in brain tissue oxygen in response to modifications of cerebral perfusion pressure: An observational study. Anesthesia Analg. 2010, 110, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lublinsky, S.; Kesler, A.; Friedman, A.; Horev, A.; Shelef, I. Quantifying response to intracranial pressure normalization in idiopathic intracranial hypertension via dynamic neuroimaging. J. Magn. Reson. Imaging. 2018, 47, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Care, N.; Cardim, D.; Schmidt, B.; Robba, C.; Donnelly, J. Transcranial Doppler Monitoring of Intracranial Pressure Plateau Waves. Neurocrit. Care 2017, 26, 330–338. [Google Scholar]
- Lalou, A.D.; Levrini, V.; Czosnyka, M.; Gergelé, L.; Garnett, M.; Kolias, A.; Hutchinson, P.J.; Czosnyka, Z. Cerebrospinal fluid dynamics in non-acute post-traumatic ventriculomegaly. Fluids Barriers CNS 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soehle, M.; Czosnyka, M.; Pickard, J.D.; Kirkpatrick, P.J. Continuous Assessment of Cerebral Autoregulation in Subarachnoid Hemorrhage. Anesthesia Analg. 2004, 98, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J. Physiology and Pathophysiology of the Cerebrospinal Fluid. J. Neurol. Neurosurg. Psychiatry 1988, 51, 469–470. [Google Scholar] [CrossRef][Green Version]
- Czosnyka, M.; Czosnyka, Z.; Agarwal-Harding, K.J.; Pickard, J.D. Modeling of cerebrospinal fluid dynamics: Legacy of Professor Anthony Marmarou. Hydrocephalus 2012, 113. [Google Scholar] [CrossRef]
- Marmarou, A.; Maset, A.L.; Ward, J.D.; Choi, S.; Brooks, D.; Lutz, H.A.; Moulton, R.J.; Muizelaar, J.P.; Desalles, A.; Young, H.F. Contribution of CSF and vascular factors to elevation of ICP in severely head-injured patients. J. Neurosurg. 1987, 66, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Abd-Elfattah Foda, M.A.; Bandoh, K.; Yoshihara, M.; Yamamoto, T.; Tsuji, O.; Zasler, N.; Ward, J.D.; Young, H.F. Posttraumatic ventriculomegaly: Hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J. Neurosurg. 1996, 85, 1026–1035. [Google Scholar] [CrossRef]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; Black, P.M. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2012, 40, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Puppo, C.; Camacho, J.; Varsos, G.V.; Yelicich, B.; Gómez, H.; Moraes, L.; Biestro, A.; Czosnyka, M. Cerebral Critical Closing Pressure: Is the Multiparameter Model Better Suited to Estimate Physiology of Cerebral Hemodynamics? Neurocrit Care 2016, 25, 446–454. [Google Scholar] [CrossRef]
- Boon, A.; Tans, J.T.J.; Delwe, E.J.; Hanlo, P.W.; Wurzer, J.A.L.; Hermans, J. Dutch normal pressure hydrocephalus study : Baseline characteristics with emphasis on clinical findings. Eur. J. Neurol. 1997, 4, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M.; Miyajima, M.; Nakajima, M.; Ogino, I.; Arai, H. Impact of cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus on the amyloid cascade. PLoS ONE 2015, 10, e0119973. [Google Scholar] [CrossRef]
- Lewis, P.M.; Smielewski, P.; Pickard, J.D.; Czosnyka, M. Dynamic cerebral autoregulation: Should intracranial pressure be taken into account? Acta Neurochir. 2007, 149, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, J.; Von Essen, C.; Zwetnow, N.N. The Pressure-volume curve of the cerebrospinal fluid space in dogs. Acta Neurol. Scand. 1973, 49, 162. [Google Scholar] [CrossRef]
- Weerakkody, R.A.; Czosnyka, M.; Zweifel, C.; Castellani, G.; Smielewski, P.; Keong, N.; Haubrich, C.; Pickard, J.; Czosnyka, Z. Slow vasogenic fluctuations of intracranial pressure and cerebral near infrared spectroscopy-an observational study. Acta Neurochir. 2010, 152, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Klinge, P.M.; Brooks, D.J.; Samii, A.; Weckesser, E.; van den Hoff, J.; Fricke, H.; Brinker, T.; Knalpp, W.H.; Berding, G. Correlates of local cerebral blood flow (CBF) in normal pressure hydrocephalus patients before and after shunting—A retrospective analysis of [15O]H2O PET-CBF studies in 65 patients. Clin. Neurol. Neurosurg. 2008, 110, 369–375. [Google Scholar] [CrossRef]
- Czosnyka, M.; Price, D.J.; Williamson, M. Monitoring of cerebrospinal dynamics using continuous analysis of intracranial pressure and cerebral perfusion pressure in head injury. Acta Neurochir. 1994, 126, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lalou, A.D.; Czosnyka, M.; Czosnyka, Z.H.; Krishnakumar, D.; Pickard, J.D.; Higgins, N.J. Coupling of CSF and sagittal sinus pressure in adult patients with pseudotumour cerebri. Acta Neurochir. 2020, 162, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Hiler, M.; Czosnyka, M.; Hutchison, P.; Balestreri, M.; Smielewski, P.; Matta, B.; Pickard, J.D. Predictive value of initial computerized tomography scan, intracranial pressure, and state of autoregulation in patients with traumatic brain injury. J. Neurosurg. 2006, 104, 731–737. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Piechnik, S.; Schmidt, E.A.; Al-Rawi, P.G.; Kirkpatrick, P.J.; Pickard, J. Hemodynamic characterization of intracranial pressure plateau waves in head-injured patients. J. Neurosurg. 1999, 91, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Matta, B.F.; Smielewski, P.; Kirkpatrick, P.J.; Pickard, J.D. Cerebral perfusion pressure in head-injured patients: A noninvasive assessment using transcranial Doppler ultrasonography. J. Neurosurg. 2009, 88, 802–808. [Google Scholar] [CrossRef]
- Lu, C.W.; Czosnyka, M.; Shieh, J.S.; Smielewska, A.; Pickard, J.D.; Smielewski, P. Complexity of intracranial pressure correlates with outcome after traumatic brain injury. Brain 2012, 135, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Momjian, S.; Schmidt, E. Calculation of the resistance to CSF outflow. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1354. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A.; Smielewski, P.; Chambers, I.; Alperin, N.; Malm, J.; Czosnyka, M.; Marmarou, A. Assessment of cerebrospinal fluid outflow resistance. Med. Biol. Eng. Comput. 2007, 45, 719–735. [Google Scholar] [CrossRef]
- Andersson, N.; Malm, J.; Eklund, A. Dependency of cerebrospinal fluid outflow resistance on intracranial pressure: Clinical article. J. Neurosurg. 2008, 109, 918–922. [Google Scholar] [CrossRef]
- Davson, H.; Domer, F.R.; Hollingsworth, J.R. The mechanism of drainage of the cerebrospinal fluid. Brain 1973, 96, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Kristensen, B.; Markgren, P.; Ekstedt, J. CSF hydrodynamics in idiopathic intracranial hypertension: A long-term study. Neurology 1992, 42, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, J. CSF hydrodynamic studies in man. 2. Normal hydrodynamic variables related to CSF pressure and flow. J. Neurol. Neurosurg. Psychiatry 1978, 41, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Semenyutin, V.; Aliev, V.; Bersnev, V.; Patzak, A.; Rozhchenko, L.; Kozlov, A.; Ramazanov, S. Informativity of pulsatility index and cerebral autoregulation in hydrocephalus. Perspect. Med. 2012, 1, 311–315. [Google Scholar] [CrossRef][Green Version]
- Albeck, M.J.; Borgesen, S.E.; Gjerris, F.; Schmidt, J.F.; Sorensen, P.S. Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J. Neurosurg. 1991, 74, 597–600. [Google Scholar] [CrossRef]
- Czosnyka, M.; Czosnyka, Z.H.; Whitfield, P.C.; Donovan, T.; Pickard, J.D. Age dependence of cerebrospinal pressure—Volume compensation in patients with hydrocephalus. J. Neurosurg. 2009, 94, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Lavinio, A.; Czosnyka, Z.; Czosnyka, M. Cerebrospinal fluid dynamics: Disturbances and diagnostics. Eur. J. Anaesthesiol. 2008, 25, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Malm, J. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Contin. Lifelong Learn. Neurol. 2016, 22, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Sundström, N.; Cesarini, K.G.; Edsbagge, M.; Kristensen, B.; Leijon, G.; Eklund, A. Implementation of a new CSF dynamic device: A multicenter feasibility study in 562 patients. Acta Neurol. Scand. 2012, 125, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Jacobsson, J.; Birgander, R.; Eklund, A. Reference values for CSF outflow resistance and intracranial pressure in healthy elderly. Neurology 2011, 76, 903–909. [Google Scholar] [CrossRef]
- Borgesen, S.E.; Gjerris, F. The predictive value of conductance to outflow of CSF in normal pressure hydrocephalus. Brain 1982, 105, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Nabbanja, E.; Czosnyka, M.; Keong, N.C.; Garnett, M.; Pickard, J.D.; Lalou, D.A.; Czosnyka, Z. Is there a link between ICP-derived infusion test parameters and outcome after shunting in normal pressure hydrocephalus? Acta Neurochir. Suppl. 2018, 126, 229–232. [Google Scholar]
- Lalou, A.D.; Asgari, S.; Garnett, M.; Nabbanja, E.; Czosnyka, M.; Czosnyka, Z. The Role of CSF Dynamics in Normal Pressure Hydrocephalus Diagnosis and Shunt Prognostication. Acta Neurochir. Suppl. 2020, in press. [Google Scholar]
- Kim, D.J.; Czosnyka, Z.; Keong, N.; Radolovich, D.K.; Smielewski, P.; Sutcliffe, M.P.; Pickard, J.D.; Czosnyka, M. Index of cerebrospinal fluid compensatory reserve in hydrocephalus. Neurosurgery 2009, 64, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Owler, B.K.; Parker, G.; Halmagyi, G.M.; Johnston, I.H.; Besser, M.; Pickard, J.D.; Higgins, J. Cranial venous outflow obstruction and psudotumor cerebri syndrome. Adv. Tech. Stand. Neurosurg. 2005, 30, 107–174. [Google Scholar]
- Karahalios, D.G.; Rekate, H.L.; Khayata, M.H.; Apostolides, P.J.; Kokmen, E.; Whisnant, J.P.; O’Fallon, W.M.; Chu, C.-P.; Beard, C.M. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996, 46, 198–202. [Google Scholar] [CrossRef]
- Higgins, J.N.P.; Owler, B.K.; Cousins, C.; Pickard, J.D. Venous sinus stenting for refractory benign intracranial hypertension. Lancet 2002, 359, 228–230. [Google Scholar] [CrossRef]
- Owler, B.K.; Parker, G.; Halmagyi, G.M.; Dunne, V.G.; Grinnell, V.; McDowell, D.; Besser, M. Pseudotumor cerebri syndrome: Venous sinus obstruction and its treatment with stent placement. J. Neurosurg. 2003, 98, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.; Owler, B.; Pickard, J.D. The Pseudotumor Cerebri Syndrome: Pseudotumor Cerebri, Idiopathic Intracranial Hypertension, Benign Intracranial Hypertension and Related Conditions; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Pickard, J.D.; Czosnyka, Z.; Czosnyka, M.; Owler, B.; Higgins, J.N. Coupling of sagittal sinus pressure and cerebrospinal fluid pressure in idiopathic intracranial hypertension—A preliminary report. Acta Neurochir. Suppl. 2008, 102, 283–285. [Google Scholar] [PubMed]
- Czosnyka, M.; Guazzo, E.; Whitehouse, M.; Smielewski, P.; Czosnyka, Z.; Kirkpatrick, P.; Piechnik, S.; Pickard, J.D. Significance of intracranial pressure waveform analysis after head injury. Acta Neurochir. 1996, 138, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, M.; Lalou, D.A.A.; Czosnyka, M.; Garnett, M.; Czosnyka, Z. Intracranial pressure, its components and cerebrospinal fluid pressure–volume compensation. Acta Neurol. Scand. 2016, 134, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K.; Sorteberg, W. Association among intracranial compliance, intracranial pulse pressure amplitude and intracranial pressure in patients with intracranial bleeds. Neurol. Res. 2007, 29, 798–802. [Google Scholar] [CrossRef]
- Carrera, E.; Kim, D.-J.; Castellani, G.; Zweifel, C.; Czosnyka, Z.; Kasprowicz, M.; Smielewski, P.; Pickard, J.D.; Czosnyka, M. What Shapes Pulse Amplitude of Intracranial Pressure? J. Neurotrauma 2010, 27, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Budohoski, K.P.; Schmidt, B.; Smielewski, P.; Kasprowicz, M.; Plontke, R.; Pickard, J.D.; Klingelhöfer, J.; Czosnyka, M. Non-Invasively Estimated ICP Pulse Amplitude Strongly Correlates with Outcome After TBI. Acta Neurochir. Suppl. 2012, 114, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, Z.; Keong, N.; Kim, D.; Radolovich, D.; Smielewski, P.; Lavinio, A.; Schmidt, E.A.; Momjian, S.; Owler, B.; Pickard, J.D.; et al. Pulse amplitude of intracranial pressure waveform in hydrocephalus. Acta Neurochir. Suppl. 2008, 102, 137–140. [Google Scholar] [PubMed]
- Lalou, A.-D.; Levrini, V.; Garnett, M.; Nabbanja, E.; Kim, D.-J.; Gergele, L.; Bjornson, A.; Czosnyka, Z.; Czosnyka, M. Validation of Davson’s equation in patients suffering from idiopathic Normal Pressure Hydrocephalus. Acta Neurochir. 2018, 160, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Maksymowicz, W.; Czosnyka, M.; Koszewski, W.; Szymanska, A.; Traczewski, W. The role of cerebrospinal compensatory parameters in the estimation of functioning of implanted shunt system in patients with communicating hydrocephalus (preliminary report). Acta Neurochir. 1989, 101, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sliwka, S. Static and Dynamic Cerebrospinal Elastance—Clinical Verification. In Intracranial Press VI; Springer: Berlin/Heidelberg, Germany, 1986; pp. 84–88. [Google Scholar]
- Tisell, M.; Edsbagge, M.; Stephensen, H.; Czosnyka, M.; Wikkelsø, C. Elastance correlates with outcome after endoscopic third ventriculostomy in adults with hydrocephalus caused by primary aqueductal stenosis. Neurosurgery 2002, 50, 70–77. [Google Scholar]
- Perry, A.; Graffeo, C.S.; Fattahi, N.; ElSheikh, M.M.; Cray, N.; Arani, A.; Ehman, R.L.; Glaser, K.J.; Manduca, A.; Meyer, F.B.; et al. Clinical Correlation of Abnormal Findings on Magnetic Resonance Elastography in Idiopathic Normal Pressure Hydrocephalus. World Neurosurg. 2017, 99, 695–700.e1. [Google Scholar] [CrossRef] [PubMed]
- Sobey, I.; Eisenträger, A.; Wirth, B.; Czosnyka, M. Multi-fluid poro-elastic modelling of the CSF infusion test. In Proceedings of the 6th World Congress of Biomechanics (WCB 2010), Singapore, 1–6 August 2010; pp. 362–365. [Google Scholar]
- Lalou, D.; Czosnyka, M.; Donnelly, J.; Lalou, D.A.; Czosnyka, M.; Donnelly, J.; Lavinio, A.; Pickard, J.D.; Garnett, M.; Czosnyka, Z. Influence of general anaesthesia on slow waves of intracranial pressure. Neurol. Res. 2016, 38, 587–592. [Google Scholar] [CrossRef]
- Krauss, J.K.; Droste, D.W.; Bohus, M.; Regel, J.P.; Scheremet, R.; Riemann, D.; Seeger, W. The relation of intracranial pressure B-waves to different sleep stages in patients with suspected normal pressure hydrocephalus. Acta Neurochir. 1995, 136, 195–203. [Google Scholar] [CrossRef]
- Eklund, A.; Ågren-Wilsson, A.; Andersson, N.; Bergenheim, A.T.; Koskinen, L.O.D.; Malm, J. Two computerized methods used to analyze intracranial pressure B waves: Comparison with traditional visual interpretation. J. Neurosurg. 2001, 94, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Eaton, B.; Paradiso, S.; Mina, M.; Hudetz, A.G.; Bolinger, L. Source of low-frequency fluctuations in functional MRI signal. J. Magn. Reson. Imaging 2008, 27, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-J.; Czosnyka, Z.; Kasprowicz, M.; Smieleweski, P.; Baledent, O.; Guerguerian, A.-M.; Pickard, J.D.; Czosnyka, M. Continuous Monitoring of the Monro-Kellie Doctrine: Is It Possible? J. Neurotrauma 2012, 29, 1354–1363. [Google Scholar] [CrossRef]
- Eide, P.K. A new method for processing of continuous intracranial pressure signals. Med. Eng. Phys. 2006, 28, 579–587. [Google Scholar] [CrossRef]
- Lee, J.K.; Kibler, K.K.; Benni, P.B.; Easley, R.B.; Czosnyka, M.; Smielewski, P.; Koehler, R.C.; Shaffner, D.H.; Brady, K.M. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 2009, 40, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Czosnyka, M.; Lavinio, A.; Castellani, G.; Kim, D.-J.; Carrera, E.; Pickard, J.D.; Kirkpatrick, P.J.; Smielewski, P. A comparison study of cerebral autoregulation assessed with transcranial Doppler and cortical laser Doppler flowmetry. Neurol. Res. 2009, 32, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Brady, K.; Reinhard, M.; Smielewski, P.; Steiner, L.A. Monitoring of cerebrovascular autoregulation: Facts, myths, and missing links. Neurocritical Care 2009, 10, 373–386. [Google Scholar] [CrossRef]
- Steiner, L.A.; Coles, J.P.; Johnston, A.J.; Chatfield, D.A.; Smielewski, P.; Fryer, T.D.; Aigbirhio, F.I.; Clark, J.C.; Pickard, J.D.; Menon, D.K.; et al. Assessment of cerebrovascular autoregulation in head-injured patients: A validation study. Stroke 2003, 34, 2404–2409. [Google Scholar] [CrossRef]
- Lewis, P.M.; Smielewski, P.; Pickard, J.D.; Czosnyka, M. Slow oscillations in middle cerebral artery cerebral blood flow velocity and aging. Neurol. Res. 2007, 29, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, H.; Andersson, N.; Eklund, A.; Malm, J.; Tisell, M.; Wikkelsö, C. Objective B wave analysis in 55 patients with non-communicating and communicating hydrocephalus. J. Neurol. Neurosurg. Psychiatry 2005, 76, 965–970. [Google Scholar] [CrossRef]
- Schuhmann, M.U.; Sood, S.; McAllister, J.P.; Jaeger, M.; Ham, S.D.; Czosnyka, Z.; Czosnyka, M. Value of overnight monitoring of intracranial pressure in hydrocephalic children. Pediatrics Neurosurg. 2008, 44, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, R.A.; Czosnyka, M.; Zweifel, C.; Castellani, G.; Smielewski, P.; Brady, K.; Pickard, J.D.; Czosnyka, Z. Near Infrared Spectroscopy as Possible Non-invasive Monitor of Slow Vasogenic ICP Waves. Acta Neurochir. Suppl. 2012, 114, 181–185. [Google Scholar] [PubMed]
- Balestreri, M.; Czosnyka, M.; Steiner, L.A.; Schmidt, E.; Smielewski, P.; Matta, B.; Pickard, J.D. Intracranial hypertension: What additional information can be derived from ICP waveform after head injury? Acta Neurochir. 2004, 146, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Czosnyka, M.; Harland, S.; Varsos, G.V.; Cardim, D.; Robba, C.; Liu, X.; Ainslie, P.N.; Smielewski, P. Cerebral haemodynamics during experimental intracranial hypertension. J. Cereb. Blood Flow Metab. 2017, 37, 694–705. [Google Scholar] [CrossRef]
- Czosnyka, M.; Pickard, J.D.; Czosnyka Marek Pickard, J.D.; Czosnyka, M.; Pickard, J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Czosnyka, M.; Adams, H.; Cardim, D.; Kolias, A.G.; Zeiler, F.A.; Lavinio, A.; Aries, M.; Robba, C.; Smielewski, P.; et al. Twenty-Five Years of Intracranial Pressure Monitoring After Severe Traumatic Brain Injury: A Retrospective, Single-Center Analysis. Neurosurgery 2018, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Castellani, G.; Zweifel, C.; Kim, D.J.; Carrera, E.; Radolovich, D.K.; Smielewski, P.; Hutchinson, P.J.; Pickard, J.D.; Czosnyka, M. Plateau waves in head injured patients requiring neurocritical care. Neurocrit. Care 2009, 11, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Israelsson, H.; Carlberg, B.; Wikkelsö, C.; Laurell, K.; Kahlon, B.; Leijon, G.; Eklund, A.; Malm, J. Vascular risk factors in INPH: A prospective case-control study (the INPH-CRasH study). Neurology 2017, 88, 577–585. [Google Scholar] [CrossRef]
- Zweifel, C.; Lavinio, A.; Steiner, L.A.; Radolovich, D.; Smielewski, P.; Timofeev, I.; Hiler, M.; Balestreri, M.; Kirkpatrick, P.J.; Pickard, J.D.; et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Czosnyka, M.; Piechnik, S.; Richards, H.K.; Kirkpatrick, P.; Smielewski, P.; Pickard, J.D. Contribution of mathematical modelling to the interpretation of bedside tests of cerebrovascular autoregulation. J. Neurol. Neurosurg. Psychiatry 1997, 63, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.A.; Pfister, D.; Strebel, S.P.; Radolovich, D.; Smielewski, P.; Czosnyka, M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocritical Care 2009, 10, 122–128. [Google Scholar] [CrossRef]
- Lemaire, J.; Khalil, T.; Cervenansky, F.; Gindre, G.; Boire, J.Y.; Bazin, J.E.; Irthum, B.; Chazal, J. Slow Pressure Waves in the Cranial Enclosure. Acta Neurochir. 2002, 144, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Balestreri, M.; Czosnyka, M.; Steiner, L.A.; Hiler, M.; Schmidt, E.A.; Matta, B.; Menon, D.; Hutchinson, P.; Pickard, J.D. Association between outcome, cerebral pressure reactivity and slow ICP waves following head injury. Acta Neurochir. Suppl. 2005, 95, 25–28. [Google Scholar] [PubMed]
- Czosnyka, M.; Smielewski, P.; Czosnyka, Z.; Piechnik, S.; Steiner, L.A.; Schmidt, E.; Gooskens, I.; Soehle, M.; Lang, E.W.; Matta, B.F.; et al. Continuous assessment of cerebral autoregulation: Clinical and laboratory experience. Acta Neurochir. Suppl. 2003, 86, 581–585. [Google Scholar] [PubMed]
- Gooskens, I.; Schmidt, E.A.; Czosnyka, M.; Piechnik, S.K.; Smielewski, P.; Kirkpatrick, P.J.; Pickard, J.D.; Piechnik, S.K. Pressure-autoregulation, CO2 reactivity and asymmetry of haemodynamic parameters in patients with carotid artery stenotic disease. A clinical appraisal. Acta Neurochir. 2003, 145, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Piechnik, S.K.; Czosnyka, M.; Richards, H.K.; Whitfield, P.C.; Pickard, J.D. Cerebral venous blood outflow: A theoretical model based on laboratory simulation. Neurosurgery 2001, 49, 1214–1223. [Google Scholar] [PubMed]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Laing, R.J.; Menon, D.; Pickard, J.D. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 1997, 41, 11–19. [Google Scholar] [CrossRef]
- Brady, K.; Joshi, B.; Zweifel, C.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Hogue, C.W. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010, 41, 1951–1956. [Google Scholar] [CrossRef]
- Guendling, K.; Smielewski, P.; Czosnyka, M.; Lewis, P.; Nortje, J.; Timofeev, I.; Hutchinson, P.J.; Pickard, J.D. Use of ICM+ software for on-line analysis of intracranial and arterial pressures in head-injured patients. Acta Neurochir. Suppl. 2006, 2005, 108–113. [Google Scholar]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Helmy, A.; Manktelow, A.; Carrera, E.; Brady, K.M.; Hutchinson, P.J.; Menon, D.K.; Pickard, J.D.; et al. Noninvasive Monitoring of Cerebrovascular Reactivity with Near Infrared Spectroscopy in Head-Injured Patients. J. Neurotrauma 2010, 27, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Hodge, C.J.; Connolly, E.S.; Kelly, D.F.; Glenn, T. Pressure autoregulation and positron emission tomography-derived cerebral blood flow acetazolamide reactivity in patients with carotid artery stenosis: Comments. Neurosurgery 2004, 55, 67–68. [Google Scholar]
- Czosnyka, M.; Czosnyka, Z.; Smielewski, P. Pressure reactivity index: Journey through the past 20 years. Acta Neurochir. 2017, 159, 2063–2065. [Google Scholar] [CrossRef]
- Szczepański, T.A.; Weiser, A.; Zub, W.L.; Jarmundowicz, W.; Koźba-Gosztyła, M.; Czapiga, B. Assessment of cerebral blood flow during infusion test in the diagnosis of normal pressure hydrocephalus. Adv. Clin. Exp. Med. 2012, 21, 55–61. [Google Scholar]
- Czosnyka, Z.H.; Czosnyka, M.; Whitfield, P.C.; Donovan, T.; Pickard, J.D. Cerebral autoregulation among patients with symptoms of hydrocephalus. Neurosurgery 2002, 50, 526–533. [Google Scholar] [PubMed]
- Tuniz, F.; Vescovi, M.C.; Bagatto, D.; Drigo, D.; De Colle, M.C.; Maieron, M.; Skrap, M. The role of perfusion and diffusion MRI in the assessment of patients affected by probable idiopathic normal pressure hydrocephalus. A cohort—Prospective preliminary study. Fluids Barriers CNS 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El Sankari, S.; Gondry-jouet, C.; Fichten, A.; Godefroy, O.; Serot, J.M.; Deramond, H.; Meyer, M.E.; Baledent, O. Cerebrospinal fluid and blood flow in mild cognitive impairment and Alzheimer’s disease: A differential diagnosis from idiopathic normal pressure hydrocephalus Cerebrospinal fluid and blood flow in mild cognitive impairment and Alzheimer’ s disease: A differential diagnosis from idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 2011, 8, 1–11. [Google Scholar]
- Silverberg, G.D.; Mayo, M.; Saul, T.; Rubenstein, E.; McGuire, D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: A hypothesis. Lancet Neurol. 2003, 2, 506–511. [Google Scholar] [CrossRef]
- Miyamoto, J.; Tatsuzawa, K.; Inoue, Y.; Imahori, Y.; Mineura, K. Oxygen metabolism changes in patients with idiopathic normal pressure hydrocephalus before and after shunting operation. Acta Neurol. Scand. 2007, 116, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.M.; Masahira, N.; Kawanishi, Y.; Fujimoto, Y.; Shimizu, K. Preoperative Acetazolamide SPECT is Useful for Predicting Outcome of Shunt Operation in Idiopathic Normal Pressure Hydrocephalus Patients. Clin. Nucl. Med. 2013, 38, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Hawkes, C.A.; Kalaria, R.N.; Werring, D.J.; Carare, R.O. White matter changes in dementia: Role of impaired drainage of interstitial fluid. Brain Pathol. 2014, 25, 63–78. [Google Scholar] [CrossRef]
- Corkill, R.G.; Garnett, M.R.; Blamire, A.M.; Rajagopalan, B.; Cadoux-Hudson, T.A.D.; Styles, P. Multi-modal MRI in normal pressure hydrocephalus identifies pre-operative haemodynamic and diffusion coefficient changes in normal appearing white matter correlating with surgical outcome. Clin. Neurol. Neurosurg. 2003, 105, 193–202. [Google Scholar] [CrossRef]
- Kristensen, B. Regional cerebral blood flow, white matter abnormalities, and cerebrospinal fluid hydroodynalitiesin patients with idiopathic adult hydrocephalus syndrome. J. Neurol. Neurosurg. Psychiatry 1996, 282–288. [Google Scholar] [CrossRef]
- Alperin, N.; Oliu, C.J.; Bagci, A.M.; Lee, S.H.; Kovanlikaya, I.; Adams, D.; Katzen, H.; Ivkovic, M.; Heier, L.; Relkin, N. Low-dose acetazolamide reverses periventricular white matter hyperintensities in iNPH. Neurology 2014, 82, 1347–1351. [Google Scholar] [CrossRef]
- Tullberg, M.; Jensen, C.; Ekholm, S.; Wikkelsø, C. Normal Pressure Hydrocephalus: Vascular White Matter Changes on MR Images Must Not Exclude Patients from Shunt Surgery. Am. J. Neuroradiol. 2001, 22, 1665–1673. [Google Scholar] [PubMed]
- Peterson, K.A.; Savulich, G.; Jackson, D.; Killikelly, C.; Pickard, J.D.; Sahakian, B.J. The effect of shunt surgery on neuropsychological performance in normal pressure hydrocephalus: A systematic review and meta-analysis. J. Neurol. 2016, 263, 1669–1677. [Google Scholar] [CrossRef]
- Toma, A.K.; Papadopoulos, M.C.; Stapleton, S.; Kitchen, N.D.; Watkins, L.D. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir. 2013, 155, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Klinge, P.; Berding, G.; Brinker, T.; Schuhmann, M.; Knapp, W.H.S.M. PET-studies in idiopathic chronic hydrocephalus before and after shunt-treatment: The role of risk factors for cerebrovascular disease (CVD) on cerebral hemodynamics. Acta Neurochir. Suppl. 2002, 81, 43–45. [Google Scholar] [PubMed]
- Boon, A.J.; Tans, J.T.; Delwel, E.J.; Egeler-Peerdeman, S.M.; Hanlo, P.W.; Wurzer, H.A.; Avezaat, C.J.; De Jong, D.A.; Gooskens, R.H.; Hermans, J. Dutch Normal-Pressure Hydrocephalus Study: Randomized comparison of low- and medium-pressure shunts. J. Neurosurg. 1998, 88, 490–495. [Google Scholar] [CrossRef]
- Yamada, S.; Kimura, T.; Jingami, N.; Atsuchi, M.; Hirai, O.; Tokuda, T.; Miyajima, M.; Kazui, H.; Mori, E.; Ishikawa, M. Disability risk or unimproved symptoms following shunt surgery in patients with idiopathic normal-pressure hydrocephalus: Post hoc analysis of SINPHONI-2. J. Neurosurg. 2016, 126, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Lundin, F.; Ledin, T.; Wikkelsø, C.; Leijon, G. Postural function in idiopathic normal pressure hydrocephalus before and after shunt surgery: A controlled study using computerized dynamic posturography (EquiTest). Clin. Neurol. Neurosurg. 2013, 115, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, Z.; Czosnyka, M.; Richards, H.; Pickard, J. Posture-related overdrainage: Comparison of the performance of 10 hydrocephalus shunts in vivo. Neurosurgery 1998, 42, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.J.; Kurlan, R.; Schwalb, J.M.; Cusimano, M.D.; Gronseth, G.; Gloss, D. Practice guideline: Idiopathic normal pressure hydrocephalus: Response to shunting and predictors of response. Neurology 2015, 85, 2063–2071. [Google Scholar] [CrossRef]
- Farahmand, D.; Qvarlander, S.; Malm, J.; Wikkelso, C.; Eklund, A.; Tisell, M. Intracranial pressure in hydrocephalus: Impact of shunt adjustments and body positions. J. Neurol. Neurosurg. Psychiatry 2015, 86, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.J.; Tans, J.T.; Delwel, E.J.; Egeler-Peerdeman, S.M.; Hanlo, P.W.; Wurzer, H.A.; Hermans, J. The Dutch Normal-Pressure Hydrocephalus Study. How to select patients for shunting? An analysis of four diagnostic criteria. Surg. Neurol. 2000, 53, 201–207. [Google Scholar] [CrossRef]
- Dupepe, E.B.; Hopson, B.; Johnston, J.M.; Rozzelle, C.J.; Oakes, W.J.; Blount, J.P.; Rocque, B.G. Rate of shunt revision as a function of age in patients with shunted hydrocephalus due to myelomeningocele. Neurosurg. Focus 2016, 41, E6. [Google Scholar] [CrossRef] [PubMed]
- Spirig, J.M.; Frank, M.N.; Regli, L.; Stieglitz, L.H. Shunt age-related complications in adult patients with suspected shunt dysfunction. A recommended diagnostic workup. Acta Neurochir. 2017, 159, 1421–1428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, A.; Sankey, E.W.; Jusué-Torres, I.; Patel, M.A.; Elder, B.D.; Goodwin, C.R.; Hoffberger, J.; Lu, J.; Rigamonti, D. Clinical outcomes after ventriculoatrial shunting for idiopathic normal pressure hydrocephalus. Clin. Neurol. Neurosurg. 2016, 143, 34–38. [Google Scholar] [CrossRef]
- Nikas, D.C.; Post, A.F.; Choudhri, A.F.; Mazzola, C.A.; Mitchell, L.; Flannery, A.M. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines. Part 10: Change in ventricle size as a measurement of effective treatment of hydrocephalus. J. Neurosurg. Pediatr. 2014, 14, 77–81. [Google Scholar] [CrossRef]
- Sugimoto, T.; Uranishi, R.; Yamada, T. Gradually Progressive Symptoms of Normal Pressure Hydrocephalus Caused by an Arachnoid Cyst in the Fourth Ventricle: A Case Report. World Neurosurg. 2016, 85, 364.e19–364.e22. [Google Scholar] [CrossRef]
- Bradley, W.G. Magnetic Resonance Imaging of Normal Pressure Hydrocephalus. Semin. Ultrasound CT MRI 2016, 37, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Harris, N.G.; Bolton, M.D.; Czosnyka, M.; Pickard, J.D. Communicating hydrocephalus: The biomechanics of progressive ventricular enlargement revisited. Acta Neurochir. Suppl. 2002, 81, 59–63. [Google Scholar]
- Yamashita, F.; Sasaki, M.; Takahashi, S.; Matsuda, H.; Kudo, K.; Narumi, S.; Terayama, Y.; Asada, T. Detection of changes in cerebrospinal fluid space in idiopathic normal pressure hydrocephalus using voxel-based morphometry. Neuroradiology 2009, 52, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Czosnyka, Z.; Czosnyka, M.; Pickard, J. A laboratory model of testing shunt performance after implantation. Br. J. Neurosurg. 2002, 16, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, Z.H.; Czosnyka, M.; Richards, H.K.; Pickard, J.D. Laboratory evaluation of the phoenix CRx diamond valve. Neurosurgery 2001, 48, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, Z.H.; Czosnyka, M.; Pickard, J.D. Shunt testing in vivo: A method based on the data from the Uk shunt evaluation laboratory. Acta Neurochir. Suppl. 2002, 81, 27–30. [Google Scholar]
- Czosnyka, M.; Whitehouse, H.; Pickard, J.D. Hydrodynamic properties of hydrocephalus shunts: United Kingdom shunt evaluation laboratory. J. Neurol. Neurosurg. Psychiatry 1997, 62, 43–50. [Google Scholar] [CrossRef]
- Czosnyka, M.; Richards, H.K.; Pickard, J.D. Laboratory testing of hydrocephalus shunts—Conclusion of the U.K. Shunt Evaluation Programme. Acta Neurochir. 2002, 144, 525–538. [Google Scholar] [CrossRef]
- Allin, D.M.; Czosnyka, Z.H.; Czosnyka, M.; Richards, H.K.; Pickard, J.D. In vitro hydrodynamic properties of the Miethke proGAV hydrocephalus shunt. Cerebrospinal Fluid Res. 2006, 3, 1–6. [Google Scholar]
- Czosnyka, Z.H.; Cieslicki, K.; Czosnyka, M.; Pickard, J.D. Hydrocephalus shunts and waves of intracranial pressure. Med. Biol. Eng. Comput. 2005, 43, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Méndez, R.; Richards, H.K.; Seeley, H.M.; Pickard, J.D.; Joannides, A.J. Current epidemiology of cerebrospinal fluid shunt surgery in the UK and Ireland (2004–2013). J. Neurol. Neurosurg. Psychiatry 2019, 90, 747–754. [Google Scholar] [CrossRef]
- Lo, T.Y.M.; Myles LMMinns, R. Long-term risks and benefits of a separate CSF access device with ventriculoperitoneal shunting in childhood hydrocephalus. Dev. Med. Child Neurol. 2003, 45, 28–33. [Google Scholar] [CrossRef]
- Bromby, A.; Czosnyka, Z.; Allin, D.; Richards, H.K.; Pickard, J.D.; Czosnyka, M. Laboratory study on “intracranial hypotension” created by pumping the chamber of a hydrocephalus shunt. Cereb. Fluid Res. 2007, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, Z.; Owler, B.; Momjian, S.; Kasprowicz, M.; Schmidt, E.A.; Smietlewski, P.; Pickalrd, J.D.; Czosnyka, M. Clinical testing of CSF circulation in hydrocephalus. Acta Neurochir. Suppl. 2005, 247–251. [Google Scholar] [CrossRef]
- Petersen, J.; Hellström, P.; Wikkelsø, C.; Lundgren-Nilsson, Å. Improvement in social function and health-related quality of life after shunt surgery for idiopathic normal-pressure hydrocephalus. J. Neurosurg. 2014, 121, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Housden, C.R.; Killikelly, C.; DeVito, E.E.; Keong, N.C.; Savulich, G.; Czosnyka, Z.; Pickard, J.D.; Sahakian, B.J. Apathy, ventriculomegaly and neurocognitive improvement following shunt surgery in normal pressure hydrocephalus. Br. J. Neurosurg. 2016, 30, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ziegelitz, D.; Arvidsson, J. In Patients With Idiopathic Normal Pressure Hydrocephalus Postoperative Cerebral Perfusion Changes Measured by Dynamic Susceptibility ContrastMagnetic Resonance Imaging Correlate With Clinical Improvement. J. Comput. Assist. Tomogr. 2015, 39, 531–540. [Google Scholar] [CrossRef]
- Eide, P.K.; Sorteberg, W. Outcome of Surgery for Idiopathic Normal Pressure Hydrocephalus: Role of Preoperative Static and Pulsatile Intracranial Pressure. World Neurosurg. 2016, 86, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lenfeldt, N.; Larsson, A.; Nyberg, L.; Birgander, R.; Eklund, A.; Malm, J. Diffusion tensor imaging reveals supplementary lesions to frontal white matter in idiopathic normal pressure hydrocephalus. Neurosurgery 2011, 68, 1586–1593. [Google Scholar] [CrossRef]
- Israelsson, H.; Birgander, R.; Ambarki, K.; Eklund, A.; Malm, J. Ventriculomegaly and balance disturbances in patients with TIA. Acta Neurol. Scand. 2012, 125, 163–170. [Google Scholar] [CrossRef] [PubMed]




| Reference | NPH | Aetiology | Rout | PPV | NPV | Other Main Findings |
|---|---|---|---|---|---|---|
| Borgensen et al. (1982) [104] | 80 | Mixed | ≥12 | 96–100% | >95% | NA |
| Borgensen et al. (1989) [9] | 183 | Mixed | ≥12 | NA | 100% | NA |
| Boon et al. (1997) [10] | 101 | iNPH | ≥12 & ≥18 | 80% & 92% | 34% | Highest LR of 3.5 for Rout 18 |
| Kahlon et al. (2002) [47] | 68 | iNPH | ≥14 | 80% | NA | Strong correlation between Rout & Outcome |
| Wikkelso et al. (2013) [11] | 115 | iNPH | ≥12 & ≥18 | 86 & 94% | 18 & 18% | No correlation of Rout & Outcome |
| Nabbanja et al. (2016) [105] | 310 | Mixed | ≥13 & ≥18 | NA | NA | Rout correlated with outcome. Kruskall-Wallis Value >6.5 and >6. |
| Lalou et al. (2020) [106] | 369 | Mixed | ≥13 & ≥18 | NA | NA | Rout > 18 highest Chi-square: 9.7 if age-adjusted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalou, A.D.; Czosnyka, M.; Placek, M.M.; Smielewski, P.; Nabbanja, E.; Czosnyka, Z. CSF Dynamics for Shunt Prognostication and Revision in Normal Pressure Hydrocephalus. J. Clin. Med. 2021, 10, 1711. https://doi.org/10.3390/jcm10081711
Lalou AD, Czosnyka M, Placek MM, Smielewski P, Nabbanja E, Czosnyka Z. CSF Dynamics for Shunt Prognostication and Revision in Normal Pressure Hydrocephalus. Journal of Clinical Medicine. 2021; 10(8):1711. https://doi.org/10.3390/jcm10081711
Chicago/Turabian StyleLalou, Afroditi Despina, Marek Czosnyka, Michal M. Placek, Peter Smielewski, Eva Nabbanja, and Zofia Czosnyka. 2021. "CSF Dynamics for Shunt Prognostication and Revision in Normal Pressure Hydrocephalus" Journal of Clinical Medicine 10, no. 8: 1711. https://doi.org/10.3390/jcm10081711
APA StyleLalou, A. D., Czosnyka, M., Placek, M. M., Smielewski, P., Nabbanja, E., & Czosnyka, Z. (2021). CSF Dynamics for Shunt Prognostication and Revision in Normal Pressure Hydrocephalus. Journal of Clinical Medicine, 10(8), 1711. https://doi.org/10.3390/jcm10081711






