Pancreatic Cancer Exposome Profile to Aid Early Detection and Inform Prevention Strategies
Abstract
| Summary Box. | |
| What is already known about risk factors for PCa | |
| Cigarette smoking, family history of PCa, diabetes mellitus, cystic lesions of pancreas, and pancreatitis are well-established risk factors for PCa. | |
| What our systematic review has revealed about the exposome for PCa | |
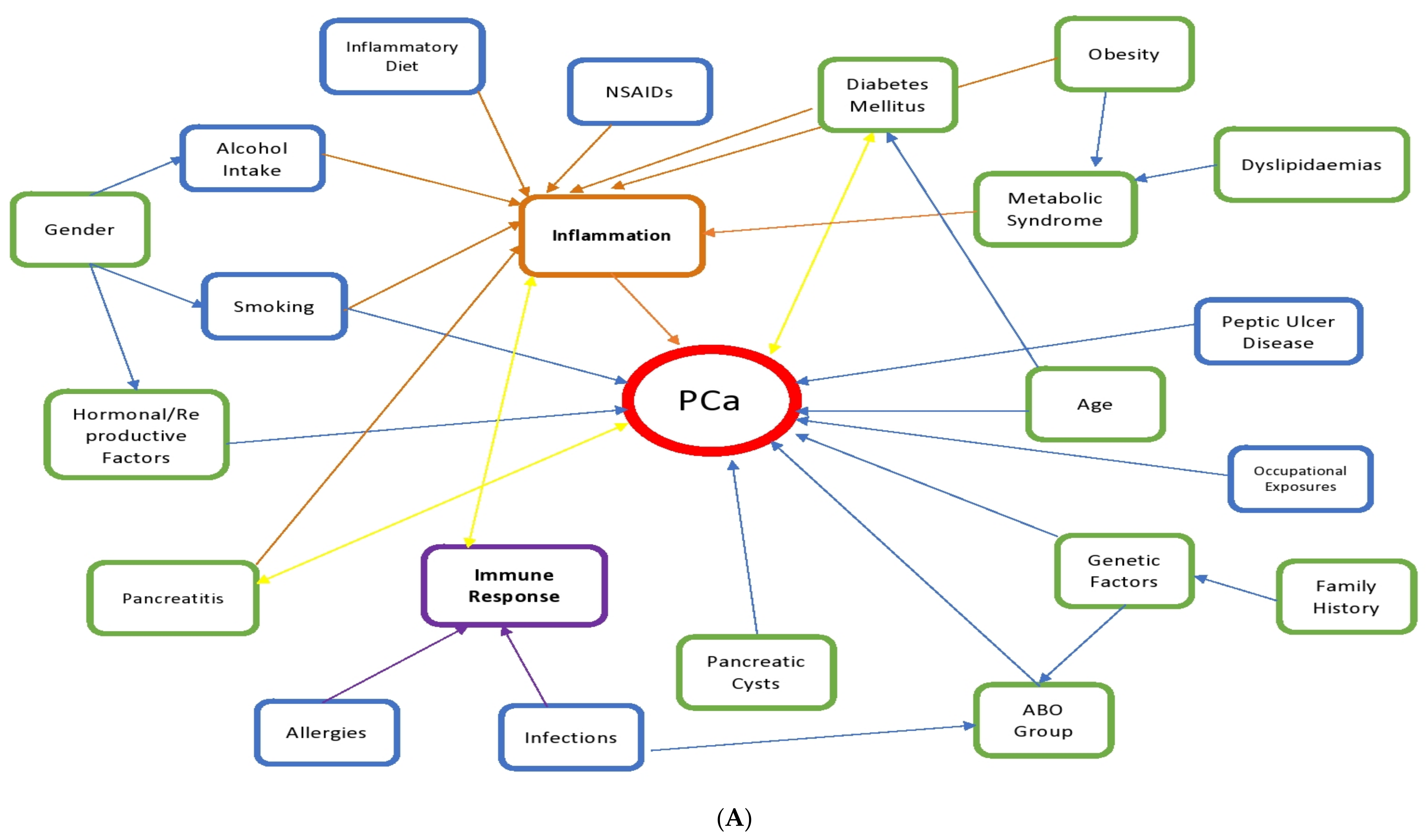
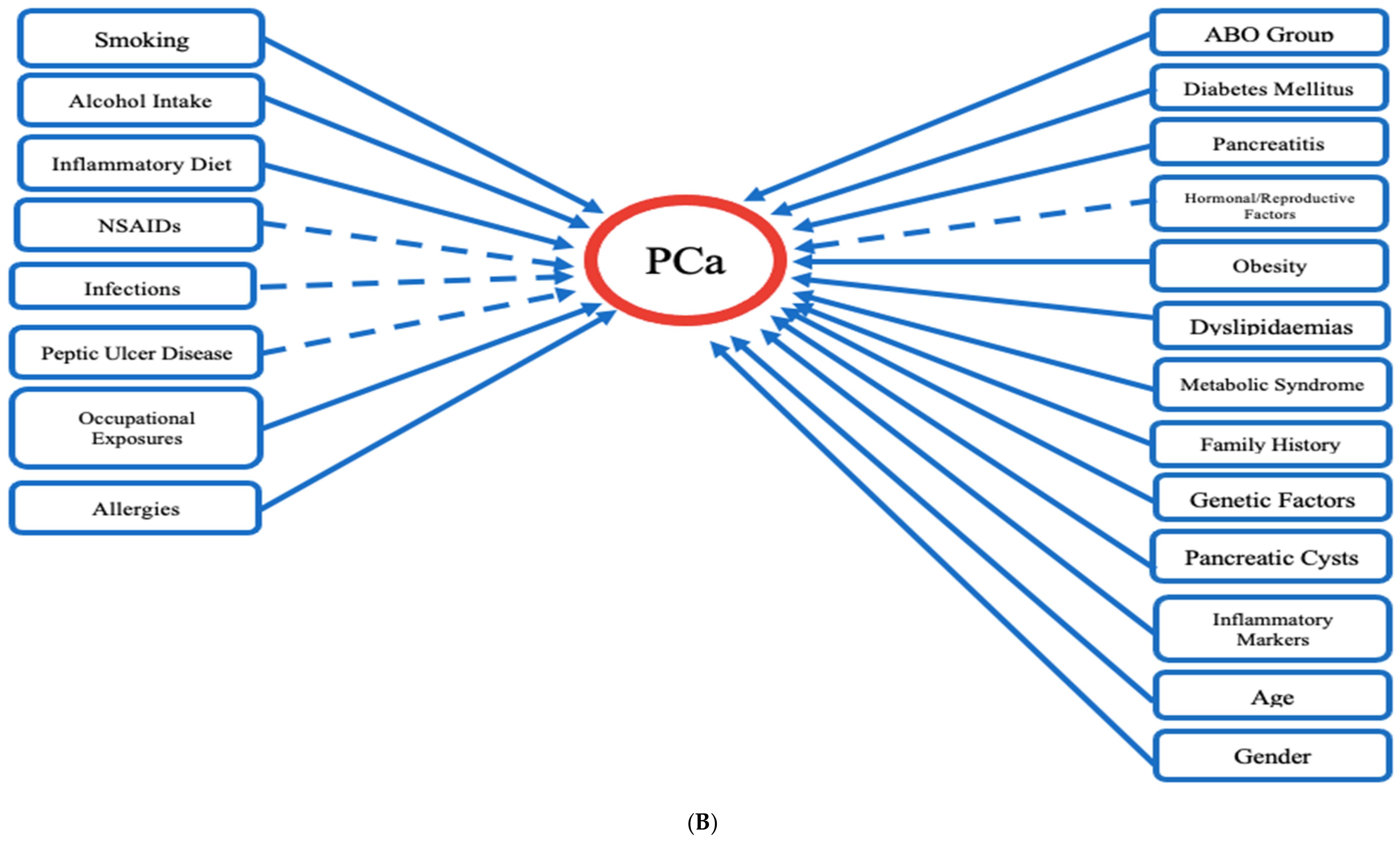
| We provided a template for a generalized approach to identify risk factors that contribute to PCa incidence and progression. We identified a list of non-modifiable factors that could alert clinicians of at-risk individuals, and modifiable factors which can be controlled in order to decrease PCa incidence. We observed that several of these factors are associated with the inflammatory pathway which suggest that PCa carcinogenesis is driven by inflammatory processes. | |
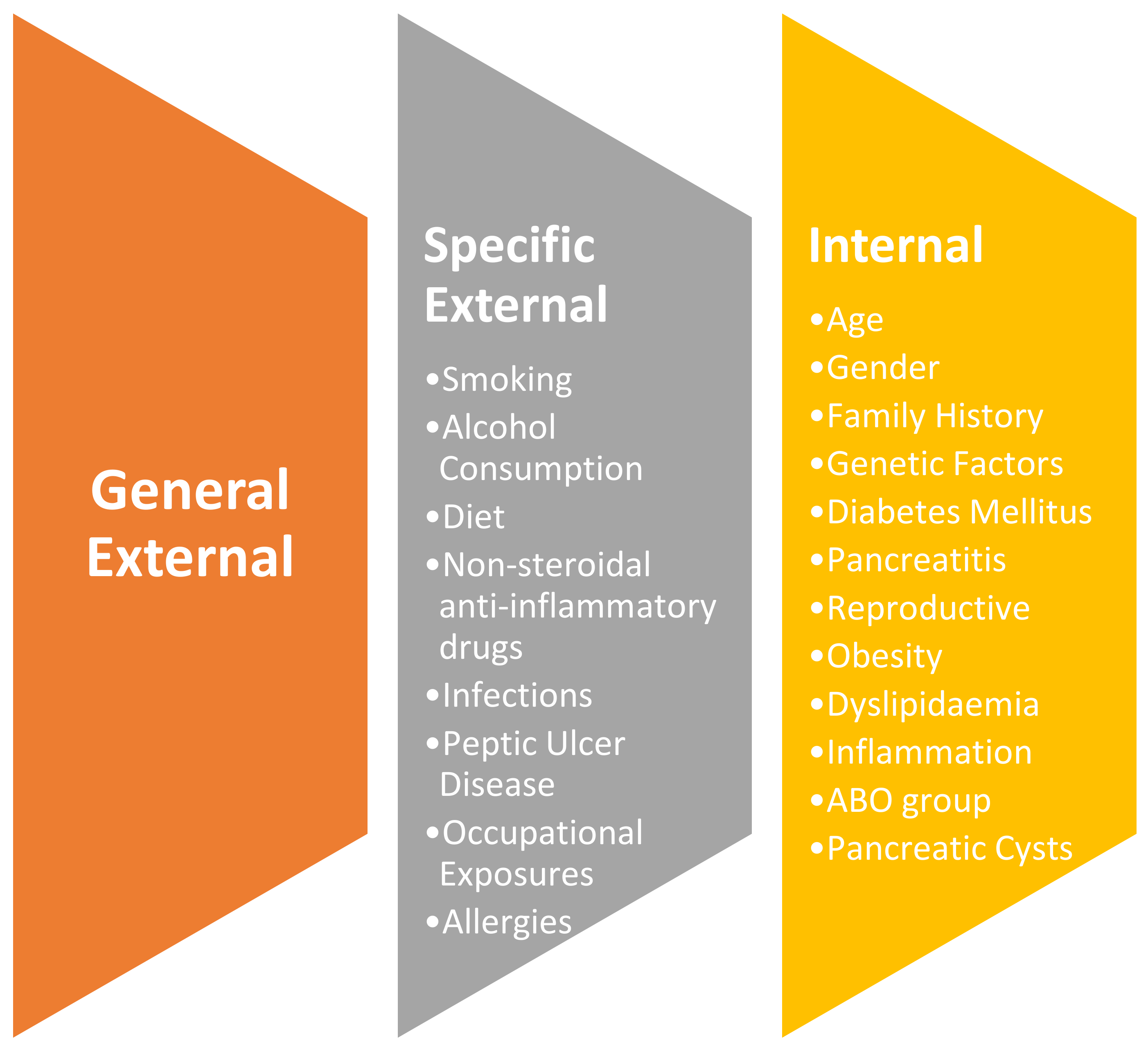
Non-Modifiable Factors:
| Modifiable Factors:
|
1. Introduction
2. Materials and Methods
3. Results
3.1. Specific External Exposures
3.1.1. Smoking
3.1.2. Alcohol Consumption
3.1.3. Diet
3.1.4. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
3.1.5. Infections
3.1.6. Peptic Ulcer Disease
3.1.7. Occupational Exposures
3.2. Internal Exposures
3.2.1. Age
3.2.2. Gender
3.2.3. Family History
3.2.4. Genetic Factors
3.2.5. Diabetes Mellitus
New-Onset Diabetes Mellitus
Late-Onset Diabetes Mellitus
Anti-Diabetic Drugs
3.2.6. Inflammation
3.2.7. Pancreatitis
3.2.8. Immune System
3.2.9. Allergies
3.2.10. Hormonal and Reproductive Factors
3.2.11. Obesity
3.2.12. Dyslipidaemia
3.2.13. ABO Group
3.2.14. Pancreatic Cysts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN es-timates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Yeo, T.P. Demographics, Epidemiology, and Inheritance of Pancreatic Ductal Adenocarcinoma. Semin. Oncol. 2015, 42, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Michalski, C.W.; Erkan, M.; Friess, H.; Kleeff, J. From tissue turnover to the cell of origin for pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 467–472. [Google Scholar] [CrossRef] [PubMed]
- PanCan. Types of Pancreatic Cancer 2020. Available online: https://www.pancan.org/facing-pancreatic-cancer/about-pancreatic-cancer/types-of-pancreatic-cancer/ (accessed on 10 February 2021).
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mario, C.; Marilisa, F.; Kryssia, I.R.C.; Pellegrino, C.; Ginevra, C.; Chiara, M.; Alberto, B.; Antonio, N.; Gioacchino, L.; Tiziana, M.; et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018, 89, 141–146. [Google Scholar]
- Guertin, K.A.; Freedman, N.D.; Loftfield, E.; Stolzenberg-Solomon, R.Z.; Graubard, B.I.; Sinha, R. A prospective study of coffee intake and pancreatic cancer: Results from the NIH-AARP Diet and Health Study. Br. J. Cancer 2015, 113, 1081–1085. [Google Scholar] [CrossRef][Green Version]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Zheng, J.; Guinter, M.A.; Merchant, A.T.; Wirth, M.D.; Zhang, J.; Stolzenberg-Solomon, R.Z.; Steck, S.E. Dietary patterns and risk of pancreatic cancer: A systematic review. Nutr. Rev. 2017, 75, 883–908. [Google Scholar] [CrossRef]
- Bosetti, C.; Bertuccio, P.; Negri, E.; La Vecchia, C.; Zeegers, M.P.; Boffetta, P. Pancreatic cancer: Overview of descriptive epidemi-ology. Mol. Carcinog. 2012, 51, 3–13. [Google Scholar] [CrossRef]
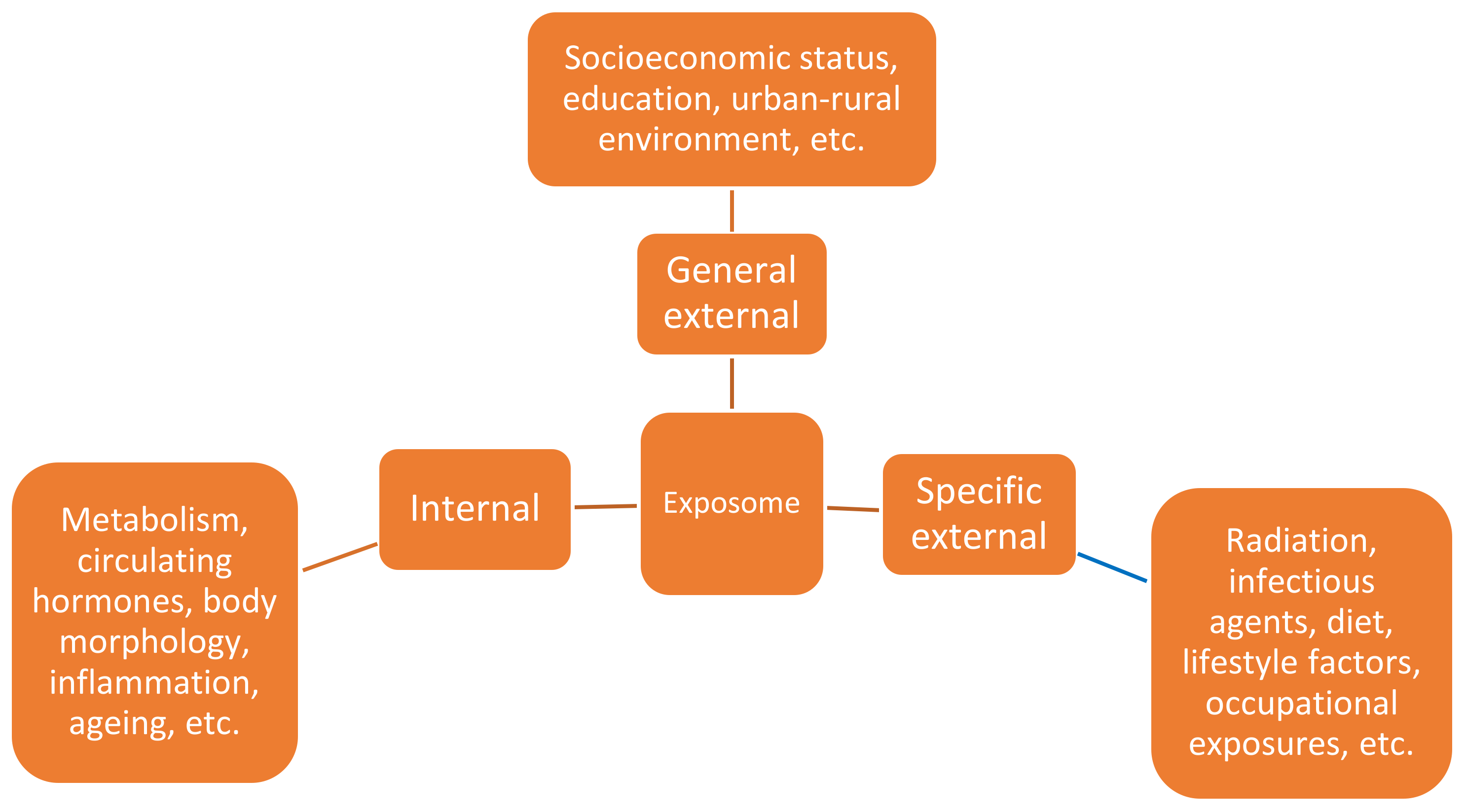
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure meas-urement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Vineis, P.; Robinson, O.; Chadeau-Hyam, M.; Dehghan, A.; Mudway, I.; Dagnino, S. What is new in the exposome? Environ. Int. 2020, 143, 105887. [Google Scholar] [CrossRef]
- Vineis, P.; Chadeau-Hyam, M.; Gmuender, H.; Gulliver, J.; Herceg, Z.; Kleinjans, J.; Kogevinas, M.; Kyrtopoulos, S.; Nieuwenhuijsen, M.; Phillips, D.; et al. The exposome in practice: Design of the EXPOsOMICS project. Int. J. Hyg. Environ. Health 2017, 220, 142–151. [Google Scholar] [CrossRef]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Habtewold, T.D.; Birhanu, M.M.; Sissay, T.A.; Tegegne, B.S.; Abuzerr, S.; Esmaillzadeh, A. Neighbourhood socioeconomic status and overweight/obesity: A systematic review and meta-analysis of epidemiological studies. Bmj Open 2019, 9, e028238. [Google Scholar] [CrossRef]
- Hsing, A.W.; Sakoda, L.C.; Chua, S.C. Obesity, metabolic syndrome, and prostate cancer. Am. J. Clin. Nutr. 2007, 86, 843S–857S. [Google Scholar] [CrossRef]
- Robinson, O.; Vrijheid, M. The Pregnancy Exposome. Curr. Environ. Health Rep. 2015, 2, 204–213. [Google Scholar] [CrossRef]
- Daiber, A.; Lelieveld, J.; Steven, S.; Oelze, M.; Kröller-Schön, S.; Sørensen, M.; Münzel, T. The “exposome” concept—how environmental risk factors influence cardiovascular health. Acta Biochim. Pol. 2019, 66, 269–283. [Google Scholar] [CrossRef]
- Dréno, B.; Bettoli, V.; Araviiskaia, E.; Viera, M.S.; Bouloc, A. The influence of exposome on acne. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 812–819. [Google Scholar] [CrossRef]
- Pham, A.; Forsmark, C. Chronic pancreatitis: Review and update of etiology, risk factors, and management. F1000Research 2018, 7, 607. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Antwi, S.O.; Oberg, A.L.; Shivappa, N.; Bamlet, W.R.; Chaffee, K.G.; Steck, S.E.; Hebert, J.R.; Petersen, G.M. Pancreatic cancer: Associations of inflammatory potential of diet, cigarette smoking and long-standing diabetes. Carcinogenesis 2016, 37, 481–490. [Google Scholar] [CrossRef]
- Hassan, M.M.; Abbruzzese, J.L.; Bondy, M.L.; Wolff, R.A.; Vauthey, J.-N.; Pisters, P.W.; Evans, D.B.; Khan, R.; Lenzi, R.; Jiao, L.; et al. Passive smoking and the use of noncigarette tobacco products in association with risk for pancreatic cancer: A case-control study. Cancer 2007, 109, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Korc, M.; Jeon, C.Y.; Edderkaoui, M.; Pandol, S.J.; Petrov, M.S. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, H.; Maeda, A.; Kanemoto, H.; Uesaka, K.; Yamazaki, K.; Hironaka, S.; Miyagi, Y.; Ikehara, H.; Ono, H.; Klein, A.; et al. Risk factors of familial pancreatic cancer in Japan: Current smoking and recent onset of diabetes. Pancreas 2011, 40, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Gomez-Rubio, P.; Márquez, M.; Rava, M.; Löhr, M.; Michalski, C.W.; Molero, X.; Farré, A.; Perea, J.; Greenhalf, W.; et al. Risk of pancreatic cancer associated with family history of cancer and other medical conditions by accounting for smoking among relatives. Int. J. Epidemiol. 2018, 47, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Pandeya, N.; Tran, B.; Fawcett, J.; Fritschi, L.; Risch, H.A.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Cigarette smoking and pancreatic cancer risk: More to the story than just pack-years. Eur. J. Cancer 2014, 50, 997–1003. [Google Scholar] [CrossRef]
- Vrieling, A.; Bueno-de-Mesquita, H.B.; Boshuizen, H.C.; Michaud, D.S.; Severinsen, M.T.; Overvad, K.; Olsen, A.; Tjønneland, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Cigarette smoking, en-vironmental tobacco smoke exposure and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nu-trition. Int. J. Cancer 2010, 126, 2394–2403. [Google Scholar]
- Kuzmickiene, I.; Everatt, R.; Virviciute, D.; Tamosiunas, A.; Radisauskas, R.; Reklaitiene, R.; Milinaviciene, E. Smoking and other risk factors for pancreatic cancer: A cohort study in men in Lithuania. Cancer Epidemiol. 2013, 37, 133–139. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, H.; Yang, X.; Guo, J. Cigarette smoking and the risk of pancreatic cancer: A case–control study. Med Oncol. 2014, 31, 1–5. [Google Scholar] [CrossRef]
- Heinen, M.M.; Verhage, B.A.; Goldbohm, R.A.; Brandt, P.A.V.D. Active and Passive Smoking and the Risk of Pancreatic Cancer in the Netherlands Cohort Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1612–1622. [Google Scholar] [CrossRef]
- Brand, R.E.; Greer, J.B.; Zolotarevsky, E.; Brand, R.; Du, H.; Simeone, D.; Zisman, A.; Gorchow, A.; Lee, S. (Connie); Roy, H.K.; et al. Pancreatic Cancer Patients Who Smoke and Drink Are Diagnosed at Younger Ages. Clin. Gastroenterol. Hepatol. 2009, 7, 1007–1012. [Google Scholar] [CrossRef]
- Muscat, J.E.; Stellman, S.D.; Hoffmann, D.; Wynder, E.L. Smoking and pancreatic cancer in men and women. Cancer Epidemiol. Biomark. Prev. 1997, 6, 15–19. [Google Scholar]
- Nilsen, T.I.L.; Vatten, L.J. A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trøndelag, Norway. Cancer Causes Control. 2000, 11, 645–652. [Google Scholar] [CrossRef]
- Hassan, M.M.; Bondy, M.L.; Wolff, R.A.; Abbruzzese, J.L.; Vauthey, J.-N.; Pisters, P.W.; Evans, D.B.; Khan, R.; Chou, T.-H.; Lenzi, R.; et al. Risk Factors for Pancreatic Cancer: Case-Control Study. Am. J. Gastroenterol. 2007, 102, 2696–2707. [Google Scholar] [CrossRef]
- Lo, A.-C.; Soliman, A.S.; El-Ghawalby, N.; Abdel-Wahab, M.; Fathy, O.; Khaled, H.M.; Omar, S.; Hamilton, S.R.; Greenson, J.K.; Abbruzzese, J.L. Lifestyle, Occupational, and Reproductive Factors in Relation to Pancreatic Cancer Risk. Pancreas 2007, 35, 120–129. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Gallo, V.; Michaud, M.; Overvad, K.; Tjønneland, A.; Clavel-Chapelon, F.; Romieu, I.; Straif, K.; Palli, D.; Pala, V.; et al. Exposure to environmental tobacco smoke in childhood and incidence of cancer in adulthood in never smokers in the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2011, 22, 487–494. [Google Scholar] [CrossRef]
- Gallicchio, L.; Kouzis, A.; Genkinger, J.M.; Burke, A.E.; Hoffman, S.C.; Diener-West, M.; Helzlsouer, K.J.; Comstock, G.W.; Alberg, A.J. Active cigarette smoking, household passive smoke exposure, and the risk of developing pancreatic cancer. Prev. Med. 2006, 42, 200–205. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Johnson, K.C.; Mao, Y.; Hanley, A.J.; Paulse, B.; Dewar, R.; Dryer, D.; Kreiger, N.; Kliewer, E.; Robson, D.; et al. Environmental tobacco smoke and the risk of pancreatic cancer: Findings from a Canadian population-based case-control study. Can. J. Public Health 2004, 95, 32–37. [Google Scholar] [CrossRef]
- Capurso, G.; Falconi, M.; Panzuto, F.; Rinzivillo, M.; Boninsegna, L.; Bettini, R.; Corleto, V.; Borgia, P.; Pederzoli, P.; Scarpa, A.; et al. Risk factors for sporadic pancreatic endocrine tumors: A case-control study of prospectively evaluated patients. Am. J. Gastroenterol. 2009, 104, 3034–3041. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bergkvist, L.; Bernstein, L.; Brandt, P.A.V.D.; English, D.R.; Freudenheim, J.L.; Fuchs, C.S.; Giles, G.G.; et al. Alcohol Intake and Pancreatic Cancer Risk: A Pooled Analysis of Fourteen Cohort Studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 765–776. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, F.; Holly, E.A.; Bracci, P.M. Risk of pancreatic cancer by alcohol dose, duration, and pattern of consumption, including binge drinking: A population-based study. Cancer Causes Control. 2010, 21, 1047–1059. [Google Scholar] [CrossRef]
- Klein, A.P.; Lindstroem, S.; Mendelsohn, J.B.; Steplowski, E.; Arslan, A.A.; Bueno-De-Mesquita, H.B.; Fuchs, C.S.; Gallinger, S.; Gross, M.; Helzlsouer, K.; et al. An Absolute Risk Model to Identify Individuals at Elevated Risk for Pancreatic Cancer in the General Population. PLoS ONE 2013, 8, e72311. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Wang, L.; Li, H.; Qian, J.M.; Deng, R.X.; Zhou, L. Establishment of risk model for pancreatic cancer in Chinese Han popu-lation. World J. Gastroenterol. 2006, 12, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Maisonneuve, P.; Bamlet, W.R.; Petersen, G.M.; Li, D.; Risch, H.; Yu, H.; Fontham, E.T.; Luckett, B.; Bosetti, C.; et al. Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas 2016, 45, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Naudin, S.; Li, K.; Jaouen, T.; Assi, N.; Kyrø, C.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.-C.; Rebours, V.; Védie, A.-L.; et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2018, 143, 801–812. [Google Scholar] [CrossRef]
- Miyasaka, K.; Kawanami, T.; Shimokata, H.; Ohta, S.; Funakoshi, A. Inactive Aldehyde Dehydrogenase-2 Increased the Risk of Pancreatic Cancer Among Smokers in a Japanese Male Population. Pancreas 2005, 30, 95–98. [Google Scholar] [CrossRef]
- Talamini, G.; Bassi, C.; Falconi, M.; Sartori, N.; Salvia, R.; Rigo, L.; Castagnini, A.; Di Francesco, V.; Frulloni, L.; Bovo, P.; et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig. Dis. Sci. 1999, 44, 1303–1311. [Google Scholar] [CrossRef]
- Bouchardy, C.; Clavel, F.; La Vecchia, C.; Raymond, L.; Boyle, P. Alcohol, beer and cancer of the pancreas. Int. J. Cancer 1990, 45, 842–846. [Google Scholar] [CrossRef]
- Falk, R.T.; Pickle, L.W.; Fontham, E.T.; Correa, P.; Fraumeni, J.F. Life-style risk factors for pancreatic cancer in louisiana: A case-control study. Am. J. Epidemiol. 1988, 128, 324–336. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Antwi, S.O.; Bamlet, W.R.; Pedersen, K.S.; Chaffee, K.G.; Risch, H.A.; Shivappa, N.; Steck, S.E.; Anderson, K.E.; Bracci, P.M.; Polesel, J.; et al. Pancreatic cancer risk is modulated by in-flammatory potential of diet and ABO genotype: A consortia-based evaluation and replication study. Carcinogenesis 2018, 39, 1056–1067. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br. J. Cancer 2012, 106, 603–607. [Google Scholar] [CrossRef]
- Shivappa, N.; Bosetti, C.; Zucchetto, A.; Serraino, D.; La Vecchia, C.; Hebert, J.R. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br. J. Nutr. 2014, 113, 292–298. [Google Scholar] [CrossRef]
- Bao, Y.; Nimptsch, K.; Wolpin, B.M.; Michaud, D.S.; Brand-Miller, J.C.; Willett, W.C.; Giovannucci, E.; Fuchs, C.S. Dietary insulin load, dietary insulin index, and risk of pancreatic cancer. Am. J. Clin. Nutr. 2011, 94, 862–868. [Google Scholar] [CrossRef]
- Lin, Y.; Tamakoshi, A.; Hayakawa, T.; Naruse, S.; Kitagawa, M.; Ohno, Y. Nutritional factors and risk of pancreatic cancer: A pop-ulation-based case-control study based on direct interview in Japan. J. Gastroenterol. 2005, 40, 297–301. [Google Scholar] [CrossRef]
- Ji, B.T.; Chow, W.H.; Gridley, G.; McLaughlin, J.K.; Dai, Q.; Wacholder, S.; Hatch, M.C.; Gao, Y.T.; Fraumeni, J.F. Dietary factors and the risk of pancreatic cancer: A case-control study in Shanghai China. Cancer Epidemiol. Biomark. Prev. 1995, 4, 885–893. [Google Scholar]
- Wang, J.; Zhang, W.; Sun, L.; Yu, H.; Ni, Q.-X.; Risch, H.A.; Gao, Y.-T. Dietary energy density is positively associated with risk of pancreatic cancer in urban Shanghai Chinese. J. Nutr. 2013, 143, 1626–1629. [Google Scholar] [CrossRef]
- Farrow, D.C.; Davis, S. Diet and the risk of pancreatic cancer in men. Am. J. Epidemiol. 1990, 132, 423–431. [Google Scholar] [CrossRef]
- Soler, M.; Chatenoud, L.; La Vecchia, C.; Franceschi, S.; Negri, E. Diet, alcohol, coffee and pancreatic cancer: Final results from an Italian study. Eur. J. Cancer Prev. 1998, 7, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Mongin, S.J.; Sinha, R.; Stolzenberg-Solomon, R.; Gross, M.D.; Ziegler, R.G.; Mabie, J.E.; Risch, A.; Kazin, S.S.; Church, T.R. Pancreatic cancer risk: Associations with meat-derived carcinogen intake in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Mol. Carcinog. 2012, 51, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Emadi, A.; Shab-Bidar, S. Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv. Nutr. 2018, 9, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Lucenteforte, E.; Talamini, R.; Bosetti, C.; Polesel, J.; Franceschi, S.; Serraino, D.; Negri, E.; La Vecchia, C. Macronutrients, fatty acids, cholesterol and pancreatic cancer. Eur. J. Cancer 2010, 46, 581–587. [Google Scholar] [CrossRef]
- Ruan, Y.; Poirier, A.E.; Hebert, L.A.; Grevers, X.; Walter, S.D.; Villeneuve, P.J.; Brenner, D.R.; Friedenreich, C.M. Estimates of the current and future burden of cancer attributable to red and processed meat consumption in Canada. Prev. Med. 2019, 122, 31–39. [Google Scholar] [CrossRef]
- Zheng, J.; Merchant, A.T.; Wirth, M.D.; Zhang, J.; Antwi, S.O.; Shoaibi, A.; Shivappa, N.; Stolzenberg-Solomon, R.Z.; Hebert, J.R.; Steck, S.E. Inflammatory potential of diet and risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Int. J. Cancer 2018, 142, 2461–2470. [Google Scholar] [CrossRef]
- Zheng, J.; Wirth, M.D.; Merchant, A.T.; Zhang, J.; Shivappa, N.; Stolzenberg-Solomon, R.Z.; Hebert, J.R.; Steck, S.E. Inflammatory Potential of Diet, Inflammation-Related Lifestyle Factors, and Risk of Pancreatic Cancer: Results from the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1266–1270. [Google Scholar] [CrossRef]
- Åsli, L.A.; Braaten, T.; Olsen, A.; Tjønneland, A.; Overvad, K.; Nilsson, L.M.; Renström, F.; Lund, E.; Skeie, G. Potato consumption and risk of pancreatic cancer in the HELGA cohort. Br. J. Nutr. 2018, 119, 1408–1415. [Google Scholar] [CrossRef]
- Mueller, N.T.; Odegaard, A.; Anderson, K.; Yuan, J.-M.; Gross, M.; Koh, W.-P.; Pereira, M.A. Soft Drink and Juice Consumption and Risk of Pancreatic Cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 447–455. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Hu, F.B.; Giovannucci, E.; Michaud, D.S.; Colditz, G.A.; Stampfer, M.J.; Fuchs, C.S. Sugar-sweetened soft drink con-sumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2098–2105. [Google Scholar] [CrossRef]
- Nöthlings, U.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Dietary glycemic load, added sugars, and carbohydrates as risk factors for pancreatic cancer: The Multiethnic Cohort Study. Am. J. Clin. Nutr. 2007, 86, 1495–1501. [Google Scholar] [CrossRef]
- Luo, J.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Otani, T.; Ye, W.; Tsugane, S. Green tea and coffee intake and risk of pancreatic cancer in a large-scale, population-based cohort study in Japan (JPHC study). Eur. J. Cancer Prev. 2007, 16, 542–548. [Google Scholar] [CrossRef]
- Turati, F.; Galeone, C.; Talamini, R.; Franceschi, S.; Manzari, M.; Gallino, G.; Polesel, J.; La Vecchia, C.; Tavani, A. Coffee, decaffeinated coffee, tea, and pancreatic cancer risk: A pooled-analysis of two Italian case-control studies. Eur. J. Cancer Prev. 2011, 20, 287–292. [Google Scholar] [CrossRef]
- Banim, P.J.R.; Luben, R.; McTaggart, A.; Welch, A.; Wareham, N.; Khaw, K.-T.; Hart, A.R. Dietary antioxidants and the aetiology of pancreatic cancer: A cohort study using data from food diaries and biomarkers. Gut 2012, 62, 1489–1496. [Google Scholar] [CrossRef]
- Lucas, A.L.; Bosetti, C.; Boffetta, P.; Negri, E.; Tavani, A.; Serafini, M.; Polesel, J.; Serraino, D.; La Vecchia, C.; Rossi, M. Dietary total antioxidant capacity and pancreatic cancer risk: An Italian case–control study. Br. J. Cancer 2016, 115, 102–107. [Google Scholar] [CrossRef]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.R.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.I.; La Vecchia, C.; et al. Vitamin D and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case–Control Consortium. Ann. Oncol. 2015, 26, 1776–1783. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Jacobs, E.J.; Arslan, A.A.; Qi, D.; Patel, A.V.; Helzlsouer, K.J.; Weinstein, S.J.; McCullough, M.L.; Purdue, M.P.; Shu, X.-O.; et al. Circulating 25-Hydroxyvitamin D and Risk of Pancreatic Cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 81–93. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Hayes, R.B.; Horst, R.L.; Anderson, K.E.; Hollis, B.W.; Silverman, D.T. Serum vitamin D and risk of pan-creatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res. 2009, 69, 1439–1447. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Stolzenberg-Solomon, R.; Ueland, P.M.; Vollset, S.E.; Midttun, Ø.; Olsen, A.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.-C.; Morois, S.; et al. A U-shaped relationship between plasma folate and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Eur. J. Cancer 2011, 47, 1808–1816. [Google Scholar] [CrossRef]
- Gong, Z.; Holly, E.A.; Bracci, P.M. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case–control study. Cancer Causes Control. 2009, 20, 1317–1325. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Albanes, D.; Nieto, F.J.; Hartman, T.J.; Tangrea, J.A.; Rautalahti, M.; Sehlub, J.; Viramo, J.; Taylor, P.R. Pancreatic cancer risk and nu-trition-related methyl-group availability indicators in male smokers. J. Natl. Cancer Inst. 1999, 91, 535–541. [Google Scholar] [CrossRef]
- Archibugi, L.; Piciucchi, M.; Stigliano, S.; Valente, R.; Zerboni, G.; Barucca, V.; Milella, M.; Maisonneuve, P.; Fave, G.D.; Capurso, G. Exclusive and Combined Use of Statins and Aspirin and the Risk of Pancreatic Cancer: A Case-Control Study. Sci. Rep. 2017, 7, 13024. [Google Scholar] [CrossRef]
- Bradley, M.C.; Hughes, C.M.; Cantwell, M.M.; Napolitano, G.; Murray, L.J. Non-steroidal anti-inflammatory drugs and pancreatic cancer risk: A nested case–control study. Br. J. Cancer 2010, 102, 1415–1421. [Google Scholar] [CrossRef]
- Coogan, P.F.; Rosenberg, L.; Palmer, J.R.; Strom, B.L.; Zauber, A.G.; Stolley, P.D.; Shapiro, S. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol. Biomark. Prev. 2000, 9, 119–123. [Google Scholar]
- Schernhammer, E.S.; Kang, J.-H.; Chan, A.T.; Michaud, D.S.; Skinner, H.G.; Giovannucci, E.; Colditz, G.A.; Fuchs, C.S. A Prospective Study of Aspirin Use and the Risk of Pancreatic Cancer in Women. J. Natl. Cancer Inst. 2004, 96, 22–28. [Google Scholar] [CrossRef]
- Khalaf, N.; Yuan, C.; Hamada, T.; Cao, Y.; Babic, A.; Morales-Oyarvide, V.; Kraft, P.; Ng, K.; Giovannucci, E.; Ogino, S.; et al. Regular Use of Aspirin or Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs Is Not Associated With Risk of Incident Pancreatic Cancer in Two Large Cohort Studies. Gastroenterology 2018, 154, 1380–1390.e5. [Google Scholar] [CrossRef]
- Xing, S.; Li, Z.-W.; Tian, Y.-F.; Zhang, L.-M.; Li, M.-Q.; Zhou, P. Chronic hepatitis virus infection increases the risk of pancreatic cancer: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 575–583. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Song, F.; Cao, S.; Yin, X.; Xie, J.; Tu, X.; Xu, J.; Xu, X.; Dong, X.; et al. Hepatitis B virus status and the risk of pancreatic cancer: A meta-analysis. Eur. J. Cancer Prev. 2013, 22, 328–334. [Google Scholar] [CrossRef]
- Song, C.; Lv, J.; Liu, Y.; Chen, J.G.; Ge, Z.; Zhu, J.; Dai, J.; Du, L.-B.; Yu, C.; Guo, Y.; et al. Associations Between Hepatitis B Virus Infection and Risk of All Cancer Types. Jama Netw. Open 2019, 2, e195718. [Google Scholar] [CrossRef]
- Chang, M.-C.; Chen, C.-H.; Liang, J.-D.; Tien, Y.-W.; Hsu, C.; Wong, J.-M.; Chang, Y.-T. Hepatitis B and C viruses are not risks for pancreatic adenocarcinoma. World J. Gastroenterol. 2014, 20, 5060–5065. [Google Scholar] [CrossRef]
- Tang, J.; Sharma, R.; Lamerato, L.; Sheehan, M.; Krajenta, R.; Gordon, S.C. Is previous exposure to hepatitis B a risk factor for pan-creatic cancer or hepatocellular carcinoma? J. Clin. Gastroenterol. 2014, 48, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A.; Lu, L.; Kidd, M.S.; Wang, J.; Zhang, W.; Ni, Q.; Gao, Y.-T.; Yu, H. Helicobacter pylori Seropositivities and Risk of Pancreatic Carcinoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.; Hua, X.; Liu, Y.; Lin, J.; Feng, Z. Preliminary Study of Pancreatic Cancer Associated with Helicobacter pylori Infection. Cell Biophys. 2015, 71, 397–400. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Llosa, A.E.; Friedmana, G.D.; Vogelman, J.H.; Orentreich, N.; Stolzenberg-Solomon, R.Z.; Parsonnet, J. Helicobacter pylori infection and development of pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1188–1194. [Google Scholar] [CrossRef]
- Hirabayashi, M.; Inoue, M.; Sawada, N.; Saito, E.; Abe, S.K.; Hidaka, A.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Tsugane, S. Helicobacter pylori infection, atrophic gastritis, and risk of pancreatic cancer: A population-based cohort study in a large Japanese population: The JPHC Study. Sci. Rep. 2019, 9, 6099. [Google Scholar] [CrossRef]
- Lindkvist, B.; Johansen, D.; Borgstrom, A.; Manjer, J. A prospective study of Helicobacter pylori in relation to the risk for pan-creatic cancer. BMC Cancer 2008, 8, 321. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Schöttker, B.; Castro, F.A.; Chen, H.; Zhang, Y.; Holleczek, B.; Brenner, H. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: A ten-year follow-up of the ESTHER cohort study. Oncotarget 2016, 7, 17182–17193. [Google Scholar] [CrossRef]
- Gerlovin, H.; Michaud, D.S.; Cozier, Y.C.; Palmer, J.R. Oral Health in Relation to Pancreatic Cancer Risk in African American Women. Cancer Epidemiol. Biomark. Prev. 2019, 28, 675–679. [Google Scholar] [CrossRef]
- Michaud, D.S.; Joshipura, K.; Giovannucci, E.; Fuchs, C.S. A Prospective Study of Periodontal Disease and Pancreatic Cancer in US Male Health Professionals. J. Natl. Cancer Inst. 2007, 99, 171–175. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Caygill, C.; Hill, M.; Braddick, M.; Sharp, J. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 1994, 343, 83–84. [Google Scholar] [CrossRef]
- Gotland, N.; Uhre, M.L.; Sandholdt, H.; Mejer, N.; Lundbo, L.F.; Petersen, A.; Larsen, A.R.; Benfield, T. Increased risk of incident primary cancer after Staphylococcus aureus bacteremia: A matched cohort study. Medicine 2020, 99, e19984. [Google Scholar] [CrossRef]
- Bao, Y.; Spiegelman, D.; Li, R.; Giovannucci, E.; Fuchs, C.S.; Michaud, D.S. History of Peptic Ulcer Disease and Pancreatic Cancer Risk in Men. Gastroenterology 2010, 138, 541–549. [Google Scholar] [CrossRef]
- Luo, J.; Nordenvall, C.; Nyrén, O.; Adami, H.-O.; Permert, J.; Ye, W. The risk of pancreatic cancer in patients with gastric or duodenal ulcer disease. Int. J. Cancer 2006, 120, 368–372. [Google Scholar] [CrossRef]
- Hwang, I.C.; Chang, J.; Park, S.M. Association between proton pump inhibitor use and the risk of pancreatic cancer: A Korean nationwide cohort study. PLoS ONE 2018, 13, e0203918. [Google Scholar] [CrossRef]
- Hicks, B.; Friis, S.; Pottegård, A. Use of proton pump inhibitors and risk of pancreatic cancer. Pharmacoepidemiol. Drug Saf. 2018, 27, 926–930. [Google Scholar] [CrossRef]
- Bosetti, C.; Lucenteforte, E.; Bracci, P.M.; Negri, E.; Neale, R.E.; Risch, H.A.; Olson, S.H.; Gallinger, S.; Miller, A.B.; Bueno-De-Mesquita, H.B.; et al. Ulcer, gastric surgery and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case–Control Consortium (PanC4). Ann. Oncol. 2013, 24, 2903–2910. [Google Scholar] [CrossRef]
- Alguacil, J.; Kauppinen, T.; Porta, M.; Partanen, T.; Malats, N.; Kogevinas, M.; Benavides, F.G.; Obiols, J.; Bernal, F.; Rifà, J.; et al. Risk of pancreatic cancer and occupational exposures in Spain. Ann. Occup. Hyg. 2000, 44, 391–403. [Google Scholar] [CrossRef]
- Andreotti, G.; Freeman, L.B.; Hou, L.; Coble, J.; Rusiecki, J.; Hoppin, J.; Silverman, D.; Alavanja, M. Abstract A120: Agricultural pesticide use and pancreatic cancer risk in the agricultural health study cohort. Int J Cancer. 2008, 124, 2495–2500. [Google Scholar] [CrossRef]
- Andreotti, G.; Silverman, D.T. Occupational risk factors and pancreatic cancer: A review of recent findings. Mol. Carcinog. 2011, 51, 98–108. [Google Scholar] [CrossRef]
- Ojajärvi, A.; Partanen, T.; Ahlbom, A.; Hakulinen, T.; Kauppinen, T.; Weiderpass, E.; Wesseling, C. Estimating the relative risk of pancreatic cancer associated with exposure agents in job title data in a hierarchical Bayesian meta-analysis. Scand. J. Work. Environ. Health 2007, 33, 325–335. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Tolbert, P.E.; Holly, E.A.; Brock, J.W.; Korrick, S.A.; Altshul, L.M.; Zhang, R.H.; Bracci, P.M.; Burse, V.W.; Needham, L.L. Pancreatic cancer and serum organochlorine levels. Cancer Epidemiol. Biomark. Prev. 2000, 9, 199–205. [Google Scholar]
- Ji, B.-T.; ScD, D.T.S.; Stewart, P.A.; Blair, A.; Swanson, G.M.; Baris, D.; Greenberg, R.S.; Hayes, R.B.; Brown, L.M.; Lillemoe, K.D.; et al. Occupational exposure to pesticides and pancreatic cancer. Am. J. Ind. Med. 2001, 39, 92–99. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P. Epidemiology and risk factors for pancreatic cancer. Best Pr. Res. Clin. Gastroenterol. 2006, 20, 197–209. [Google Scholar] [CrossRef]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef]
- Antwi, S.O.; Eckert, E.C.; Sabaque, C.V.; Leof, E.R.; Hawthorne, K.M.; Bamlet, W.R.; Chaffee, K.G.; Oberg, A.L.; Petersen, G.M. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control. 2015, 26, 1583–1591. [Google Scholar] [CrossRef]
- Kachuri, L.; Harris, M.A.; MacLeod, J.S.; Tjepkema, M.; Peters, P.A.; Demers, P.A. Cancer risks in a population-based study of 70,570 agricultural workers: Results from the Canadian census health and Environment cohort (CanCHEC). BMC Cancer 2017, 17, 343. [Google Scholar] [CrossRef]
- Kauppinen, T.; Partanen, T.; Degerth, R.; Ojajdrvi, A. Pancreatic Cancer and Occupational Exposures. Epidemiol. 1995, 6, 498–502. [Google Scholar] [CrossRef]
- Review SCS. SEER Cancer Statistics Review 1975-2017 National Cancer Institue, Bethesda, MD2018. Available online: https://seer.cancer.gov/csr/1975_2017/browse_csr.php (accessed on 10 February 2021).
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef]
- Fernandez, E.; La Vecchia, C.; D’Avanzo, B.; Negri, E.; Franceschi, S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 1994, 3, 209–212. [Google Scholar]
- Catts, Z.A.-K.; Baig, M.K.; Milewski, B.; Keywan, C.; Guarino, M.; Petrelli, N. Statewide Retrospective Review of Familial Pancreatic Cancer in Delaware, and Frequency of Genetic Mutations in Pancreatic Cancer Kindreds. Ann. Surg. Oncol. 2016, 23, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.-X.; Cong, L.; Zhao, Y.-P.; Zhang, T.-P.; Chen, G. Risk factors for the occurrence of insulinoma: A case-control study. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 324–328. [Google Scholar] [CrossRef]
- Schenk, M.; Schwartz, A.G.; O’Neal, E.; Kinnard, M.; Greenson, J.K.; Fryzek, J.P.; Ying, G.S.; Garabrant, D.H. Familial Risk of Pancreatic Cancer. J. Natl. Cancer Inst. 2001, 93, 640–644. [Google Scholar] [CrossRef]
- Fernandez, E.; La Vecchia, C.; DeCarli, A. Attributable risks for pancreatic cancer in northern Italy. Cancer Epidemiol. Biomark. Prev. 1996, 5, 23–27. [Google Scholar]
- Paiella, S.; Capurso, G.; Cavestro, G.M.; Butturini, G.; Pezzilli, R.; Salvia, R.; Signoretti, M.; Crippa, S.; Carrara, S.; Frigerio, I.; et al. Results of First-Round of Surveillance in Individuals at High-Risk of Pancreatic Cancer from the AISP (Italian Association for the Study of the Pancreas) Registry. Am. J. Gastroenterol. 2019, 114, 665–670. [Google Scholar] [CrossRef]
- Mocci, E.; Guillen-Ponce, C.; Earl, J.; Marquez, M.; Solera, J.; Salazar-López, M.T.; Calcedo-Arnáiz, C.; Vázquez-Sequeiros, E.; Montans, J.; Muñoz-Beltrán, M.; et al. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur. J. Cancer 2015, 51, 1911–1917. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, R.; He, Y.; Sun, X.; Wang, N.; Chen, T.; Chen, W. Risk Factors for Pancreatic Cancer in China: A Multicenter Case-Control Study. J. Epidemiol. 2016, 26, 64–70. [Google Scholar] [CrossRef]
- Schulte, A.; Pandeya, N.; Fawcett, J.; Fritschi, L.; Klein, K.; Risch, H.A.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Association between family cancer history and risk of pancreatic cancer. Cancer Epidemiol. 2016, 45, 145–150. [Google Scholar] [CrossRef]
- Hamada, T.; Yuan, C.; Yurgelun, M.B.; Perez, K.; Khalaf, N.; Morales-Oyarvide, V.; Babic, A.; Nowak, J.A.; Rubinson, D.A.; Giannakis, M.; et al. Family history of cancer, Ashkenazi Jewish ancestry, and pancreatic cancer risk. Br. J. Cancer 2019, 120, 848–854. [Google Scholar] [CrossRef]
- Klein, A.P.; Brune, K.A.; Petersen, G.M.; Goggins, M.; Tersmette, A.C.; Offerhaus, G.J.A.; Griffin, C.; Cameron, J.L.; Yeo, C.J.; Kern, S.; et al. Prospective Risk of Pancreatic Cancer in Familial Pancreatic Cancer Kindreds. Cancer Res. 2004, 64, 2634–2638. [Google Scholar] [CrossRef]
- Piciucchi, M.; Capurso, G.; Valente, R.; Larghi, A.; Archibugi, L.; Signoretti, M.; Stigliano, S.; Zerboni, G.; Barucca, V.; La Torre, M.; et al. Early onset pancreatic cancer: Risk factors, presentation and outcome. Pancreatol. 2015, 15, 151–155. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Takaori, K.; Morizane, C.; Maguchi, H.; Mizuma, M.; Takahashi, H.; Wada, K.; Hosoi, H.; Yachida, S.; Suzuki, M.; et al. Familial pancreatic cancer: Concept, management and issues. World J. Gastroenterol. 2017, 23, 935–948. [Google Scholar] [CrossRef]
- Moran, A.; O’Hara, C.; Khan, S.; Shack, L.; Woodward, E.; Maher, E.R.; Lalloo, F.; Evans, D.G.R. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam. Cancer 2012, 11, 235–242. [Google Scholar] [CrossRef]
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated withBRCA1andBRCA2mutations other than breast and ovarian. Cancer 2015, 121, 269–275. [Google Scholar] [CrossRef]
- Bannon, S.A.; Montiel, M.F.; Goldstein, J.B.; Dong, W.; Mork, M.E.; Borras, E.; Hasanov, M.; Varadhachary, G.R.; Maitra, A.; Katz, M.H.G.; et al. High Prevalence of Hereditary Cancer Syndromes and Outcomes in Adults with Early-Onset Pancreatic Cancer. Cancer Prev. Res. 2018, 11, 679–686. [Google Scholar] [CrossRef]
- Axilbund, J.E.; Argani, P.; Kamiyama, M.; Palmisano, E.; Raben, M.; Borges, M.; Brune, K.A.; Goggins, M.; Hruban, R.H.; Klein, A.P. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Biol. Ther. 2009, 8, 131–135. [Google Scholar] [CrossRef]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.L.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM Mutations in Patients with Hereditary Pancreatic Cancer. Cancer Discov. 2011, 2, 41–46. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Slater, E.P.; Langer, P.; Niemczyk, E.; Strauch, K.; Butler, J.; Habbe, N.; Neoptolemos, J.P.; Greenhalf, W.; Bartsch, D.K. PALB2 mutations in European familial pancreatic cancer families. Clin. Genet. 2010, 78, 490–494. [Google Scholar] [CrossRef]
- Lynch, H.T.; Deters, C.A.; Snyder, C.L.; Lynch, J.F.; Villeneuve, P.; Silberstein, J.; Martin, H.; Narod, S.A.; Brand, R.E. BRCA1 and pancreatic cancer: Pedigree findings and their causal relationships. Cancer Genet. Cytogenet. 2005, 158, 119–125. [Google Scholar] [CrossRef]
- Vasen, H.F.; Gruis, N.A.; Frants, R.R.; Van Der Velden, P.A.; Hille, E.T.; Bergman, W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int. J. Cancer 2000, 87, 809–811. [Google Scholar] [CrossRef]
- Lynch, H.T.; Fusaro, R.M.; Lynch, J.F.; Brand, R. Pancreatic cancer and the FAMMM syndrome. Fam. Cancer 2007, 7, 103–112. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz–Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; DiMagno, E.P.; Elitsur, Y.; Gates, L.K.; Perrault, J.; Whitcomb, D.C. International Hereditary Pancreatitis Study Group Hereditary Pancreatitis and the Risk of Pancreatic Cancer. J. Natl. Cancer Inst. 1997, 89, 442–446. [Google Scholar] [CrossRef]
- Howes, N.; Lerch, M.M.; Greenhalf, W.; Stocken, D.D.; Ellis, I.; Simon, P.; Truninger, K.; Ammann, R.; Cavallini, G.; Charnley, R.M.; et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin. Gastroenterol. Hepatol. 2004, 2, 252–261. [Google Scholar] [CrossRef]
- Rebours, V.; Boutron-Ruault, M.-C.; Schnee, M.; Férec, C.; Maire, F.; Hammel, P.; Ruszniewski, P.; Lévy, P. Risk of Pancreatic Adenocarcinoma in Patients With Hereditary Pancreatitis: A National Exhaustive Series. Am. J. Gastroenterol. 2008, 103, 111–119. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Applebaum, S.; Martin, S.P. Hereditary Pancreatitis and Pancreatic Carcinoma. Ann. New York Acad. Sci. 1999, 880, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Aarnio, M.; Sankila, R.; Pukkala, E.; Salovaara, R.; Aaltonen, L.A.; De La Chapelle, A.; Mecklin, J.-P. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int. J. Cancer 1999, 81, 214–218. [Google Scholar] [CrossRef]
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Er, K.C.; Hsu, C.Y.; Lee, Y.K.; Huang, M.Y.; Su, Y.C. Effect of glycemic control on the risk of pancreatic cancer: A nationwide cohort study. Medicine 2016, 95, e3921. [Google Scholar] [CrossRef]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Bragg, F.; Yang, L.; Bian, Z.; Chen, Y.; Iona, A.; Millwood, I.Y.; Lv, J.; et al. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million C hinese adults and a meta-analysis of 22 cohort studies. Int. J. Cancer 2017, 140, 1781–1788. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Bao, Y.; Qian, Z.R.; Wu, C.; Kraft, P.; Ogino, S.; Stampfer, M.J.; Sato, K.; Ma, J.; Buring, J.E.; et al. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J. Natl. Cancer Inst. 2013, 105, 1027–1035. [Google Scholar] [CrossRef]
- Austin, M.A.; Kuo, E.; Eeden, S.K.V.D.; Mandelson, M.T.; Brentnall, T.A.; Kamineni, A.; Potter, J.D. Family History of Diabetes and Pancreatic Cancer as Risk Factors for Pancreatic Cancer: The PACIFIC Study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1913–1917. [Google Scholar] [CrossRef]
- Perrin, M.C.; Terry, M.B.; Kleinhaus, K.; Deutsch, L.; Yanetz, R.; Tiram, E.; Calderon, R.; Friedlander, Y.; Paltiel, O.; Harlap, S. Gestational diabetes as a risk factor for pancreatic cancer: A prospective cohort study. BMC Med. 2007, 5, 25. [Google Scholar] [CrossRef]
- Ben, Q.; Cai, Q.; Li, Z.; Yuan, Y.; Ning, X.; Deng, S.; Wang, K. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: A case–control study. Eur. J. Cancer 2011, 47, 248–254. [Google Scholar] [CrossRef]
- Johnson, J.A.; Bowker, S.L.; Richardson, K.; Marra, C.A. Time-varying incidence of cancer after the onset of type 2 diabetes: Evidence of potential detection bias. Diabetol. 2011, 54, 2263–2271. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Stram, D.O.; Porcel, J.; Chari, S.T.; Maskarinec, G.; Le Marchand, L.; Wilkens, L.R.; Haiman, C.A.; Pandol, S.J.; Monroe, K.R. Pancreatic Cancer Following Incident Di-abetes in African Americans and Latinos: The Multiethnic Cohort. J. Natl. Cancer Inst. 2019, 111, 27–33. [Google Scholar] [CrossRef]
- Wang, F.; Gupta, S.; Holly, E.A. Diabetes Mellitus and Pancreatic Cancer in a Population-Based Case-Control Study in the San Francisco Bay Area, California. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1458–1463. [Google Scholar] [CrossRef]
- Li, D. Diabetes and pancreatic cancer. Mol. Carcinog. 2011, 51, 64–74. [Google Scholar] [CrossRef]
- Song, S.; Wang, B.; Zhang, X.; Hao, L.; Hu, X.; Li, Z.; Sun, S. Long-Term Diabetes Mellitus Is Associated with an Increased Risk of Pancreatic Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0134321. [Google Scholar] [CrossRef]
- Elena, J.W.; Steplowski, E.; Yu, K.; Hartge, P.; Tobias, G.S.; Brotzman, M.J.; Chanock, S.J.; Stolzenberg-Solomon, R.Z.; Arslan, A.A.; Bueno-De-Mesquita, H.B.; et al. Diabetes and risk of pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control. 2013, 24, 13–25. [Google Scholar] [CrossRef]
- Michaud, D.S.; Vrieling, A.; Jiao, L.; Mendelsohn, J.B.; Steplowski, E.; Lynch, S.M.; Wactawski-Wende, J.; Arslan, A.A.; Bueno-De-Mesquita, H.B.; Fuchs, C.S.; et al. Alcohol intake and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium (PanScan). Cancer Causes Control. 2010, 21, 1213–1225. [Google Scholar] [CrossRef]
- Janghorbani, M.; Dehghani, M.; Salehi-Marzijarani, M. Systematic Review and Meta-analysis of Insulin Therapy and Risk of Cancer. Horm. Cancer 2012, 3, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.J.; Poole, C.D.; Gale, E.A.M. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetol. 2009, 52, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Soranna, D.; Scotti, L.; Zambon, A.; Bosetti, C.; Grassi, G.; Catapano, A.; La Vecchia, C.; Mancia, G.; Corrao, G. Cancer Risk Associated with Use of Metformin and Sulfonylurea in Type 2 Diabetes: A Meta-Analysis. Oncologist 2012, 17, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Rosato, V.; Li, D.; Silverman, D.T.; Petersen, G.M.; Bracci, P.M.; Neale, R.E.; Muscat, J.E.; Anderson, K.E.; Gallinger, S.; et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2014, 25, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Karp, I.; Sivaswamy, A.; Booth, C. Does the use of incretin-based medications increase the risk of cancer in patients with type-2 diabetes mellitus? Pharmacoepidemiol. Drug Saf. 2019, 28, 489–499. [Google Scholar] [CrossRef]
- Buse, J.B.; Bethel, M.A.; Green, J.B.; Stevens, S.R.; Lokhnygina, Y.; Aschner, P.; Grado, C.R.; Tankova, T.; Wainstein, J.; Josse, R.; et al. Pancreatic Safety of Sitagliptin in the TECOS Study. Diabetes Care 2017, 40, 164–170. [Google Scholar] [CrossRef]
- Lee, M.; Sun, J.; Han, M.; Cho, Y.; Lee, J.-Y.; Nam, C.M.; Kang, E.S. Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients with Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors. Diabetes Care 2019, 42, 2057–2064. [Google Scholar] [CrossRef]
- Raz, I.; Bhatt, D.L.; Hirshberg, B.; Mosenzon, O.; Scirica, B.M.; Umez-Eronini, A.; Im, K.; Stahre, C.; Buskila, A.; Iqbal, N.; et al. Incidence of Pancreatitis and Pancreatic Cancer in a Randomized Controlled Multicenter Trial (SAVOR-TIMI 53) of the Dipeptidyl Peptidase-4 Inhibitor Saxagliptin. Diabetes Care 2014, 37, 2435–2441. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Holmberg, L.; Garmo, H.; Hammar, N.; Walldius, G.; Binda, E.; Lambe, M.; Jungner, I. Association between levels of C-reactive protein and leukocytes and cancer: Three repeated measurements in the Swedish AMORIS study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 428–437. [Google Scholar] [CrossRef]
- Ghuman, S.; Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; Wulaningsih, W. Serum inflammatory markers and colorectal cancer risk and survival. Br. J. Cancer 2017, 116, 1358–1365. [Google Scholar] [CrossRef]
- Sollie, S.; Michaud, D.S.; Sarker, D.; Karagiannis, S.N.; Josephs, D.H.; Hammar, N.; Santaolalla, A.; Walldius, G.; Garmo, H.; Holmberg, L.; et al. Chronic inflammation markers are associated with risk of pancreatic cancer in the Swedish AMORIS cohort study. BMC Cancer 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Grote, V.A.; Kaaks, R.; Nieters, A.; Tjønneland, A.; Halkjær, J.; Overvad, K.; Nielsen, M.R.S.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Racine, A.; et al. Inflammation marker and risk of pancreatic cancer: A nested case–control study within the EPIC cohort. Br. J. Cancer 2012, 106, 1866–1874. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Ekbom, A.; McLaughlin, J.K.; Karlsson, B.M.; Nyrén, O.; Gridley, G.; Adami, H.O.; Fraumeni, J.F., Jr. Pancreatitis and pancreatic cancer: A popu-lation-based study. J. Natl. Cancer Inst. 1994, 86, 625–627. [Google Scholar] [CrossRef]
- Goldacre, M.J.; Wotton, C.J.; Yeates, D.; Seagroatt, V.; Collier, J. Liver cirrhosis, other liver diseases, pancreatitis and subsequent cancer: Record linkage study. Eur. J. Gastroenterol. Hepatol. 2008, 20, 384–392. [Google Scholar] [CrossRef]
- Fernandez, E.; La Vecchia, C.; Porta, M.; Negri, E.; D’avanzo, B.; Boyle, P. Pancreatitis and the Risk of Pancreatic Cancer. Pancreas 1995, 11, 185–189. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; DiMagno, E.P.; Andren-Sandberg, A.; Domellof, L.; International Pancreatitis Study Group. Pancreatitis and the Risk of Pancreatic Cancer. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- Malka, D.; Hammel, P.; Maire, F.; Rufat, P.; Madeira, I.; Pessione, F.; Lévy, P.; Ruszniewski, P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 2002, 51, 849–852. [Google Scholar] [CrossRef]
- Midha, S.; Sreenivas, V.; Kabra, M.; Chattopadhyay, T.K.; Joshi, Y.K.; Garg, P.K. Genetically Determined Chronic Pancreatitis but not Alcoholic Pancreatitis Is a Strong Risk Factor for Pancreatic Cancer. Pancreas 2016, 45, 1478–1484. [Google Scholar] [CrossRef]
- Duell, E.J.; Lucenteforte, E.; Olson, S.H.; Bracci, P.M.; Li, D.; Risch, H.A.; Silverman, D.T.; Ji, B.T.; Gallinger, S.; Holly, E.A.; et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 2012, 23, 2964–2970. [Google Scholar] [CrossRef]
- Bansal, P.; Sonnenberg, A. Pancreatitis is a risk factor for pancreatic cancer. Gastroenterology 1995, 109, 247–251. [Google Scholar] [CrossRef]
- Bracci, P.M.; Wang, F.; Hassan, M.M.; Gupta, S.; Li, D.; Holly, E.A. Pancreatitis and pancreatic cancer in two large pooled case–control studies. Cancer Causes Control. 2009, 20, 1723–1731. [Google Scholar] [CrossRef]
- Karlson, B.M.; Ekbom, A.; Josefsson, S.; McLaughlin, J.K.; Fraumeni, J.F., Jr.; Nyren, O. The risk of pancreatic cancer following pan-creatitis: An association due to confounding? Gastroenterology 1997, 113, 587–592. [Google Scholar] [CrossRef]
- Talamini, G.; Falconi, M.; Bassi, C.; Sartori, N.; Salvia, R.; Caldiron, E.; Frulloni, L.; Di Francesco, V.; Vaona, B.; Bovo, P.; et al. Incidence of cancer in the course of chronic pancreatitis. Am. J. Gastroenterol. 1999, 94, 1253–1260. [Google Scholar] [CrossRef]
- Ueda, J.; Tanaka, M.; Ohtsuka, T.; Tokunaga, S.; Shimosegawa, T. Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: A multicenter retrospective analysis. Surgery 2013, 153, 357–364. [Google Scholar] [CrossRef]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aeti-ology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Pak, L.M.; Schattner, M.A.; Balachandran, V.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R.; Allen, P.J. The clinical utility of immuno-globulin G4 in the evaluation of autoimmune pancreatitis and pancreatic adenocarcinoma. HPB 2018, 20, 182–187. [Google Scholar] [CrossRef]
- Raina, A.; Krasinskas, A.M.; Greer, J.B.; Lamb, J.; Fink, M.E.; Moser, A.J.; Iii, H.J.Z.; Slivka, A.; Whitcomb, D.C. Serum Immunoglobulin G Fraction 4 Levels in Pancreatic Cancer: Elevations Not Associated With Autoimmune Pancreatitis. Arch. Pathol. Lab. Med. 2008, 132, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, T.; Law, R.; Hart, P.; Smyrk, T.C.; Chari, S.T. Serum IgG4 elevation in pancreatic cancer: Diagnostic and prognostic signifi-cance and association with autoimmune pancreatitis. Pancreas 2015, 44, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Liang, P.-C.; Jan, S.; Yang, C.-Y.; Tien, Y.-W.; Wei, S.-C.; Wong, J.-M.; Chang, Y.-T. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels. Pancreatol. 2014, 14, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Talar-Wojnarowska, R.; Gasiorowska, A.; Olakowski, M.; Dranka-Bojarowska, D.; Lampe, P.; Śmigielski, J.; Kujawiak, M.; Grzegorczyk, J.; Małecka-Panas, E. Utility of serum IgG, IgG4 and carbonic anhydrase II antibodies in distinguishing autoimmune pancreatitis from pancreatic cancer and chronic pancreatitis. Adv. Med Sci. 2014, 59, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Sollie, S.; Santaolalla, A.; Michaud, D.S.; Sarker, D.; Karagiannis, S.N.; Josephs, D.H.; Hammar, N.; Walldius, G.; Garmo, H.; Holmberg, L.; et al. Serum Immunoglobulin G Is Associated with Decreased Risk of Pancreatic Cancer in the Swedish AMORIS Study. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Turner, M.C. Epidemiology: Allergy history, IgE, and cancer. Cancer Immunol. Immunother. 2011, 61, 1493–1510. [Google Scholar] [CrossRef]
- Anderson, L.N.; Cotterchio, M.; Gallinger, S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control. 2009, 20, 825–834. [Google Scholar] [CrossRef][Green Version]
- Cotterchio, M.; Lowcock, E.; Hudson, T.J.; Greenwood, C.; Gallinger, S. Association between Allergies and Risk of Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 469–480. [Google Scholar] [CrossRef]
- Gandini, S.; Lowenfels, A.B.; Jaffee, E.M.; Armstrong, T.D.; Maisonneuve, P. Allergies and the risk of pancreatic cancer: A me-ta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1908–1916. [Google Scholar] [CrossRef]
- Gomez-Rubio, P.; Zock, J.-P.; Rava, M.; Marquez, M.; Sharp, L.; Hidalgo, M.; Carrato, A.; Ilzarbe, L.; Michalski, C.; Molero, X.; et al. Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut 2015, 66, 314–322. [Google Scholar] [CrossRef]
- Eppel, A.; Cotterchio, M.; Gallinger, S. Allergies are associated with reduced pancreas cancer risk: A population-based case–control study in Ontario, Canada. Int. J. Cancer 2007, 121, 2241–2245. [Google Scholar] [CrossRef]
- Olson, S.H. Selected medical conditions and risk of pancreatic cancer. Mol. Carcinog. 2011, 51, 75–97. [Google Scholar] [CrossRef]
- Kreiger, N.; Lacroix, J.; Sloan, M. Hormonal Factors and Pancreatic Cancer in Women. Ann. Epidemiol. 2001, 11, 563–567. [Google Scholar] [CrossRef]
- Lucenteforte, E.; Zucchetto, A.; Bosetti, C.; Talamini, R.; Negri, E.; Serraino, D.; Franceschi, S.; Lipworth, L.; La Vecchia, C. Reproductive and hormonal factors and pan-creatic cancer risk in women. Pancreas 2011, 40, 460–463. [Google Scholar] [CrossRef]
- Skinner, H.G.; Michaud, D.S.; Colditz, G.A.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol. Biomark. Prev. 2003, 12, 433–438. [Google Scholar]
- Zhang, Y.; Coogan, P.F.; Palmer, J.R.; Strom, B.L.; Rosenberg, L. A case-control study of reproductive factors, female hormone use, and risk of pancreatic cancer. Cancer Causes Control. 2010, 21, 473–478. [Google Scholar] [CrossRef]
- Karlson, B.M.; Wuu, J.; Hsieh, C.C.; Lambe, M.; Ekbom, A. Parity and the risk of pancreatic cancer: A nested case-control study. Int. J. Cancer 1998, 77, 224–227. [Google Scholar] [CrossRef]
- Andersson, G.; Borgquist, S.; Jirström, K. Hormonal factors and pancreatic cancer risk in women: The Malmö Diet and Cancer Study. Int. J. Cancer 2018, 143, 52–62. [Google Scholar] [CrossRef]
- Bueno de Mesquita, H.B.; Maisonneuve, P.; Moerman, C.J.; Walker, A.M. Anthropometric and reproductive variables and exocrine carcinoma of the pancreas: A population-based case-control study in The Netherlands. Int. J. Cancer 1992, 52, 24–29. [Google Scholar] [CrossRef]
- Fernández, E.; La Vecchia, C.; D’Avanzo, B.; Negri, E. Menstrual and reproductive factors and pancreatic cancer risk in women. Int. J. Cancer 1995, 62, 11–14. [Google Scholar] [CrossRef]
- Duell, E.J.; Holly, E.A. Reproductive and menstrual risk factors for pancreatic cancer: A population-based study of San Francisco Bay Area women. Am. J. Epidemiol. 2005, 161, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Berrington de Gonzalez, A.; Sweetland, S.; Spencer, E. A meta-analysis of obesity and the risk of pancreatic cancer. Br. J. Cancer 2003, 89, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Schairer, C.; Moore, S.; Hollenbeck, A.; Silverman, D.T. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 2013, 98, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int. J. Cancer 2007, 120, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; De Gonzalez, A.B.; Hartge, P.; Pfeiffer, R.M.; Park, Y.; Freedman, D.M.; Gail, M.H.; Alavanja, M.C.R.; Albanes, D.; Freeman, L.E.B.; et al. Body mass index, effect modifiers, and risk of pancreatic cancer: A pooled study of seven prospective cohorts. Cancer Causes Control. 2010, 21, 1305–1314. [Google Scholar] [CrossRef]
- Noor, N.M.; Banim, P.J.; Luben, R.N.; Khaw, K.T.; Hart, A.R. Investigating Physical Activity in the Etiology of Pancreatic Cancer: The Age at Which This Is Measured Is Important and Is Independent of Body Mass Index. Pancreas 2016, 45, 388–393. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Chlebowski, R.T.; Vitolins, M.Z.; Wassertheil-Smoller, S.; Rohan, T.E. Serum lipids and risk of obesity-related cancers in postmenopausal women. Cancer Causes Control. 2017, 29, 13–24. [Google Scholar] [CrossRef]
- La Torre, G.; Sferrazza, A.; Gualano, M.R.; De Waure, C.; Clemente, G.; De Rose, A.M.; Nicolotti, N.; Nuzzo, G.; Siliquini, R.; Boccia, A.; et al. Investigating the synergistic interaction of diabetes, tobacco smoking, alcohol consumption, and hypercholesterolemia on the risk of pancreatic cancer: A case-control study in Italy. Biomed. Res. Int. 2014, 2014, 481019. [Google Scholar] [CrossRef]
- Rosato, V.; Tavani, A.; Bosetti, C.; Pelucchi, C.; Talamini, R.; Polesel, J.; Serraino, D.; Negri, E.; La Vecchia, C. Metabolic syndrome and pancreatic cancer risk: A case-control study in Italy and meta-analysis. Metabolism 2011, 60, 1372–1378. [Google Scholar] [CrossRef]
- Zaleska, M.; Mozenska, O.; Bil, J. Statins use and cancer: An update. Futur. Oncol. 2018, 14, 1497–1509. [Google Scholar] [CrossRef]
- Bradley, M.C.; Hughes, C.M.; Cantwell, M.M.; Murray, L.J. Statins and pancreatic cancer risk: A nested case–control study. Cancer Causes Control. 2010, 21, 2093–2100. [Google Scholar] [CrossRef]
- Carey, F.J.; Little, M.W.; Pugh, T.F.G.; Ndokera, R.; Ing, H.; Clark, A.; Dennison, A.; Metcalfe, M.S.; Robinson, R.J.; Hart, A.R. The Differential Effects of Statins on the Risk of Developing Pancreatic Cancer: A Case–Control Study in Two Centres in the United Kingdom. Dig. Dis. Sci. 2013, 58, 3308–3312. [Google Scholar] [CrossRef]
- Chiu, H.F.; Chang, C.C.; Ho, S.C.; Wu, T.N.; Yang, C.Y. Statin use and the risk of pancreatic cancer: A population-based case-control study. Pancreas 2011, 40, 669–672. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Y.; Chen, M.; Li, J.; Liao, X.; Shen, J.; Shi, M.; Li, W.; Zheng, H.; Jiang, B. Statin use and risk of pancreatic cancer: A meta-analysis. Cancer Causes Control. 2012, 23, 1099–1111. [Google Scholar] [CrossRef]
- Kirkegård, J.; Lund, J.L.; Mortensen, F.V.; Cronin-Fenton, D. Statins and pancreatic cancer risk in patients with chronic pancreatitis: A Danish nationwide population-based cohort study. Int. J. Cancer 2020, 146, 610–616. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: Association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Inoue, M.; Noda, M.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Iso, H.; Tsugane, S. Impact of metabolic factors on subsequent cancer risk: Results from a large-scale population-based cohort study in Japan. Eur. J. Cancer Prev. 2009, 18, 240–247. [Google Scholar] [CrossRef]
- Risch, H.A.; Yu, H.; Lu, L.; Kidd, M.S. ABO Blood Group, Helicobacter pylori Seropositivity, and Risk of Pancreatic Cancer: A Case-Control Study. J. Natl. Cancer Inst. 2010, 102, 502–505. [Google Scholar] [CrossRef]
- Risch, H.A.; Lu, L.; Wang, J.; Zhang, W.; Ni, Q.; Gao, Y.-T.; Yu, H. ABO Blood Group and Risk of Pancreatic Cancer: A Study in Shanghai and Meta-Analysis. Am. J. Epidemiol. 2013, 177, 1326–1337. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Kraft, P.; Gross, M.; Helzlsouer, K.; Bueno-De-Mesquita, H.B.; Steplowski, E.; Stolzenberg-Solomon, R.Z.; Arslan, A.A.; Jacobs, E.J.; Lacroix, A.; et al. Pancreatic Cancer Risk and ABO Blood Group Alleles: Results from the Pancreatic Cancer Cohort Consortium. Cancer Res. 2010, 70, 1015–1023. [Google Scholar] [CrossRef]
- Tada, M.; Kawabe, T.; Arizumi, M.; Togawa, O.; Matsubara, S.; Yamamoto, N.; Nakai, Y.; Sasahira, N.; Hirano, K.; Tsujino, T.; et al. Pancreatic Cancer in Patients With Pancreatic Cystic Lesions: A Prospective Study in 197 Patients. Clin. Gastroenterol. Hepatol. 2006, 4, 1265–1270. [Google Scholar] [CrossRef]
- Matsubara, S.; Tada, M.; Akahane, M.; Yagioka, H.; Kogure, H.; Sasaki, T.; Arizumi, T.; Togawa, O.; Nakai, Y.; Sasahira, N.; et al. Incidental Pancreatic Cysts Found by Magnetic Resonance Imaging and Their Relationship with Pancreatic Cancer. Pancreas 2012, 41, 1241–1246. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zambirinis, C.P.; Pushalkar, S.; Saxena, D.; Miller, G. Pancreatic Cancer, Inflammation, and Microbiome. Cancer J. 2014, 20, 195–202. [Google Scholar] [CrossRef]
- Singh, G.K.; Jemal, A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J. Environ. Public Health 2017, 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-Y.; Shu, L.; Shen, S.-S.; Chen, X.-J.; Zhang, X.-Y. Dietary Patterns and Pancreatic Cancer Risk: A Meta-Analysis. Nutrients 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
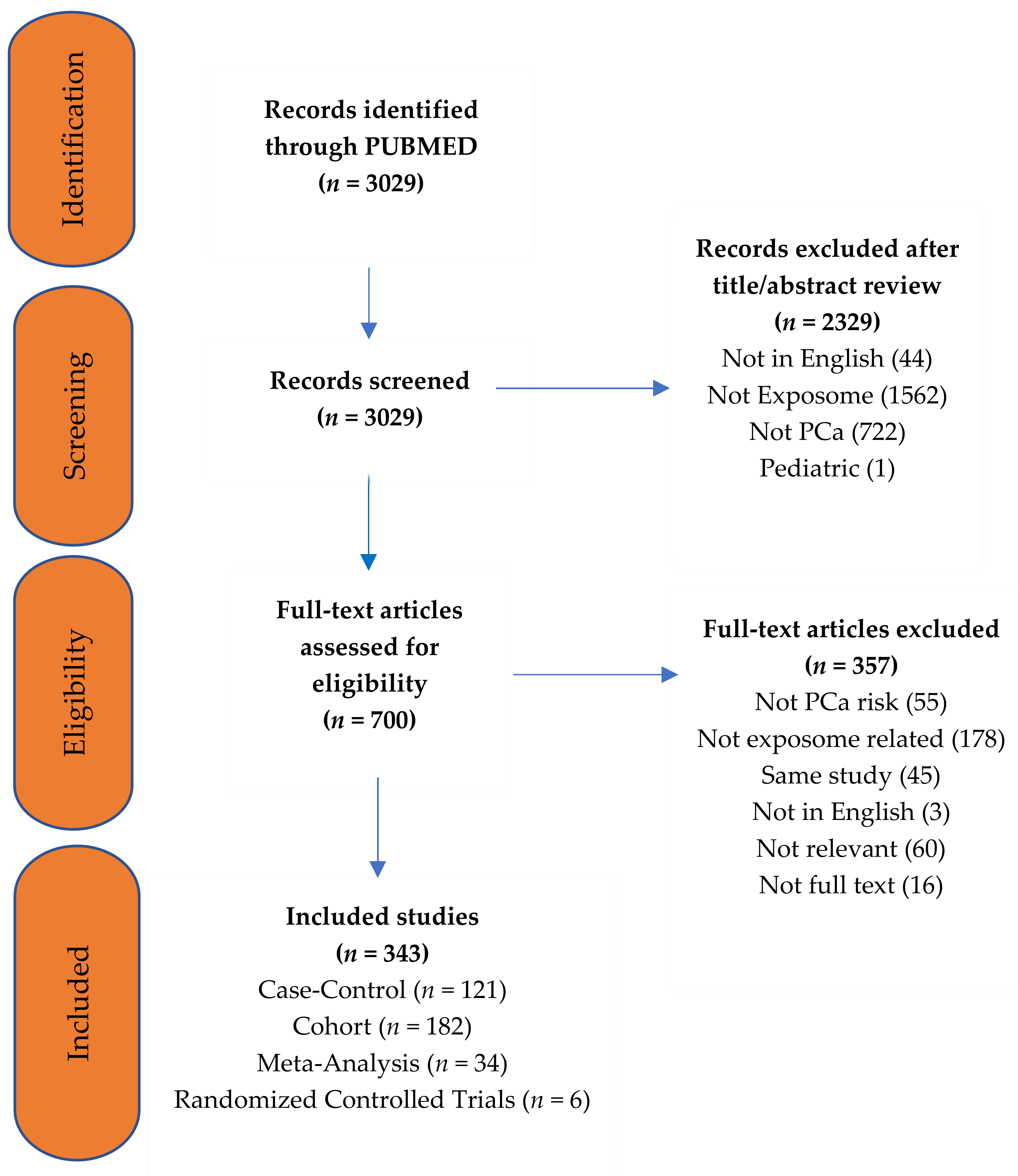
| Search No. | Search Terms | Hits |
|---|---|---|
| Disease | ||
| #1 | “Pancreatic Neoplasms”[Mesh] OR “cancer of the pancreas” [Title/Abstract] OR “pancreatic cancer” [Title/Abstract] OR “pancreatic tumour” [Title/Abstract] | 69,527 |
| Risk Factors | ||
| #2 | (“Risk Factors”[Mesh] OR “Biomarkers”[Mesh] OR “Exposome”[Mesh] OR “Causality”[Mesh] AND “etiology” [Subheading]) | 756,580 |
| #3 | #1 AND #2 | 5872 |
| Exclusions | ||
| #4 | “Animals”[Mesh] NOT “Humans”[Mesh] | 3,503,479 |
| #5 | “Adult”[Mesh] | 5,373,576 |
| Total | ||
| #6 | (#3 NOT #4) AND #5 | 3390 |
| Full text (#6) | 3029 | |
| Exposure | Studies | Summary of Findings |
|---|---|---|
| Specific External Exposures | ||
| Smoking | 9 |
|
| Alcohol Consumption | 10 |
|
| Dietary Patterns | 25 |
|
| NSAIDs | 5 |
|
| Infectious Agents | 18 |
|
| Peptic Ulcer Disease | 3 |
|
| Occupational Exposures | 6 |
|
| Allergies | 6 |
|
| Internal exposures | ||
| ABO group | 6 |
|
| Diabetes Mellitus | 18 |
|
| Pancreatitis | 12 |
|
| Hormonal and Reproductive Factors | 10 |
|
| Obesity | 8 |
|
| Physical Activity | 1 |
|
| Metabolic Syndrome | 2 |
|
| Dyslipidaemias | 2 |
|
| Statin Use | 6 |
|
| Family History of PCa | 12 |
|
| Inflammatory Markers | 2 |
|
| Genetic Factors | 12 |
|
| Cystic Lesions | 2 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy-Iglesias, M.J.; Dolly, S.; Sarker, D.; Thillai, K.; Van Hemelrijck, M.; Santaolalla, A. Pancreatic Cancer Exposome Profile to Aid Early Detection and Inform Prevention Strategies. J. Clin. Med. 2021, 10, 1665. https://doi.org/10.3390/jcm10081665
Monroy-Iglesias MJ, Dolly S, Sarker D, Thillai K, Van Hemelrijck M, Santaolalla A. Pancreatic Cancer Exposome Profile to Aid Early Detection and Inform Prevention Strategies. Journal of Clinical Medicine. 2021; 10(8):1665. https://doi.org/10.3390/jcm10081665
Chicago/Turabian StyleMonroy-Iglesias, Maria J., Saoirse Dolly, Debashis Sarker, Kiruthikah Thillai, Mieke Van Hemelrijck, and Aida Santaolalla. 2021. "Pancreatic Cancer Exposome Profile to Aid Early Detection and Inform Prevention Strategies" Journal of Clinical Medicine 10, no. 8: 1665. https://doi.org/10.3390/jcm10081665
APA StyleMonroy-Iglesias, M. J., Dolly, S., Sarker, D., Thillai, K., Van Hemelrijck, M., & Santaolalla, A. (2021). Pancreatic Cancer Exposome Profile to Aid Early Detection and Inform Prevention Strategies. Journal of Clinical Medicine, 10(8), 1665. https://doi.org/10.3390/jcm10081665







