The Importance of Platelet Glycoside Residues in the Haemostasis of Patients with Immune Thrombocytopaenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Collection and Preparation of Samples
2.3. Determination of Platelet Activation Markers
2.4. Lectin Binding Studies
2.5. Measurement of Apoptosis Markers in Platelets
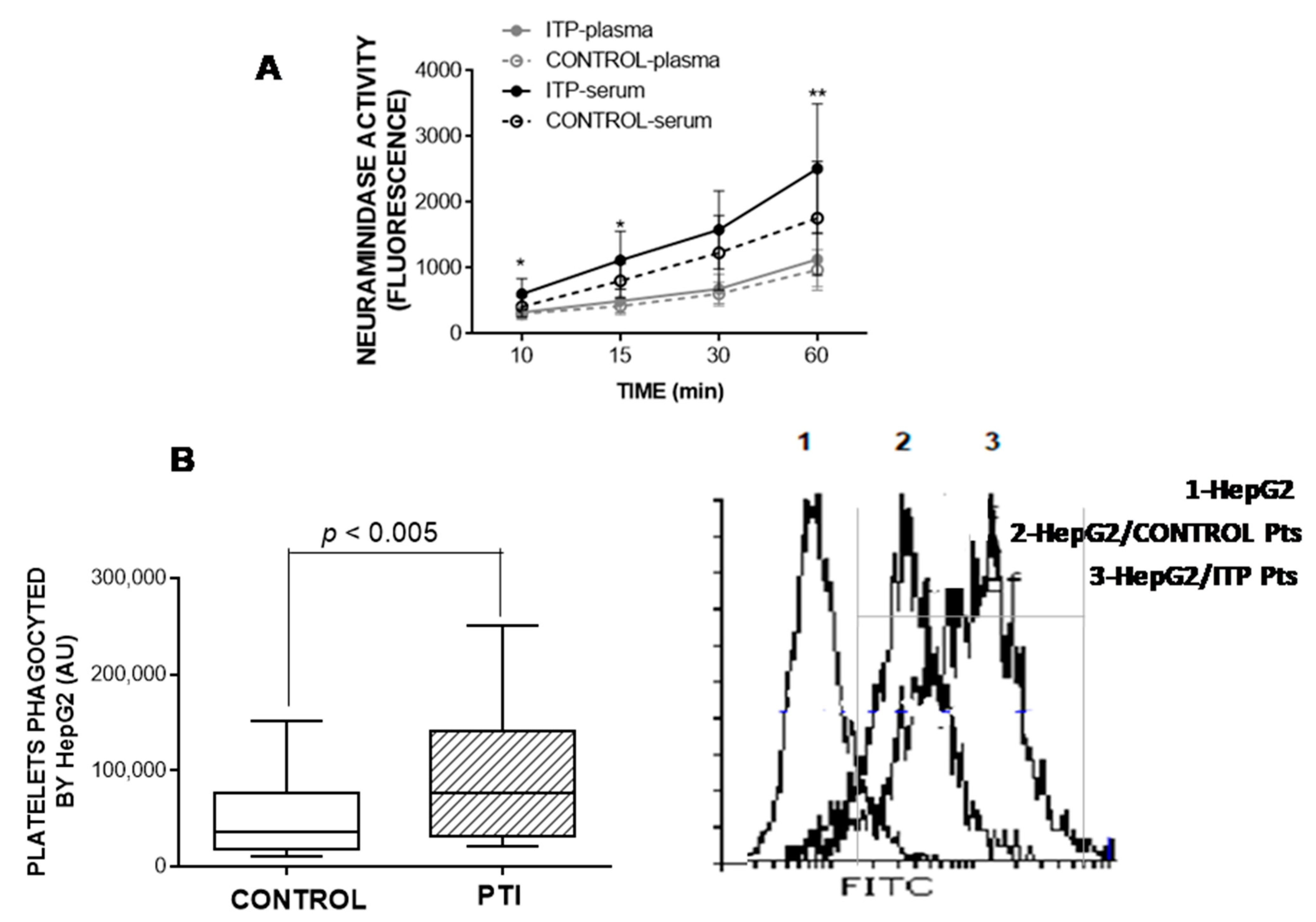
2.6. Neuraminidase Activity
2.7. HepG2 Uptake of Platelets
2.8. Statistical Analysis
3. Results
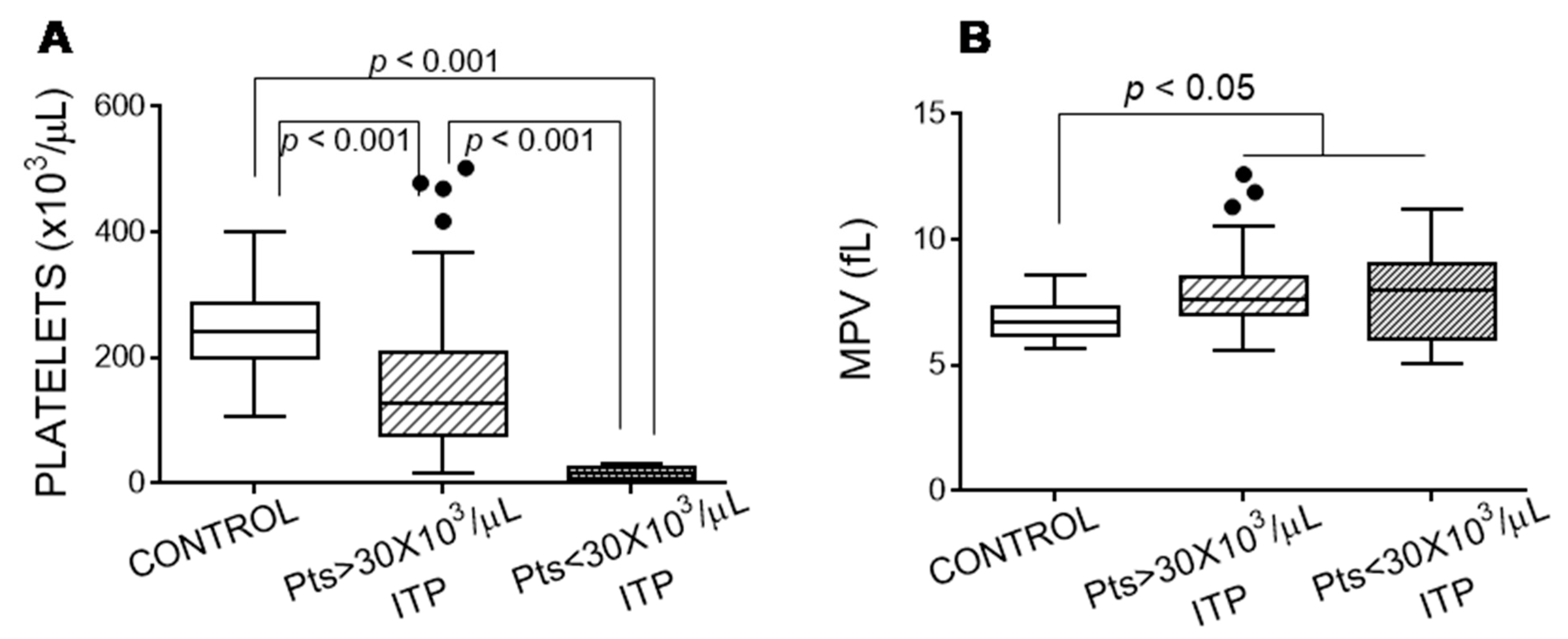
3.1. Features of Patients with Immune Thrombocytopaenia
3.2. Exposure of Glycoside Residues on the Platelet Surface
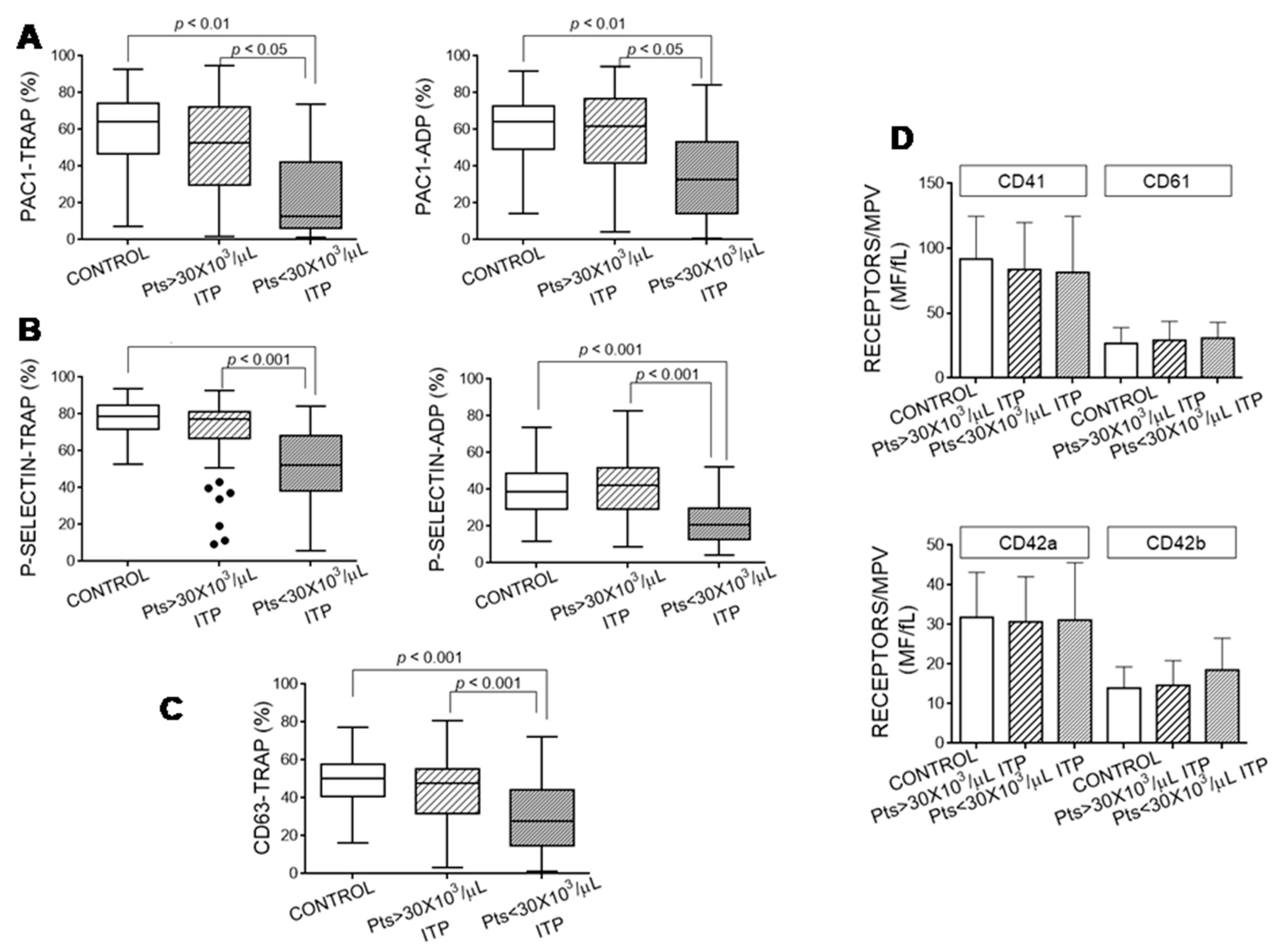
3.3. Platelet Activation Markers
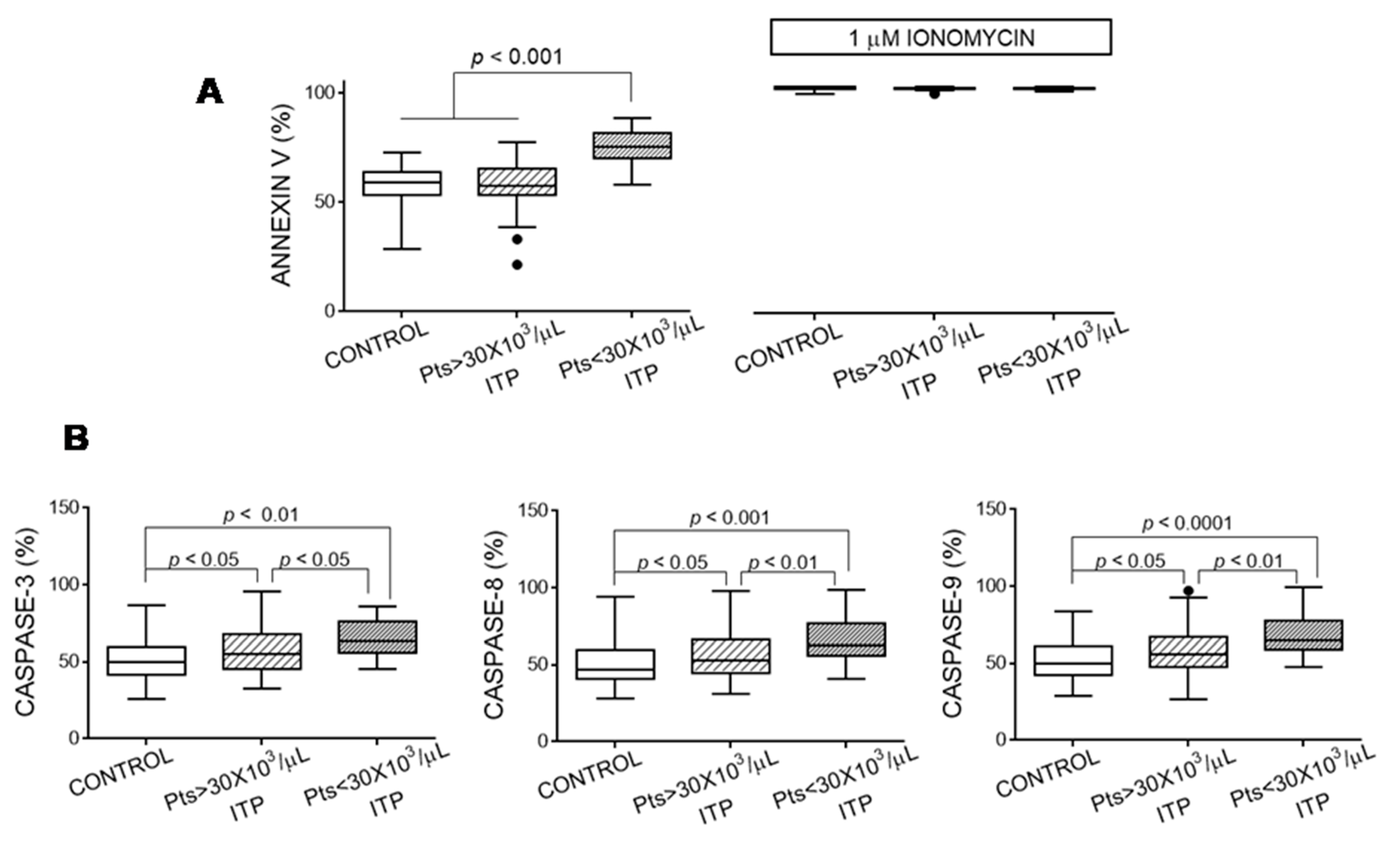
3.4. Apoptosis Markers of Platelets
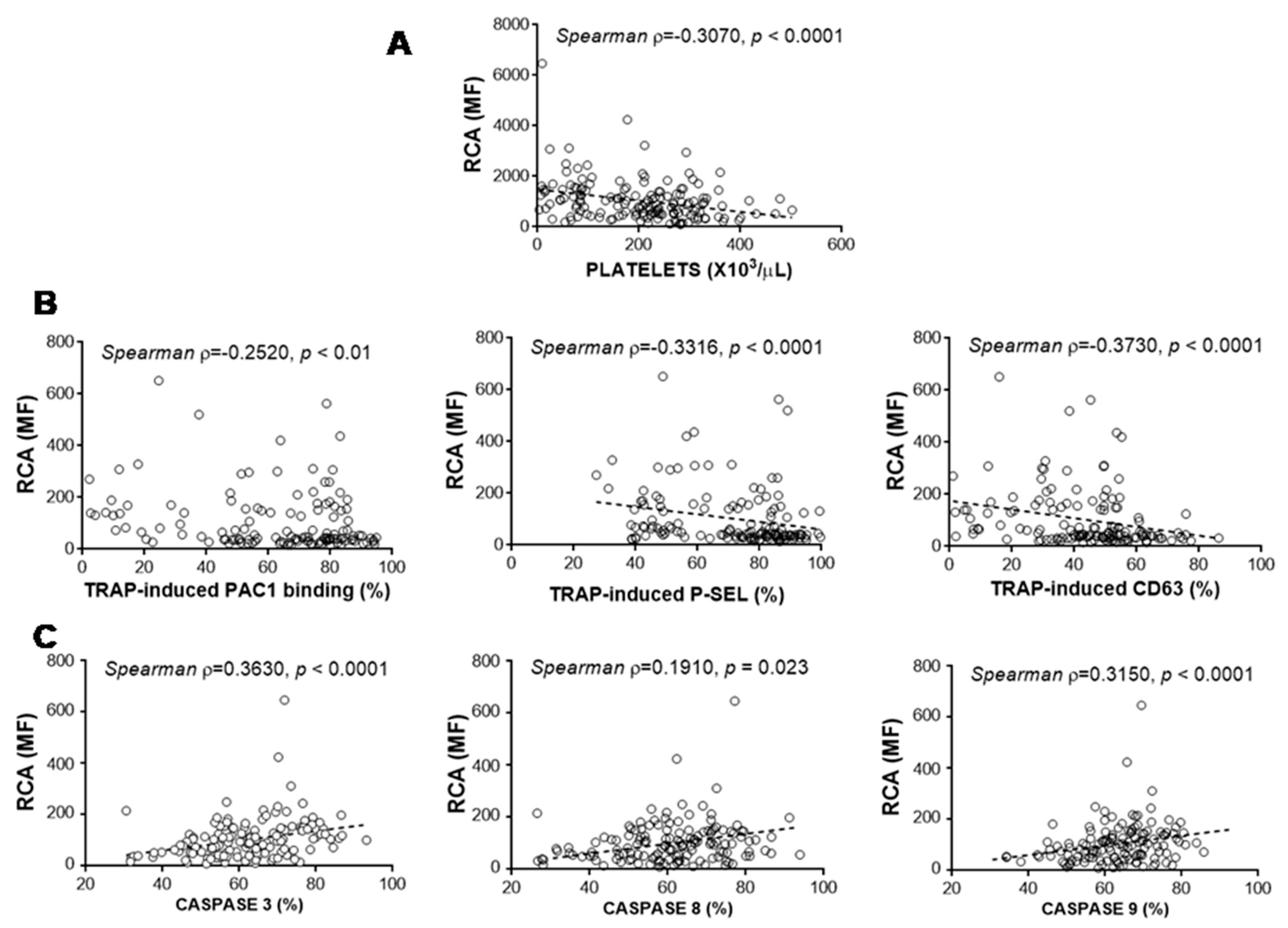
3.5. Relationship between Glycosylation and Platelet Functional Characteristics
3.6. Neuraminidase Activity in Plasma and Serum
3.7. HepG2-Based Platelet Ingestion Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of Terminology, Definitions and Outcome Criteria in Immune Thrombocytopenic Purpura of Adults and Children: Report from an International Working Group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Cooper, N.; Ghanima, W. Immune Thrombocytopenia. N. Engl. J. Med. 2019, 381, 945–955. [Google Scholar] [CrossRef]
- Swinkels, M.; Rijkers, M.; Voorberg, J.; Vidarsson, G.; Leebeek, F.W.G.; Jansen, A.J.G. Emerging Concepts in Immune Thrombocytopenia. Front. Immunol. 2018, 9, 880. [Google Scholar] [CrossRef]
- MonzonManzano, E.; Alvarez Roman, M.T.; Justo Sanz, R.; Fernandez Bello, I.; Hernandez, D.; Martin Salces, M.; Valor, L.; Rivas Pollmar, I.; Butta, N.V.; Jimenez Yuste, V. Platelet and Immune Characteristics of Immune Thrombocytopaenia Patients Non-Responsive to Therapy Reveal Severe Immune Dysregulation. Br. J. Haematol. 2020, 189, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Audia, S.; Mahevas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of Immune Thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Jaeken, J.; Gudermann, T.; Braun, A. Platelets and Defective N-Glycosylation. Int. J. Mol. Sci. 2020, 21, 5630. [Google Scholar] [CrossRef]
- Lauc, G.; Pezer, M.; Rudan, I.; Campbell, H. Mechanisms of Disease: The human N-glycome. Biochim. Biophys. Acta 2016, 1860, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Kunicki, T.J.; Cheli, Y.; Moroi, M.; Furihata, K. The influence of N-linked Glycosylation on the Function of Platelet Glycoprotein VI. Blood 2005, 106, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jobe, S.M.; Ding, X.; Choo, H.; Archer, D.R.; Mi, R.; Ju, T.; Cummings, R.D. Platelet Biogenesis and Functions Require Correct Protein O-Glycosylation. Proc. Natl. Acad. Sci. USA 2012, 109, 16143–16148. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hoffmeister, K.M.; Falet, H. Glycans and the Platelet Life Cycle. Platelets 2016, 27, 505–511. [Google Scholar] [CrossRef]
- Rumjantseva, V.; Grewal, P.K.; Wandall, H.H.; Josefsson, E.C.; Sorensen, A.L.; Larson, G.; Marth, J.D.; Hartwig, J.H.; Hoffmeister, K.M. Dual Roles for Hepatic Lectin Receptors in the Clearance of Chilled Platelets. Nat. Med. 2009, 15, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell Receptor Regulates Hepatic Thrombopoietin Production via JAK2-STAT3 Signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Roman, M.T.; Rivas Pollmar, M.I.; Bernardino, J.I.; Lozano, M.L.; Martin-Salces, M.; Fernandez-Bello, I.; Jimenez-Yuste, V.; Butta, N.V. Thrombopoietin Receptor Agonists in Conjunction with Oseltamivir for Immune Thrombocytopenia. Aids 2016, 30, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Revilla, N.; Corral, J.; Minano, A.; Mingot-Castellano, M.E.; Campos, R.M.; Velasco, F.; Gonzalez, N.; Galvez, E.; Berrueco, R.; Fuentes, I.; et al. Multirefractory Primary Immune Thrombocytopenia; Targeting the Decreased Sialic Acid Content. Platelets 2019, 30, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wu, Y.; Zhou, H.; Qin, P.; Ni, H.; Peng, J.; Hou, M. Successful Treatment with Oseltamivir Phosphate in a Patient with Chronic Immune Thrombocytopenia Positive for Anti-GPIb/IX Autoantibody. Platelets 2015, 26, 495–497. [Google Scholar] [CrossRef]
- van der Wal, D.E.; Davis, A.M.; Mach, M.; Marks, D.C. The Role of Neuraminidase 1 and 2 in Glycoprotein Ibalpha-Mediated Integrin Alphaiibbeta3 Activation. Haematologica 2020, 105, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Erlandsen, S.L.; Greet Bittermann, A.; White, J.; Leith, A.; Marko, M. High-Resolution CryoFESEM of Individual Cell Adhesion Molecules (CAMs) in the Glycocalyx of Human Platelets: Detection of P-Selectin (CD62P), GPI-IX Complex (CD42A/CD42B alpha,B beta), and Integrin GPIIbIIIa (CD41/CD61) by Immunogold Labeling and Stereo Imaging. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2001, 49, 809–819. [Google Scholar] [CrossRef]
- Li, J.; van der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation is a Mechanism of Fc-Independent Platelet Clearance and a Therapeutic Target in Immune Thrombocytopenia. Nat. Commun. 2015, 6, 7737. [Google Scholar] [CrossRef]
- Miyagi, T.; Yamaguchi, K. Mammalian Sialidases: Physiological and Pathological Roles in Cellular Functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef]
- Monti, E.; Miyagi, T. Structure and Function of Mammalian Sialidases. Top. Curr. Chem. 2015, 366, 183–208. [Google Scholar] [CrossRef]
- Li, J.; Callum, J.L.; Lin, Y.; Zhou, Y.; Zhu, G.; Ni, H. Severe Platelet Desialylation in a Patient with Glycoprotein Ib/IX Antibody-Mediated Immune Thrombocytopenia and Fatal Pulmonary Hemorrhage. Haematologica 2014, 99, e61–e63. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, X.; Li, X.; Zhang, X.; Han, P.; Zhou, H.; Shao, L.; Hou, Y.; Min, Y.; Kong, Z.; et al. CD8(+) T Cells Induce Platelet Clearance in the Liver via Platelet Desialylation in Immune Thrombocytopenia. Sci. Rep. 2016, 6, 27445. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.M.; Brown, S.A. Oligosaccharide Structures of von Willebrand Factor and Their Potential Role in von Willebrand Disease. Blood Rev. 2006, 20, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.L.; Rumjantseva, V.; Nayeb-Hashemi, S.; Clausen, H.; Hartwig, J.H.; Wandall, H.H.; Hoffmeister, K.M. Role of Sialic Acid for Platelet Life Span: Exposure of Beta-Galactose Results in the Rapid Clearance of Platelets from the Circulation by Asialoglycoprotein Receptor-Expressing Liver Macrophages and Hepatocytes. Blood 2009, 114, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, D.E.; Verhoef, S.; Schutgens, R.E.; Peters, M.; Wu, Y.; Akkerman, J.W. Role of Glycoprotein Ibalpha Mobility in Platelet Function. Thromb. Haemost. 2010, 103, 1033–1043. [Google Scholar] [CrossRef]
- Riswari, S.F.; Tunjungputri, R.N.; Kullaya, V.; Garishah, F.M.; Utari, G.S.R.; Farhanah, N.; Overheul, G.J.; Alisjahbana, B.; Gasem, M.H.; Urbanus, R.T.; et al. Desialylation of Platelets Induced by Von Willebrand Factor is a Novel Mechanism of Platelet Clearance in Dengue. PLoS Pathog. 2019, 15, e1007500. [Google Scholar] [CrossRef]
- Li, L.; Qu, C.; Lu, Y.; Gong, Y.; You, R.; Miao, L.; Guo, S. The Platelet Surface Glycosylation Caused by Glycosidase has a Strong Impact on Platelet Function. Blood Coagul. Fibrinolysis 2019, 30, 217–223. [Google Scholar] [CrossRef]
- Bellucci, S.; Huisse, M.G.; Boval, B.; Hainaud, P.; Robert, A.; Fauvel-Lafeve, F.; Jandrot-Perrus, M. Defective Collagen-Induced Platelet Activation in Two Patients with Malignant Haemopathies is Related to a Defect in the GPVI-Coupled Signalling Pathway. Thromb. Haemost. 2005, 93, 130–138. [Google Scholar] [CrossRef]
- Toonstra, C.; Hu, Y.; Zhang, H. Deciphering the Roles of N-Glycans on Collagen-Platelet Interactions. J. Proteome Res. 2019, 18, 2467–2477. [Google Scholar] [CrossRef]
- Cai, X.; Thinn, A.M.M.; Wang, Z.; Shan, H.; Zhu, J. The importance of N-glycosylation on beta3 Integrin Ligand Binding and Conformational Regulation. Sci. Rep. 2017, 7, 4656. [Google Scholar] [CrossRef]
- Wandall, H.H.; Rumjantseva, V.; Sorensen, A.L.; Patel-Hett, S.; Josefsson, E.C.; Bennett, E.P.; Italiano, J.E., Jr.; Clausen, H.; Hartwig, J.H.; Hoffmeister, K.M. The Origin and Function of Platelet Glycosyltransferases. Blood 2012, 120, 626–635. [Google Scholar] [CrossRef]
- Grewal, P.K.; Uchiyama, S.; Ditto, D.; Varki, N.; Le, D.T.; Nizet, V.; Marth, J.D. The Ashwell Receptor Mitigates the Lethal Coagulopathy of Sepsis. Nat. Med. 2008, 14, 648–655. [Google Scholar] [CrossRef]
- Lee-Sundlov, M.B.R.; Grozovsky, R.; Giannini, S.; Rivadeneyra, L.; Zheng, Y.; Glabere, S.; Kahr, W.H.; Abdi, R.; Wang, D.; Hoffmeister, K.M. Plasmacytoid Dendritic Cells Surveil Megakaryocyte Sialic Acid to Regulate Thrombopoiesis. Blood 2020, 136, 12–13. [Google Scholar] [CrossRef]
- Lee-Sundlov, M.M.; Stowell, S.R.; Hoffmeister, K.M. Multifaceted role of glycosylation in transfusion medicine, platelets, and red blood cells. J. Thromb. Haemost. 2020, 18, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Ramstrom, S.; O’Neill, S.; Dunne, E.; Kenny, D. Annexin V Binding to Platelets is Agonist, Time and Temperature Dependent. Platelets 2010, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Grodzielski, M.; Goette, N.P.; Glembotsky, A.C.; Pietto, M.C.B.; Mendez-Huergo, S.P.; Pierdominici, M.S.; Montero, V.S.; Rabinovich, G.A.; Molinas, F.C.; Heller, P.G.; et al. Multiple Concomitant Mechanisms Contribute to Low Platelet Count in Patients with Immune Thrombocytopenia. Sci. Rep. 2019, 9, 2208. [Google Scholar] [CrossRef]
- Hoffmeister, K.M.; Josefsson, E.C.; Isaac, N.A.; Clausen, H.; Hartwig, J.H.; Stossel, T.P. Glycosylation Restores Survival of Chilled Blood Platelets. Science 2003, 301, 1531–1534. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Gebhard, H.H.; Stossel, T.P.; Hartwig, J.H.; Hoffmeister, K.M. The Macrophage Alphambeta2 Integrin Alpham Lectin Domain Mediates the Phagocytosis of Chilled Platelets. J. Biol. Chem. 2005, 280, 18025–18032. [Google Scholar] [CrossRef] [PubMed]
- Cedzynski, M.; Swierzko, A.S. Components of the Lectin Pathway of Complement in Haematologic Malignancies. Cancers 2020, 12, 1792. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Fumagalli, S.; Csaszar, E.; Toth, K.; De Blasio, D.; Zangari, R.; Lenart, N.; Denes, A.; De Simoni, M.G. Mannose-Binding Lectin Drives Platelet Inflammatory Phenotype and Vascular Damage After Cerebral Ischemia in Mice via IL (Interleukin)-1alpha. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Saevarsdottir, S.; Vikingsdottir, T.; Valdimarsson, H. The Potential Role of Mannan-Binding Lectin in the Clearance of Self-Components Including Immune Complexes. Scand. J. Immunol. 2004, 60, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Castelli, R.; LambertenghiDelilliers, G.; Gidaro, A.; Cicardi, M.; Bergamaschini, L. Complement Activation in Patients with Immune Thrombocytopenic Purpura According to Phases of Disease Course. Clin. Exp. Immunol. 2020, 201, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Peerschke, E.I.; Andemariam, B.; Yin, W.; Bussel, J.B. Complement Activation on Platelets Correlates with a Decrease in Circulating Immature Platelets in Patients with Immune Thrombocytopenic Purpura. Br. J. Haematol. 2010, 148, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R. Siglecs in Innate Immunity. Curr. Opin. Pharmacol. 2005, 5, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Cobb, B.A. The Direct and Indirect Effects of Glycans on Immune Function. Glycobiology 2017, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the Immune System and The Altered Glycan Theory of Autoimmunity: A Critical Review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef]

| Lectin | Aleuria Aurantia | Concavalin A | Datura Stramonium | Ricinus Communis Agglutinin I | Wheat Germ Agglutinin |
|---|---|---|---|---|---|
| Abbreviation | AA | C | DS | RCA | WGA |
| Sugar specificity | α1,6-Fucose | α-Mannose | GalNAc | Galactose GalNAc | β-GlcNAc |
| <30,000 Platelets/µL | >30,000 Platelets/µL | |||||
|---|---|---|---|---|---|---|
| Gender (%) | Age Mean ± SD | Concomitant Medication (Nº Patients) | Gender (%) | Age Mean ± SD | Concomitant Medication (Nº Patients) | |
| No treatment | M: 2 (50) F: 2 (50) | 49 ± 21.6 | - | M: 11(34) F: 21 (66) | 56 ± 19 | +Anticoagulant: 3 |
| Eltrombopag | M: 3 (50) F: 3 (50) | 46 ± 11.8 | +IGIV (2) +Corticosteroids (1) +Corticosteroids + azathioprine (2) | M: 3 (21) F: 11 (79) | 54 ± 21.5 | +IGIV: 1 +Anticoagulant: 5 |
| Romiplostim | M: 3(50) F: 3(50) | 58 ± 25.9 | - | M: 9 (56) F: 7 (44) | 57 ± 19.5 | +IGIV: 1 +Corticosteroids: 5 |
| IGIV | M: 0 (0) F: 1(100) | 62 | - | - | - | - |
| Corticosteroids | - | - | - | M: 0 F: 3(100) | 51 ± 2.5 | +IGIV: 2 +Rituximab: 1 |
| Recognised Glycoside Residue (Lectin) | α-1,6 Fucose (AA) | α-Mannose (C) | GalNAc (DS) | β-GluNAc (WGA) |
|---|---|---|---|---|
| Lectin binding vs. Platelet count (Spearman ρ, p) | −0.3946; 0.0002 | −0.3964; 0.0002 | −0.4091; 0.0001 | −0.2868; 0.0177 |
| Lectin binding vs. TRAP-PAC1 binding (Spearman ρ, p) | −0.2297; 0.392 | −0.2567; 0.040 | −0.2581; 0.0185 | −0.3737; 0.0007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-López, A.; Álvarez Román, M.T.; Monzón Manzano, E.; Acuña, P.; Arias-Salgado, E.G.; Martín Salces, M.; Rivas Pollmar, M.I.; Jiménez Yuste, V.; Justo Sanz, R.; García Barcenilla, S.; et al. The Importance of Platelet Glycoside Residues in the Haemostasis of Patients with Immune Thrombocytopaenia. J. Clin. Med. 2021, 10, 1661. https://doi.org/10.3390/jcm10081661
Ramírez-López A, Álvarez Román MT, Monzón Manzano E, Acuña P, Arias-Salgado EG, Martín Salces M, Rivas Pollmar MI, Jiménez Yuste V, Justo Sanz R, García Barcenilla S, et al. The Importance of Platelet Glycoside Residues in the Haemostasis of Patients with Immune Thrombocytopaenia. Journal of Clinical Medicine. 2021; 10(8):1661. https://doi.org/10.3390/jcm10081661
Chicago/Turabian StyleRamírez-López, Andrés, María Teresa Álvarez Román, Elena Monzón Manzano, Paula Acuña, Elena G. Arias-Salgado, Mónica Martín Salces, María Isabel Rivas Pollmar, Víctor Jiménez Yuste, Raul Justo Sanz, Sara García Barcenilla, and et al. 2021. "The Importance of Platelet Glycoside Residues in the Haemostasis of Patients with Immune Thrombocytopaenia" Journal of Clinical Medicine 10, no. 8: 1661. https://doi.org/10.3390/jcm10081661
APA StyleRamírez-López, A., Álvarez Román, M. T., Monzón Manzano, E., Acuña, P., Arias-Salgado, E. G., Martín Salces, M., Rivas Pollmar, M. I., Jiménez Yuste, V., Justo Sanz, R., García Barcenilla, S., Cebanu, T., González Zorrilla, E., & Butta, N. V. (2021). The Importance of Platelet Glycoside Residues in the Haemostasis of Patients with Immune Thrombocytopaenia. Journal of Clinical Medicine, 10(8), 1661. https://doi.org/10.3390/jcm10081661







