Secular Trends in Ablation Therapy for Graves’ Disease: An Analysis of a 15-Year Experience at a Tertiary Hospital in South Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Definition of the Clinical Outcomes of GD
2.3. Statistical Analysis
3. Results
3.1. Trends in Ablation Therapy for GD during the 15-Year Period
3.2. Changes in the Reasons for Ablation Therapy for GD during the 15-Year Period
3.3. Changes in the Clinical Characteristics of Patients during the 15-Year Period
3.4. Clinical Characteristics of Patients According to the Treatment Modality
3.5. Clinical Outcomes of Ablation Therapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N. American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.H.; Moon, J.H.; Kim, I.; Bom, H.; Lee, J.; Chung, W.Y.; Chuang, J.H.; Shong, Y.K. The Diagnosis and Management of Hyperthyroidism Consensus-Report of the Korean Thyroid Association. J. Korean Thyroid Assoc. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Sundaresh, V.; Brito, J.P.; Thapa, P.; Bahn, R.S.; Stan, M.N. Comparative Effectiveness of Treatment Choices for Graves’ Hyperthyroidism: A Historical Cohort Study. Thyroid 2017, 27, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kornelius, E.; Yang, Y.S.; Huang, C.N.; Wang, Y.H.; Lo, S.C.; Lai, Y.R.; Chiou, J.-Y. The Trends of Hyperthyroidism Treatment in Taiwan: A Nationwide Population-Based Study. Endocr. Pract. 2018, 24, 573–579. [Google Scholar] [CrossRef]
- Bartalena, L.; Burch, H.B.; Burman, K.D.; Kahaly, G.J. A 2013 European survey of clinical practice patterns in the management of Graves’ disease. Clin. Endocrinol. 2016, 84, 115–120. [Google Scholar] [CrossRef]
- Burch, H.B.; Burman, K.D.; Cooper, D.S. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J. Clin. Endocrinol. Metab. 2012, 97, 4549–4558. [Google Scholar] [CrossRef]
- Cho, B.Y.; Koh, C.S. Current Trends in The Diagnosis and Treatment of Graves’ Disease in Korea. J. Korean Soc. Endocrinol. 1992, 7, 216–227. [Google Scholar]
- Moon, J.H.; Yi, K.H. The diagnosis and management of hyperthyroidism in Korea: Consensus report of the korean thyroid association. Endocrinol. Metab. 2013, 28, 275–279. [Google Scholar] [CrossRef]
- Seo, G.H.; Kim, S.W.; Chung, J.H. Incidence & Prevalence of Hyperthyroidism and Preference for Therapeutic Modalities in Korea. J. Korean Thyroid Assoc. 2013, 6, 56. [Google Scholar]
- Sundaresh, V.; Brito, J.P.; Wang, Z.; Prokop, L.J.; Stan, M.N.; Murad, M.H.; Bahn, R.S. Comparative effectiveness of therapies for Graves’ hyperthyroidism: A systematic review and network meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3671–3677. [Google Scholar] [CrossRef]
- Kim, Y.A.; Cho, S.W.; Choi, H.S.; Moon, S.; Moon, J.H.; Kim, K.W.; Park, D.J.; Yi, K.H.; Park, Y.J.; Cho, B.Y. The Second Antithyroid Drug Treatment Is Effective in Relapsed Graves’ Disease Patients: A Median 11-Year Follow-Up Study. Thyroid 2017, 27, 491–496. [Google Scholar] [CrossRef]
- Bartalena, L.; Baldeschi, L.; Boboridis, K.; Eckstein, A.; Kahaly, G.J.; Marcocci, C.; Perros, P.; Salvi, M.; Wiersinga, W.M. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur. Thyroid. J. 2016, 5, 9–26. [Google Scholar] [CrossRef]
- Moon, J.H.; Hyun, M.K.; Lee, J.Y.; Shim, J.I.; Kim, T.H.; Choi, H.S.; Ahn, H.Y.; Kim, K.W.; Park, D.J.; Park, Y.J.; et al. Prevalence of thyroid nodules and their associated clinical parameters: A large-scale, multicenter-based health checkup study. Korean J. Intern. Med. 2018, 33, 753–762. [Google Scholar] [CrossRef]
- Yi, K.H.; Lee, E.K.; Kang, H.-C.; Koh, Y.; Kim, S.W.; Kim, I.J.; Na, D.G.; Ryu, J.-S.; Park, S.Y.; Park, I.A.; et al. Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer. Int. J. Thyroidol. 2016, 9, 59. [Google Scholar] [CrossRef]
- Jia, Q.; Li, X.; Liu, Y.; Li, L.; Kwong, J.S.; Ren, K.; Jiang, Y.; Sun, X.; Tian, H.; Li, S. Incidental thyroid carcinoma in surgery-treated hyperthyroid patients with Graves’ disease: A systematic review and meta-analysis of cohort studies. Cancer Manag. Res. 2018, 10, 1201–1207. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, H.; Chang, H.S.; Park, C.S. Papillary thyroid microcarcinomas are different from latent papillary thyroid carcinomas at autopsy. J. Korean Med. Sci. 2014, 29, 676–679. [Google Scholar] [CrossRef]
- Leboulleux, S.; Tuttle, R.M.; Pacini, F.; Schlumberger, M. Papillary thyroid microcarcinoma: Time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol. 2016, 4, 933–942. [Google Scholar] [CrossRef]
- Ergin, A.B.; Saralaya, S.; Olansky, L. Incidental papillary thyroid carcinoma: Clinical characteristics and prognostic factors among patients with Graves’ disease and euthyroid goiter, Cleveland Clinic experience. Am. J. Otolaryngol. 2014, 35, 784–790. [Google Scholar] [CrossRef]
- Kikuchi, S.; Noguchi, S.; Yamashita, H.; Uchino, S.; Kawamoto, H. Prognosis of small thyroid cancer in patients with Graves’ disease. Br. J. Surg. 2006, 93, 434–439. [Google Scholar] [CrossRef]
- Abraham-Nordling, M.; Torring, O.; Hamberger, B.; Lundell, G.; Tallstedt, L.; Calissendorff, J. Graves’ disease: A long-term quality-of-life follow up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid 2005, 15, 1279–1286. [Google Scholar] [CrossRef]
- Li, H.X.; Xiang, N.; Hu, W.K.; Jiao, X.L. Relation between therapy options for Graves’ disease and the course of Graves’ ophthalmopathy: A systematic review and meta-analysis. J. Endocrinol. Investig. 2016, 39, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Elfenbein, D.M.; Schneider, D.F.; Havlena, J.; Chen, H.; Sippel, R.S. Clinical and socioeconomic factors influence treatment decisions in Graves’ disease. Ann. Surg. Oncol. 2015, 22, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Tellez, M.; Cooper, J.; Edmonds, C. Graves’ ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin. Endocrinol. 1992, 36, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Khong, J.J.; Finch, S.; De Silva, C.; Rylander, S.; Craig, J.E.; Selva, D.; Ebeling, P.R. Risk Factors for Graves’ Orbitopathy; the Australian Thyroid-Associated Orbitopathy Research (ATOR) Study. J. Clin. Endocrinol. Metab. 2016, 101, 2711–2720. [Google Scholar] [CrossRef]
- Genovese, B.M.; Noureldine, S.I.; Gleeson, E.M.; Tufano, R.P.; Kandil, E. What is the best definitive treatment for Graves’ disease? A systematic review of the existing literature. Ann. Surg. Oncol. 2013, 20, 660–667. [Google Scholar] [CrossRef]
- Feroci, F.; Rettori, M.; Borrelli, A.; Coppola, A.; Castagnoli, A.; Perigli, G.; Cianchi, F.; Scatizzi, M. A systematic review and meta-analysis of total thyroidectomy versus bilateral subtotal thyroidectomy for Graves’ disease. Surgery 2014, 155, 529–540. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, P.; Liu, Z.; Si, Y.; Jin, M. Total thyroidectomy vs bilateral subtotal thyroidectomy in patients with Graves’ diseases: A meta-analysis of randomized clinical trials. Clin. Endocrinol. 2013, 79, 739–746. [Google Scholar]
- Sung, T.Y.; Kim, Y.S.; Lee, S.H.; Yoon, J.H.; Hong, S.J. Surgical Treatment of Graves’ Disease: Comparison between Total Thyroidectomy and Subtotal Thyroidectomy. J. Korean Surg. Soc. 2009, 77, 82–87. [Google Scholar] [CrossRef]
- Sung, T.Y.; Lee, Y.M.; Yoon, J.H.; Chung, K.W.; Hong, S.J. Long-Term Effect of Surgery in Graves’ Disease: 20 Years Experience in a Single Institution. Int. J. Endocrinol. 2015, 2015, 542641. [Google Scholar] [CrossRef]
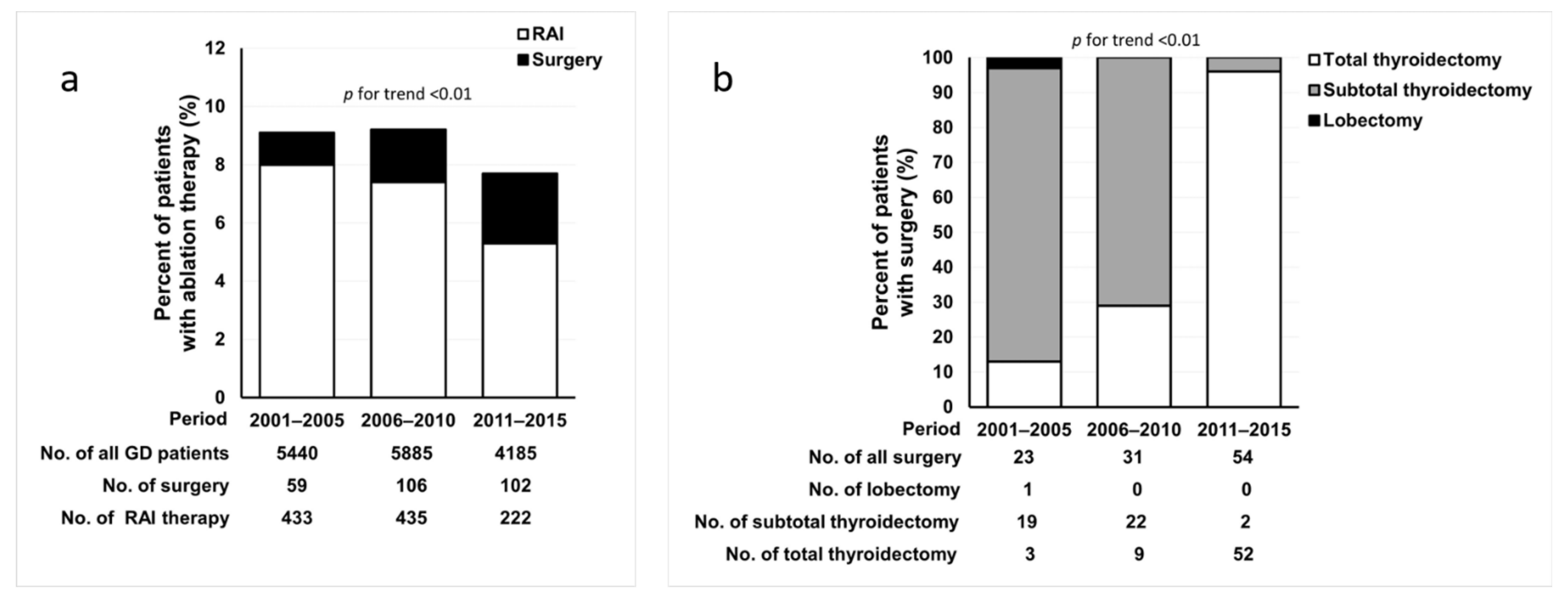
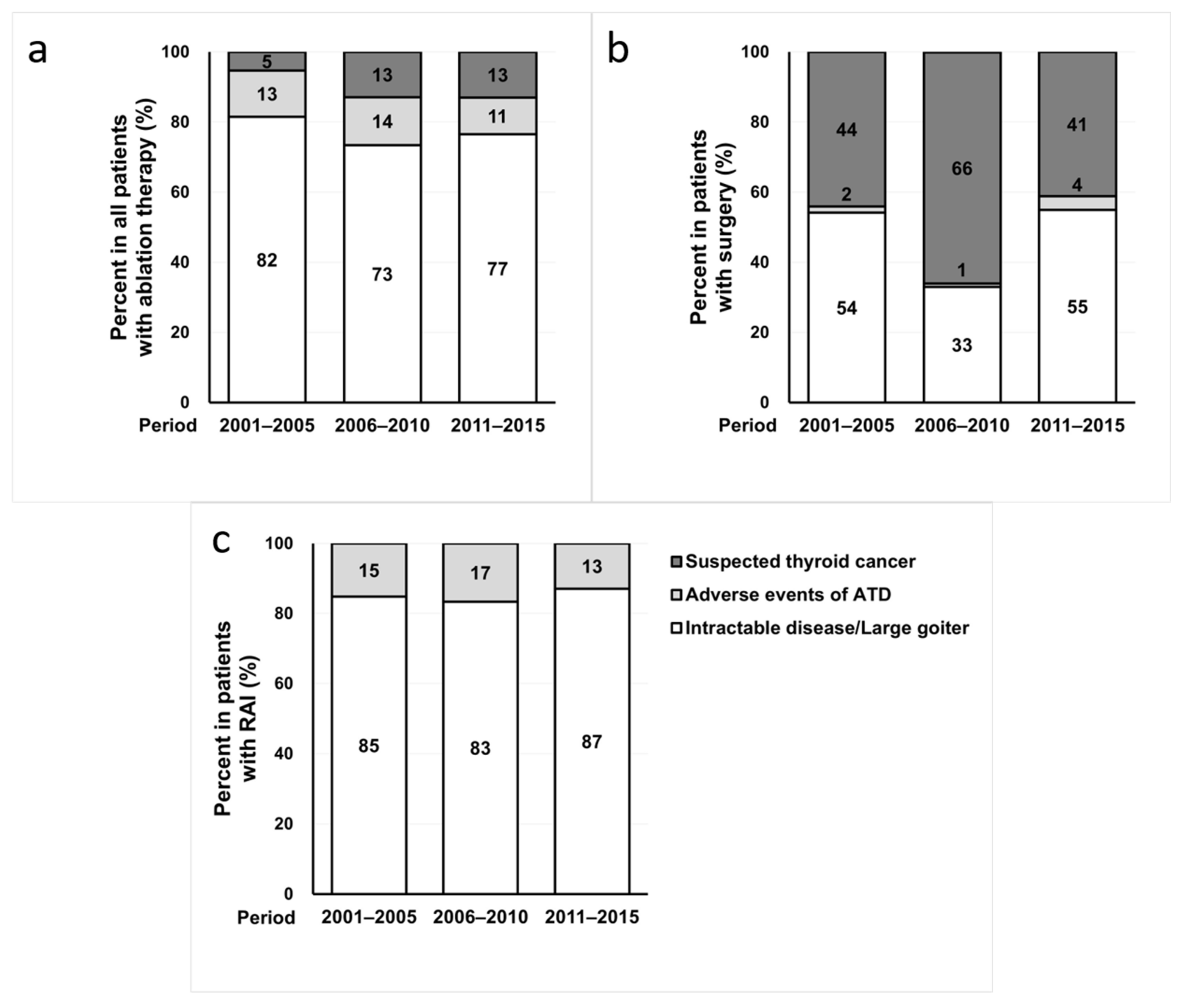
| Characteristics | Total (n = 1201) | 2001–2005 (n = 432) | 2006–2010 (n = 474) | 2011–2015 (n = 295) | p for trend |
|---|---|---|---|---|---|
| Age at ablation therapy (years) | 42 ± 13 | 39 ± 12 | 42 ± 13 | 46 ± 14 | <0.001 |
| Sex, female, n (%) | 855 (71) | 309 (72) | 322 (68) | 224 (76) | 0.300 |
| Time from diagnosis to ablation therapy (years) | 5.1 ± 5.2 | 4.4 ± 4.8 | 4.6 ± 4.8 | 6.6 ± 5.9 | <0.001 |
| GO, n (%) | 89 (7) | 17 (4) | 43 (9) | 29 (10) | 0.002 |
| Moderate-to-severe GO, n (%) | 16 (1.3) | 5 (1.2) | 7 (1.5) | 4 (1.4) | 0.915 |
| Characteristics | RAI (n = 968) | Surgery for GD (n = 106) | Surgery for Cancer (n = 127) |
|---|---|---|---|
| Age at ablation therapy (years) | 41 ± 13 | 39 ± 14 | 48 ± 14 a,b |
| Sex, female, n (%) | 663 (69) | 91 (86) a | 101 (80) a |
| Time from diagnosis to ablation therapy (years) | 4.9 ± 4.8 | 6.9 ± 6.9 a | 4.4 ± 6.0 |
| Follow-up duration (years) | 7.2 ± 4.4 | 5.5 ± 3.3 a | 7.2 ± 3.1 b |
| GO, n (%) | 67 (7) | 10 (9) | 12 (9) |
| Moderate-to-severe GO, n (%) | 12 (1.2) | 4 (3.8) a | 0 (0) |
| Reason for ablation therapy, n (%) | |||
| Intractable disease ± large goiter | 820 (85) | 100 (94) a | |
| Adverse events of ATD | 148 (15) | 6 (6) a | |
| Suspected thyroid cancer | 127 (100) a,b |
| Characteristics | Incidental Cancer (Surgery for GD) (n = 11) | Suspected Cancer (Surgery for Cancer) (n = 110) | p |
|---|---|---|---|
| Mean age at surgery (years) | 42 ± 13 | 48 ± 13 | 0.19 |
| <55 years | 9 (82) | 74 (67) | 0.50 |
| ≥55 years | 2 (18) | 36 (33) | |
| Sex, female, n (%) | 10 (91) | 86 (78) | 0.46 |
| Tumor type, n (%) | |||
| PTC | 9 (82) | 97 (88) | 0.46 |
| FTC | 2 (18) | 9 (8) | |
| PDTC | 0 (0) | 2 (2) | |
| MTC | 0 (0) | 2 (2) | |
| Tumor size (cm), n (%) | 0.7 ± 0.7 | 1.3 ± 1.2 | <0.01 |
| ≤0.5 cm | 8 (73) | 25 (23) | <0.01 |
| 0.6–1.0 cm | 1 (9) | 39 (35) | |
| 1.1–2.0 cm | 1 (9) | 29 (26) | |
| >2.0 cm | 1(9) | 17 (16) | |
| Multiplicity, n (%) | 3 (27) | 40 (36) | 0.75 |
| ETE, n (%) | 1 (9) | 54 (50) | 0.01 |
| LN metastasis, n (%) | 1 (9) | 25 (23) | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Kim, Y.A.; Cho, S.W.; Kim, S.-j.; Lee, K.E.; Park, Y.J.; Park, D.J.; Cho, B.Y. Secular Trends in Ablation Therapy for Graves’ Disease: An Analysis of a 15-Year Experience at a Tertiary Hospital in South Korea. J. Clin. Med. 2021, 10, 1629. https://doi.org/10.3390/jcm10081629
Kim MJ, Kim YA, Cho SW, Kim S-j, Lee KE, Park YJ, Park DJ, Cho BY. Secular Trends in Ablation Therapy for Graves’ Disease: An Analysis of a 15-Year Experience at a Tertiary Hospital in South Korea. Journal of Clinical Medicine. 2021; 10(8):1629. https://doi.org/10.3390/jcm10081629
Chicago/Turabian StyleKim, Min Joo, Ye An Kim, Sun Wook Cho, Su-jin Kim, Kyu Eun Lee, Young Joo Park, Do Joon Park, and Bo Youn Cho. 2021. "Secular Trends in Ablation Therapy for Graves’ Disease: An Analysis of a 15-Year Experience at a Tertiary Hospital in South Korea" Journal of Clinical Medicine 10, no. 8: 1629. https://doi.org/10.3390/jcm10081629
APA StyleKim, M. J., Kim, Y. A., Cho, S. W., Kim, S.-j., Lee, K. E., Park, Y. J., Park, D. J., & Cho, B. Y. (2021). Secular Trends in Ablation Therapy for Graves’ Disease: An Analysis of a 15-Year Experience at a Tertiary Hospital in South Korea. Journal of Clinical Medicine, 10(8), 1629. https://doi.org/10.3390/jcm10081629






