Molecular Landscape of the Epithelial–Mesenchymal Transition in Endometrioid Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Materials
2.3. RNA Isolation
2.4. The mRNA Microarray Analysis
2.5. The miRNA Microarray Analysis
2.6. Reverse-Transcription Quantitative Polymerase Chain Reaction
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. The mRNA Microarray Profile of Genes Involved in EMT in the Tissue and Whole Blood Samples
3.2. Results of Real-Time Quantitative Polymerase Chain Reaction
3.3. Results of miRNA Microarray
3.4. Predictive Analysis of Expression Regulation of Genes Connected with EMT Selected as Differentiating through the miR-144, miR-106a, and miR-30d Molecules
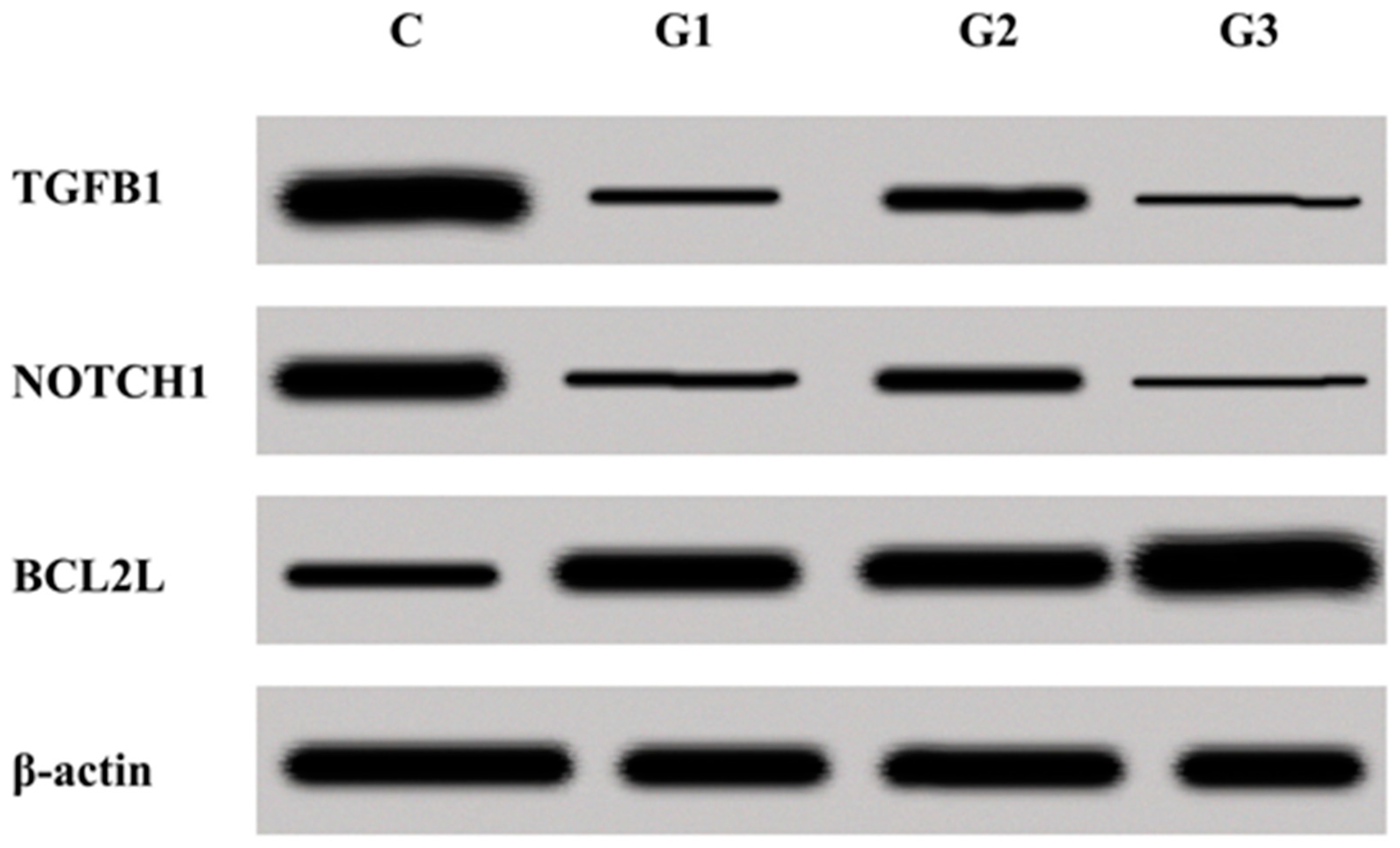
3.5. Concentration of TGFβ1, NOTCH1, and BCL2L Determined by the Western Blot Technique
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Diepenbruck, M.; Christofori, G. Epithelial–mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A. The Regulatory Role of MicroRNAs in EMT and Cancer. J. Oncol. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, X.; Yu, L.; Zhou, R.; Li, A.; Li, M.; Yang, W. Differential expression and clinical significance of epithelial-mesenchymal transition markers among different histological types of triple-negative breast cancer. J. Cancer 2018, 9, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.-H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef]
- Liao, T.-T.; Yang, M.-H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Karlsson, M.C.; Gonzalez, S.F.; Welin, J.; Fuxe, J. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Mol. Oncol. 2017, 11, 781–791. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.-P.; Lièvre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of Breast Cancer Stem Cells through Epithelial-Mesenchymal Transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Hosseinahli, N.; Aghapour, M.; Duijf, P.H.G.; Baradaran, B. Treating cancer with microRNA replacement therapy: A literature review. J. Cell. Physiol. 2018, 233, 5574–5588. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P. MicroRNAs as biomarkers for clinical studies. Exp. Biol. Med. 2017, 243, 283–290. [Google Scholar] [CrossRef]
- Wang, H.; An, X.; Yu, H.; Zhang, S.; Tang, B.; Zhang, X.; Li, Z. MiR-29b/TET1/ZEB2 signaling axis regulates metastatic properties and epithelial-mesenchymal transition in breast cancer cells. Oncotarget 2017, 8, 102119–102133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, X.; Zeng, X.; Peng, X. The Role of Mir-148a in Cancer. J. Cancer 2016, 7, 1233–1241. [Google Scholar] [CrossRef]
- Shi, W.; Wang, X.; Ruan, L.; Fu, J.; Liu, F.; Qu, J. MiR-200a promotes epithelial-mesenchymal transition of endometrial cancer cells by negatively regulating FOXA2 expression. Die Pharm. 2017, 72, 694–699. [Google Scholar]
- Jing, X.; Peng, J.; Dou, Y.; Sun, J.; Ma, C.; Wang, Q.; Zhang, L.; Luo, X.; Kong, B.; Zhang, Y.; et al. Macrophage ERα promoted invasion of endometrial cancer cell by mTOR/KIF5B-mediated epithelial to mesenchymal transition. Immunol. Cell Biol. 2019, 97, 563–576. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (accessed on 13 March 2021).
- Michalak, M.; Florczak, Z.W.; Staszewska-Nowak, A.; Roszak, A. Adjuvant therapy for early endometrial cancer—who benefits the most from a radiation therapy? Gin. Pol. 2020, 91, 13–19. [Google Scholar] [CrossRef]
- Helpman, L.; Kupets, R.; Covens, A.; Saad, R.S.; Khalifa, M.A.; Ismiil, N.; Ghorab, Z.; Dubé, V.; Nofech-Mozes, S. Assessment of endometrial sampling as a predictor of final surgical pathology in endometrial cancer. Br. J. Cancer 2014, 110, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hosen, I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. BioMed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef]
- Zhu, B.; Song, N.; Shen, R.; Arora, A.; Machiela, M.J.; Song, L.; Landi, M.T.; Ghosh, D.; Chatterjee, N.; Baladandayuthapani, V.; et al. Integrating Clinical and Multiple Omics Data for Prognostic Assessment across Human Cancers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Oplawski, M.; Dziobek, K.; Zmarzły, N.; Grabarek, B.; Halski, T.; Januszyk, P.; Kuś-Kierach, A.; Adwent, I.A.; Dąbruś, D.; Kiełbasiński, K.; et al. Expression Profile of VEGF-C, VEGF-D, and VEGFR-3 in Different Grades of Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 20, 1004–1010. [Google Scholar] [CrossRef]
- Mathai, R.A.; Vidya, R.V.S.; Reddy, B.S.; Thomas, L.; Udupa, K.; Kolesar, J.; Rao, M. Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology. J. Clin. Med. 2019, 8, 373. [Google Scholar] [CrossRef]
- Zmarzły, N.; Hermyt, E.; Witek, A.; Gola, J.; Mazurek, U. Liquid biopsy in endometrial cancer. Curr. Gynecol. Oncol. 2019, 17, 27–42. [Google Scholar] [CrossRef]
- Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Das, V.; Bhattacharya, S.; Chikkaputtaiah, C.; Hazra, S.; Pal, M. The basics of epithelial–mesenchymal transition (EMT): A study from a structure, dynamics, and functional perspective. J. Cell. Physiol. 2019, 234, 14535–14555. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef] [PubMed]
- Esebanmen, G.E.; Langridge, W.H.R. The role of TGF-beta signaling in dendritic cell tolerance. Immunol. Res. 2017, 65, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Ling, L.; van Dam, H.; Zhou, F.; Zhang, L. TGF-β signaling in cancer metastasis. Acta Biochim. Biophys. Sin. 2018, 50, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Shrihari, T.G. Dual role of inflammatory mediators in cancer. Ecancermedicalscience 2017, 11, 721. [Google Scholar] [CrossRef]
- Soleimani, A.; Khazaei, M.; Ferns, G.A.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Role of TGF-β signaling regulatory microRNAs in the pathogenesis of colorectal cancer. J. Cell. Physiol. 2019, 234, 14574–14580. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Yuan, Z.; Wang, S.; Du, H.; Liu, X.; Wang, Q.; Zhu, X. Human adipose-derived mesenchymal stem cells promote breast cancer MCF7 cell epithelial-mesenchymal transition by cross interacting with the TGF-β/Smad and PI3K/AKT signaling pathways. Mol. Med. Rep. 2018, 19, 177–186. [Google Scholar] [CrossRef]
- Fuxe, J.; Vincent, T.; de Herreros, A.G. Transcriptional crosstalk between TGFβ and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010, 9, 2363–2374. [Google Scholar] [CrossRef]
- Perlino, E.; Loverro, G.; Maiorano, E.; Giannini, T.; Cazzolla, A.; Napoli, A.; Fiore, M.G.; Ricco, R.; Marra, E.; Selvaggi, L. Down-regulated expression of transforming growth factor beta 1 mRNA in endometrial carcinoma. Br. J. Cancer 1998, 77, 1260–1266. [Google Scholar] [CrossRef]
- Soufla, G.; Sifakis, S.; Porichis, F.; Spandidos, D.A. Prognostic value of tgfb1 protein in endometrioid adenocarcinoma. Eur. J. Clin. Investig. 2012, 43, 79–90. [Google Scholar] [CrossRef]
- Delforce, S.J.; Lumbers, E.R.; De Meaultsart, C.C.; Wang, Y.; Proietto, A.; Otton, G.; Scurry, J.; Verrills, N.M.; Scott, R.J.; Pringle, K.G. Expression of renin–angiotensin system (RAS) components in endometrial cancer. Endocr. Connect. 2017, 6, 9–19. [Google Scholar] [CrossRef]
- Alghamdi, S.; Alghaashamy, K.; Pinto, A. Expression of SMAD4 is Retained in Most Gynecologic Tumors with Mucinous Differentiation. Int. J. Gynecol. Pathol. 2019, 39, 493–497. [Google Scholar] [CrossRef]
- Giglio, S.; Annibali, V.; Cirombella, R.; Faruq, O.; Volinia, S.; De Vitis, C.; Pesce, M.; Caserta, D.; Pettinato, A.; Fraggetta, F.; et al. miRNAs as Candidate Biomarker for the Accurate Detection of Atypical Endometrial Hyperplasia/Endometrial Intraepithelial Neoplasia. Front. Oncol. 2019, 9, 526. [Google Scholar] [CrossRef]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef]
- Wasniewski, T.; Kiezun, J.; Krazinski, B.E.; Kowalczyk, A.E.; Szostak, B.; Wierzbicki, P.M.; Kiewisz, J. WNT5A gene and protein expression in endometrial cancer. Folia Histochem. Cytobiol. 2019, 57, 84–93. [Google Scholar] [CrossRef]
- McDonald, S.L.; Silver, A. The opposing roles of Wnt-5a in cancer. Br. J. Cancer 2009, 101, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Bénard, J.; Gaasterland, T.; Willert, K.; Cappellen, D. WNT5A Encodes Two Isoforms with Distinct Functions in Cancers. PLoS ONE 2013, 8, e80526. [Google Scholar] [CrossRef]
- Cao, M.; Chan, R.W.S.; Cheng, F.H.C.; Li, J.; Li, T.; Pang, R.T.K.; Lee, C.-L.; Li, R.H.W.; Ng, E.H.Y.; Chiu, P.C.N.; et al. Myometrial Cells Stimulate Self-Renewal of Endometrial Mesenchymal Stem-Like Cells Through WNT5A/β-Catenin Signaling. Stem Cells 2019, 37, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Jha, N.K.; Jha, S.K.; Sharma, A.; Dholpuria, S.; Asthana, N.; Chaurasiya, K.; Singh, V.K.; Burgee, S.; Nand, P. A “NOTCH” Deeper into the Epithelial-To-Mesenchymal Transition (EMT) Program in Breast Cancer. Genes 2019, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Chen, R.; Cao, Y.; Wang, Y.; Song, K.; Zhang, Y.; Yang, J. Emodin suppresses TGF-β1-induced epithelial-mesenchymal transition in alveolar epithelial cells through Notch signaling pathway. Toxicol. Appl. Pharmacol. 2017, 318, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.-H.; Wei, H.; Xie, J.; Zheng, D.-P.; Luo, E.-L.; Huang, X.-Y.; Xie, J.; Zhao, Y.; Ding, L.-C.; Su, B.-H.; et al. Notch1 regulates tongue cancer cells proliferation, apoptosis and invasion. Cell Cycle 2017, 17, 216–224. [Google Scholar] [CrossRef]
- Jonusiene, V.; Sasnauskiene, A.; Lachej, N.; Kanopiene, D.; Dabkeviciene, D.; Sasnauskiene, S.; Kazbariene, B.; Didziapetriene, J. Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med. Oncol. 2013, 30, 1–7. [Google Scholar] [CrossRef]
- Sasnauskienė, A.; Jonušienė, V.; Krikštaponienė, A.; Butkytė, S.; Dabkevičienė, D.; Kanopienė, D.; Didžiapetrienė, J. NOTCH1, NOTCH3, NOTCH4, and JAG2 protein levels in human endometrial cancer. Medicina 2014, 50, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Dolka, I.; Król, M.; Sapierzyński, R. Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: An immunohistochemical and prognostic study. Res. Vet. Sci. 2016, 105, 124–133. [Google Scholar] [CrossRef]
- Samani, S.M.; Bojnordi, T.E.; Zarghampour, M.; Merat, S.; Fouladi, D.F. Expression of p53, Bcl-2 and Bax in endometrial carcinoma, endometrial hyperplasia and normal endometrium: A histopathological study. J. Obstet. Gynaecol. 2018, 38, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Kiełbasiński, K.; Peszek, W.; Grabarek, B.O.; Boroń, D.; Wierzbik-Strońska, M.; Oplawski, M. Effect of Salinomycin on Expression Pattern of Genes Associated with Apoptosis in Endometrial Cancer Cell Line. Curr. Pharm. Biotechnol. 2020, 21, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef]
- Couzigou, J.-M.; Lauressergues, D.; André, O.; Gutjahr, C.; Guillotin, B.; Bécard, G.; Combier, J.-P. Positive Gene Regulation by a Natural Protective miRNA Enables Arbuscular Mycorrhizal Symbiosis. Cell Host Microbe 2017, 21, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, Y.; Guo, G.; Li, L.; Dou, D.; Ge, X.; Lv, P.; Wang, F.; Gu, Y. microRNA-30d mediated breast cancer invasion, migration, and EMT by targeting KLF11 and activating STAT3 pathway. J. Cell. Biochem. 2018, 119, 8138–8145. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, L.; Li, J.; Chen, W.; Li, X. miR-30d Blocked Transforming Growth Factor β1–Induced Epithelial-Mesenchymal Transition by Targeting Snail in Ovarian Cancer Cells. Int. J. Gynecol. Cancer 2015, 25, 1574–1581. [Google Scholar] [CrossRef]
- Özcan, S. MiR-30 family and EMT in human fetal pancreatic islets. Islets 2009, 1, 283–285. [Google Scholar] [CrossRef]
- Tang, W.; Li, J.; Liu, H.; Zhou, F.; Liu, M. MiR-106a promotes tumor growth, migration, and invasion by targeting BCL2L11 in human endometrial adenocarcinoma. Am. J. Transl. Res. 2017, 9, 4984–4993. [Google Scholar] [PubMed]
- Wang, W.; Ge, L.; Xu, X.-J.; Yang, T.; Yuan, Y.; Ma, X.-L.; Zhang, X.-H. LncRNA NEAT1 promotes endometrial cancer cell proliferation, migration and invasion by regulating the miR-144-3p/EZH2 axis. Radiol. Oncol. 2019, 53, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Hutt, S.; Tailor, A.; Ellis, P.; Michael, A.; Butler-Manuel, S.; Chatterjee, J. The role of biomarkers in endometrial cancer and hyperplasia: A literature review. Acta Oncol. 2019, 58, 342–352. [Google Scholar] [CrossRef] [PubMed]
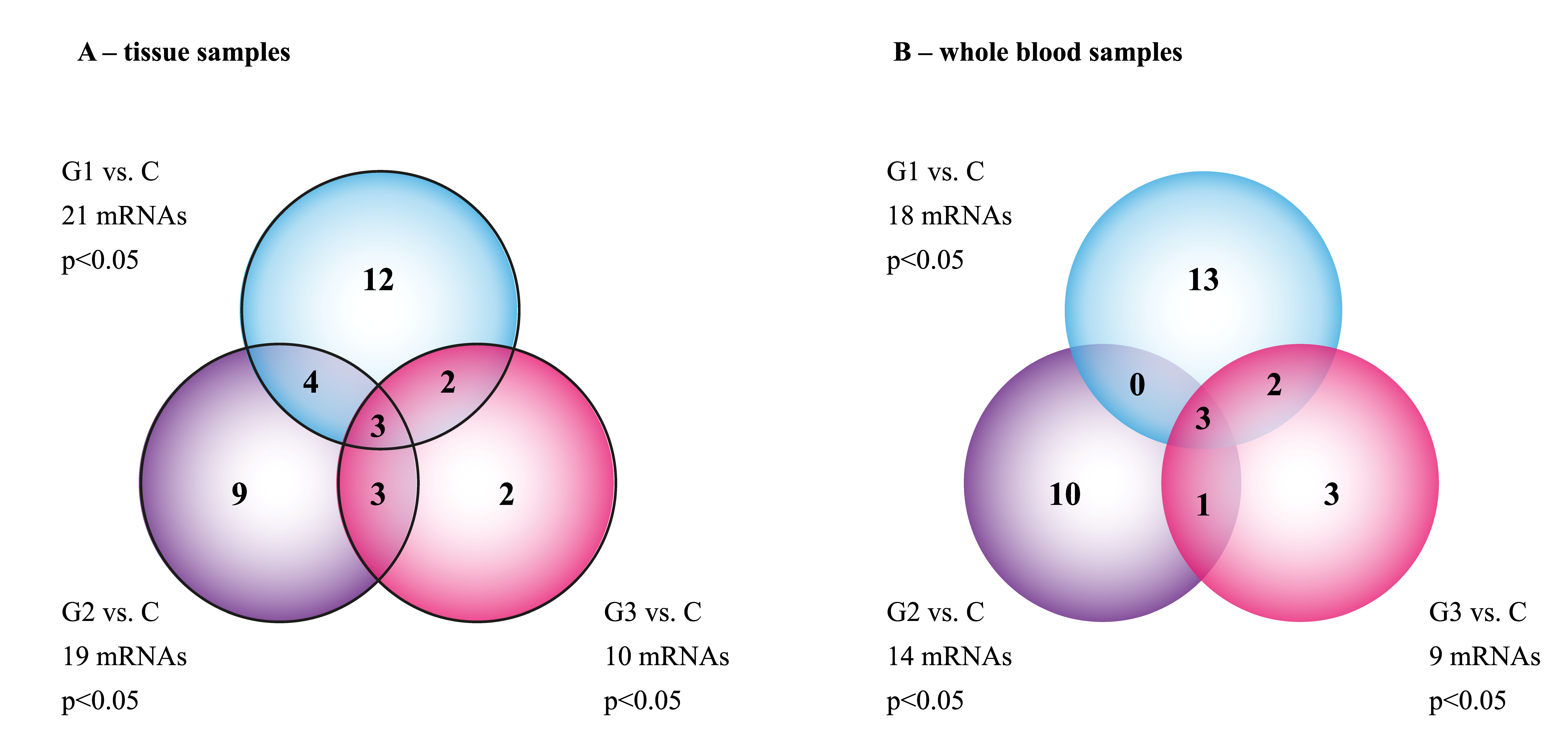
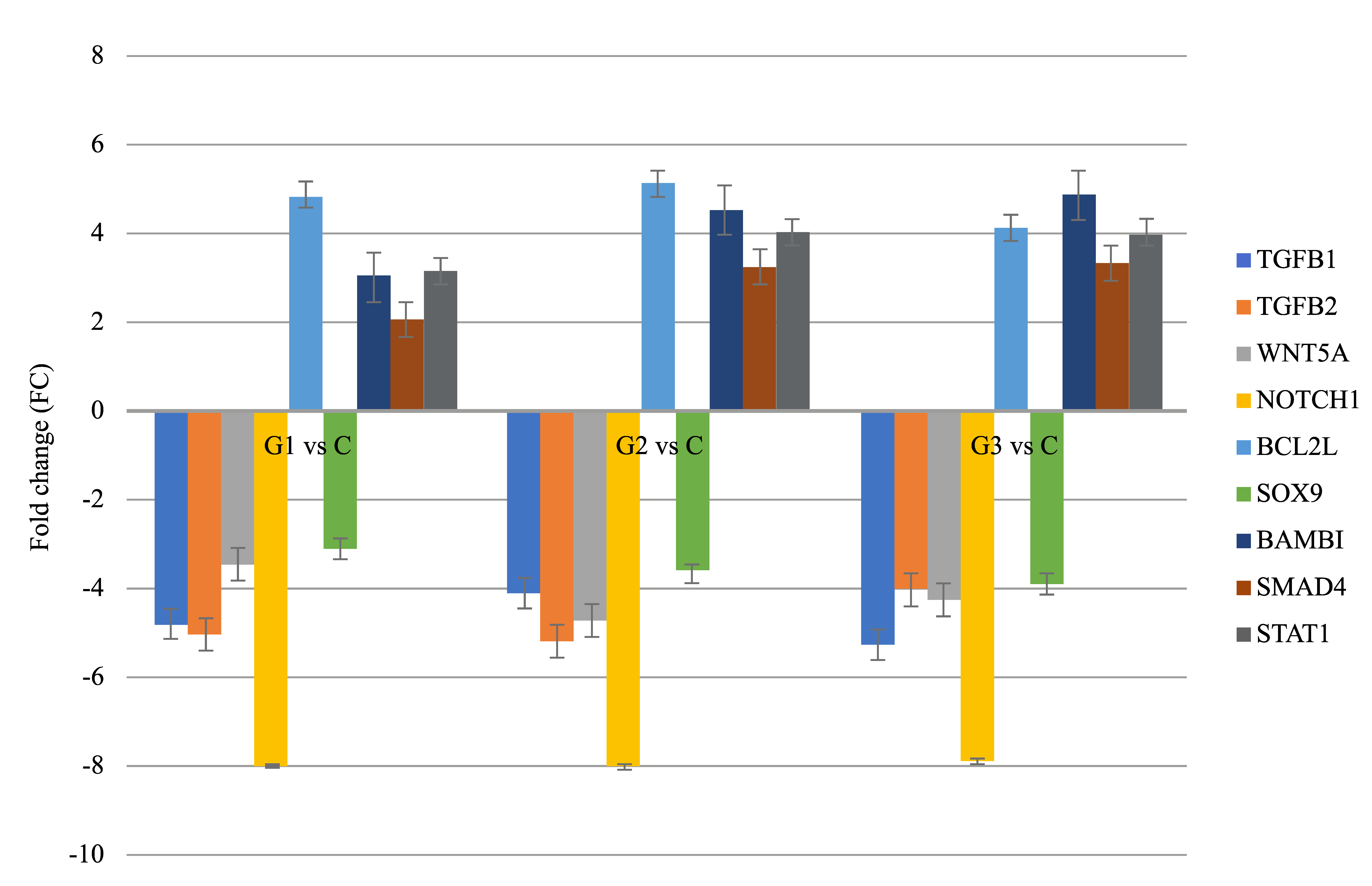

| C (n = 30 Cases) | G1 (n = 15 Cases) | G2 (n = 8 Cases) | G3 (n = 7 Cases) | |
|---|---|---|---|---|
| Age (years) | 65.36 ± 10.29 | 67.22 ± 8.04 | 68.4 ± 10.09 | 64.88 ± 12.02 |
| Height (m) | 1.63 ± 0.14 | 1.59 ± 0.08 | 1.62 ± 0.05 | 1.59 ± 0.04 |
| Weight (kg) | 72.99± 13.95 | 74.41 kg ± 11.79 | 85.77 ± 21.99 | 85.22 ± 13.11 |
| BMI | 28.77 ± 7.14—overweight | 29.01 ± 3.14—overweight | 36.15 ± 10.44—1st degree of obesity | 33.18 ± 5.44—1st degree of obesity |
| mRNA | Primer Sequence (Forward, Reverse) |
|---|---|
| TGFβ1 | Forward: 5′-TGAACCGGCCTTTCCTGCTTCTCATG-3′ Reverse: 5′-GCGGAAGTCAATGTACAGCTGCCGC-3′ |
| TGFβ2 | Forward: 5′-TACTACGCCAAGGAGGTTTACAAA-3′ Reverse: 5′-TTGTTCAGGCACTCTGGCTTT-3′ |
| WNT5A | Forward 5′-CGTTAGCAGCATCAGTCCAC-3′ Reverse 5′-ACGGCATCTCTCTTTCACCA-3′ |
| NOTCH1 | Forward 5′-ACTGTGAGGACCTGGTGGAC-3′ Reverse: 5′TTGTAGGTGTTGGGGAGGTC-3′ |
| BCL2L | Forward:5′-GTAGTTGGAGATGAGTTCGAGATTC-3′ Reverse:5′-TTCATCGAAAACCTAAATAAAACGT-3′ |
| SOX9 | Forward 5′-TTGAGCCTTAAAACGGTGCT-3′ Reverse: 5′CTGGTGTTCTGAGAGGCACA-3′ |
| BAMBI | Forward 5′-GCTGGAACTTGGTGCAAAAT-3′ Reverse: 5′GCCATTTTTCTCGGAATCAA-3′ |
| SMAD4 | Forward 5′-TTGCTTCCACTTGAATGCTG-3′ Reverse: 5′-CTTCAAAGGGGACACCAAAA-3′ |
| STAT1 | Forward 5′-CCGTTTTCATGACCTCCTGT-3′ Reverse: 5′-TGAATATTCCCCGACTGAGC-3′ |
| ACTB | Forward: 5′-TCACCCACACTGTGCCCATCTACGA-3′ Reverse: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ |
| Group | C | G1 | G2 | G3 |
|---|---|---|---|---|
| C | 60 | 21 A | 19 A | 10 A |
| G1 | 22 | 60 | 17 B | 24 B |
| G2 | 12 | 44 | 60 | 21 C |
| G3 | 31 | 32 | 22 | 60 |
| Group | C | G1 | G2 | G3 |
|---|---|---|---|---|
| C | 54 | 21 A | 18 A | 9 A |
| G1 | 20 | 51 | 14 B | 23 B |
| G2 | 11 | 39 | 51 | 20 C |
| G3 | 28 | 34 | 19 | 49 |
| Groups Compared | ID | mRNA | FC | Expression |
|---|---|---|---|---|
| G1 vs. C | 203085_s_at | TGFβ1 | −4.89 | Decreased |
| 220407_s_at | TGFβ2 | −4.71 | Decreased | |
| 205990_s_at | WNT5A | −3.98 | Decreased | |
| 218902_at | NOTCH1 | −7.77 | Decreased | |
| G2 vs. C | 207005_s_at | BCL2L | +5.04 | Increased |
| 202935_s_at | SOX9 | −3.11 | Decreased | |
| 203304_at | BAMBI | +3.66 | Increased | |
| 202527_s_at | SMAD4 | +3.01 | Increased | |
| G3 vs. C | AFFX-HUMISGF3A/M97935_5_at | STAT1 | +4.01 | Increased |
| 203085_s_at | TGFβ1 | −5.07 | Decreased |
| Tissue | Whole Blood | |||||
|---|---|---|---|---|---|---|
| miR-144 | miR-106a | miR-30d | miR-144 | miR-106a | miR-30d | |
| G1 vs. C | +8.21 | +11.44 | −5.22 | +8.44 | +10.14 | −5.14 |
| G2 vs. C | +9.18 | +9.14 | −7.11 | +8.36 | +9.88 | −7.44 |
| G3 vs. C | +9.04 | +8.11 | −8.01 | +10.02 | +7.99 | −9.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opławski, M.; Nowakowski, R.; Średnicka, A.; Ochnik, D.; Grabarek, B.O.; Boroń, D. Molecular Landscape of the Epithelial–Mesenchymal Transition in Endometrioid Endometrial Cancer. J. Clin. Med. 2021, 10, 1520. https://doi.org/10.3390/jcm10071520
Opławski M, Nowakowski R, Średnicka A, Ochnik D, Grabarek BO, Boroń D. Molecular Landscape of the Epithelial–Mesenchymal Transition in Endometrioid Endometrial Cancer. Journal of Clinical Medicine. 2021; 10(7):1520. https://doi.org/10.3390/jcm10071520
Chicago/Turabian StyleOpławski, Marcin, Robert Nowakowski, Agata Średnicka, Dominika Ochnik, Beniamin Oskar Grabarek, and Dariusz Boroń. 2021. "Molecular Landscape of the Epithelial–Mesenchymal Transition in Endometrioid Endometrial Cancer" Journal of Clinical Medicine 10, no. 7: 1520. https://doi.org/10.3390/jcm10071520
APA StyleOpławski, M., Nowakowski, R., Średnicka, A., Ochnik, D., Grabarek, B. O., & Boroń, D. (2021). Molecular Landscape of the Epithelial–Mesenchymal Transition in Endometrioid Endometrial Cancer. Journal of Clinical Medicine, 10(7), 1520. https://doi.org/10.3390/jcm10071520







