Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases
Abstract
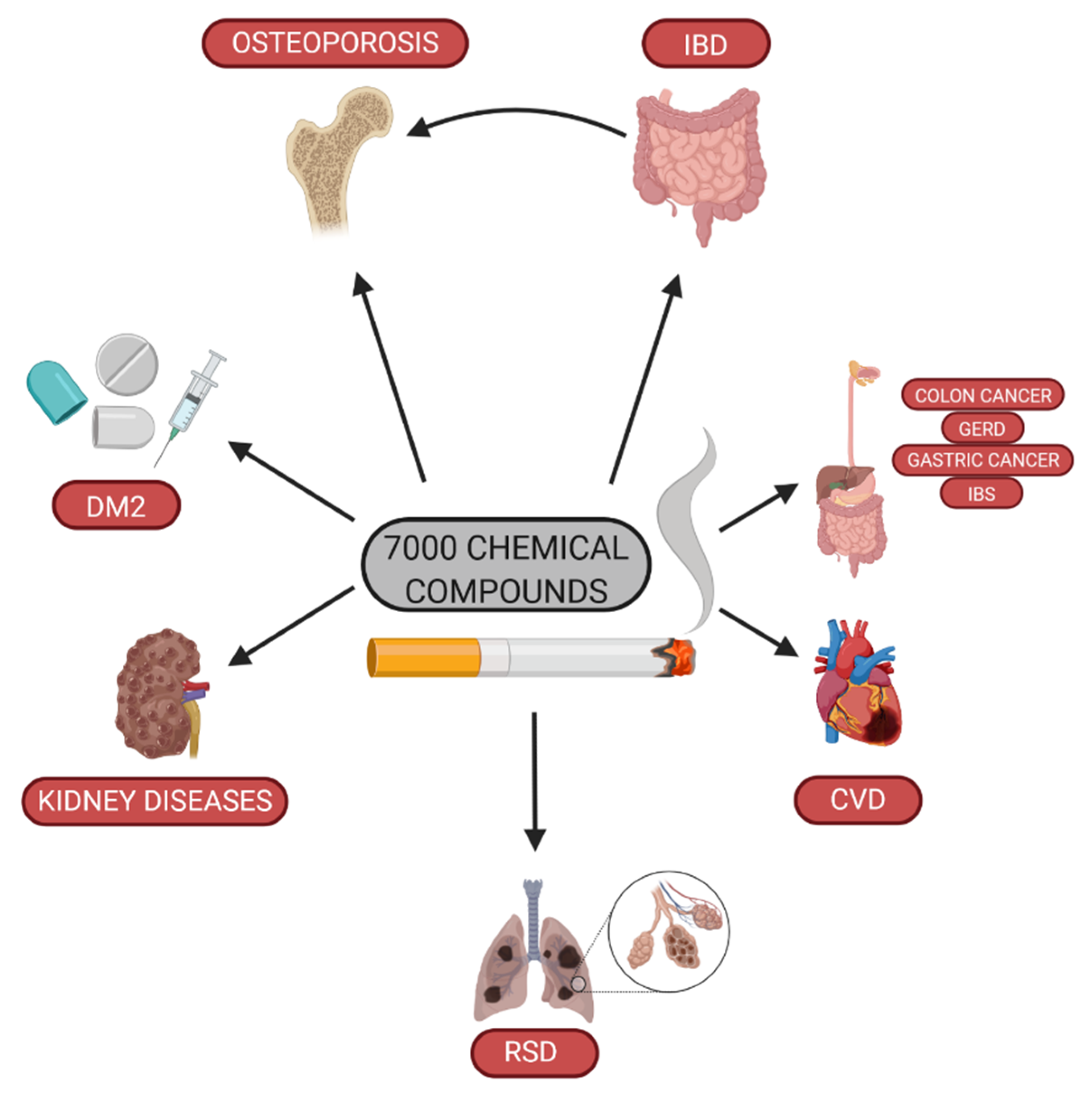
1. Introduction
2. Pathophysiological Mechanisms of Smoking on Bone Tissue
3. Cigarette Smoking and Osteoporosis
4. Pathophysiological Mechanisms of Smoking on Colon and Small Intestine
5. Cigarette Smoking and IBD
6. Conclusions
- Cigarette smoking is a risk factor of the development of and a deterioration in the course of Crohn’s disease. Tobacco use protects from the development of ulcerative colitis. However, due to other smoking-related conditions, cigarette smoking is not recommended both in CD and UC [139].
- Guidelines of AACE/ACE (American Association Of Clinical Endocrinologists And American College Of Endocrinology) report clearly that cigarette smoking constitutes a risk factor of osteoporosis development, and all patients should be persuaded to quit smoking [140].
- European guidelines also contain data concerning cigarette smoking as a risk of osteoporosis [141].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.J.; Jeon, C.; Jee, S.H. The Effect of Smoking on Lung Cancer: Ethnic Differences and the Smoking Paradox. Epidemiol. Health 2016, 38. [Google Scholar] [CrossRef]
- Li, L.F.; Chan, R.L.Y.; Lu, L.; Shen, J.; Zhang, L.; Wu, W.K.K.; Wang, L.; Hu, T.; Li, M.X.; Cho, C.H. Cigarette Smoking and Gastrointestinal Diseases: The Causal Relationship and Underlying Molecular Mechanisms (Review). Int. J. Mol. Med. 2014, 34, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B.; Brask-Lindemann, D.; Rubin, K.H.; Schwarz, P. A Review of Lifestyle, Smoking and Other Modifiable Risk Factors for Osteoporotic Fractures. BoneKEy Rep. 2014, 3. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B.; Wu, T. Relation of Active, Passive, and Quitting Smoking with Incident Diabetes: A Meta-Analysis and Systematic Review. Lancet Diabetes Endocrinol. 2015, 3, 958–967. [Google Scholar] [CrossRef]
- Xia, J.; Wang, L.; Ma, Z.; Zhong, L.; Wang, Y.; Gao, Y.; He, L.; Su, X. Cigarette Smoking and Chronic Kidney Disease in the General Population: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2017, 32, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, D.; Korytkowski, P.; Makowiec-Dąbrowska, T. Palenie papierosów w populacji osób czynnych zawodowo. Med. PR 2014, 64, 359–371. [Google Scholar] [CrossRef]
- Joehanes, R.; Just, A.C.; Marioni, R.E.; Pilling, L.C.; Reynolds, L.M.; Mandaviya, P.R.; Guan, W.; Xu, T.; Elks, C.E.; Aslibekyan, S.; et al. Epigenetic Signatures of Cigarette Smoking. Circ. Cardiovasc. Genet. 2016, 9, 436–447. [Google Scholar] [CrossRef]
- Astuti, Y.; Wardhana, A.; Watkins, J.; Wulaningsih, W. Cigarette Smoking and Telomere Length: A Systematic Review of 84 Studies and Meta-Analysis. Environ. Res. 2017, 158, 480–489. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Ghadermarzi, S.; Raza, A.; O’Connell, D.; Xiao, A.; Ayyaz, F.; Zhi, M.; Zhang, Y.; Parekh, N.K.; et al. Smoking and Inflammatory Bowel Disease: A Comparison of China, India, and the USA. Dig. Dis. Sci. 2018, 63, 2703–2713. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, Y.; Wang, X.; Wang, J.; Yan, Z.; Gong, G.; Li, G.; Li, C. Meta-Analysis of Prospective Cohort Studies of Cigarette Smoking and the Incidence of Colon and Rectal Cancers. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2015, 24, 6–15. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kubo, M.; Kohata, Y.; Machida, H.; Okazaki, H.; Yamagami, H.; Tanigawa, T.; Watanabe, K.; Watanabe, T.; Tominaga, K.; et al. Cigarette Smoking and Its Association with Overlapping Gastroesophageal Reflux Disease, Functional Dyspepsia, or Irritable Bowel Syndrome. Intern. Med. Tokyo Jpn. 2011, 50, 2443–2447. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.-W.; Cao, S.-S.; Wang, H.-Q.; Hu, R.-Y. Cigarette Smoking and Electronic Cigarettes Use: A Meta-Analysis. Int. J. Environ. Res. Public. Health 2016, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoran, S.; Glantz, S.A. E-Cigarettes and Smoking Cessation in Real-World and Clinical Settings: A Systematic Review and Meta-Analysis. Lancet Respir. Med. 2016, 4, 116–128. [Google Scholar] [CrossRef]
- MacDonald, A.; Middlekauff, H.R. Electronic Cigarettes and Cardiovascular Health: What Do We Know so Far? Vasc. Health Risk Manag. 2019, 15, 159–174. [Google Scholar] [CrossRef]
- Wiraszka, G.; Obierzyńska, A. The level of nicotine addiction and motivation to stop smoking among young adults – students from the Świętokrzyskie region. Nurs. Public Health 2019, 9, 15–22. [Google Scholar] [CrossRef]
- WHO | WHO Global Report on Trends in Tobacco Smoking 2000–2025—First Edition. Available online: http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/ (accessed on 26 March 2020).
- Al-Saleh, Y.; Al-Daghri, N.M.; Sabico, S.; Alessa, T.; Al Emadi, S.; Alawadi, F.; Al Qasaabi, S.; Alfutaisi, A.; Al Izzi, M.; Mukhaimer, J.; et al. Diagnosis and Management of Osteoporosis in Postmenopausal Women in Gulf Cooperation Council (GCC) Countries: Consensus Statement of the GCC Countries’ Osteoporosis Societies under the Auspices of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Arch. Osteoporos. 2020, 15, 109. [Google Scholar] [CrossRef]
- Pang, K.-L.; Low, N.Y.; Chin, K.-Y. A Review on the Role of Denosumab in Fracture Prevention. Drug Des. Devel. Ther. 2020, 14, 4029–4051. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef]
- Zhu, S.; Häussling, V.; Aspera-Werz, R.H.; Chen, T.; Braun, B.; Weng, W.; Histing, T.; Nussler, A.K. Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model. Int. J. Mol. Sci. 2020, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Wysocka, E.; Szymczak, A.; Eder, P.; Michalak, M.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Klimczak, K.; Linke, K.; Horst-Sikorska, W. Osteoprotegerin, s-RANKL, and Selected Interleukins in the Pathology of Bone Metabolism in Patients with Crohn’s Disease. Przeglad Gastroenterol. 2016, 11, 30–34. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL–RANK–OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Ludwiczek, O.; Gabriel, M.; Obrist, P.; Wolf, A.M.; Tilg, H. The RANKL/OPG System Is Activated in Inflammatory Bowel Disease and Relates to the State of Bone Loss. Gut 2005, 54, 479–487. [Google Scholar] [CrossRef]
- Blaschke, M.; Koepp, R.; Cortis, J.; Komrakova, M.; Schieker, M.; Hempel, U.; Siggelkow, H. IL-6, IL-1β, and TNF-α Only in Combination Influence the Osteoporotic Phenotype in Crohn’s Patients via Bone Formation and Bone Resorption. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.H.; Fitzsimmons, T.R.; Bartold, P.M. Effect of Smoking on Concentrations of Receptor Activator of Nuclear Factor Kappa B Ligand and Osteoprotegerin in Human Gingival Crevicular Fluid. J. Clin. Periodontol. 2009, 36, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Lappin, D.F.; Sherrabeh, S.; Jenkins, W.M.M.; Macpherson, L.M.D. Effect of Smoking on Serum RANKL and OPG in Sex, Age and Clinically Matched Supportive-Therapy Periodontitis Patients. J. Clin. Periodontol. 2007, 34, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin Ligand Is a Cytokine That Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The Effects of Smoking on Bone Metabolism. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2012, 23, 2081–2092. [Google Scholar] [CrossRef]
- Dobnig, H.; Hofbauer, L.C.; Viereck, V.; Obermayer-Pietsch, B.; Fahrleitner-Pammer, A. Changes in the RANK Ligand/Osteoprotegerin System Are Correlated to Changes in Bone Mineral Density in Bisphosphonate-Treated Osteoporotic Patients. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2006, 17, 693–703. [Google Scholar] [CrossRef]
- D’Amelio, P.; Grimaldi, A.; Cristofaro, M.A.; Ravazzoli, M.; Molinatti, P.A.; Pescarmona, G.P.; Isaia, G.C. Alendronate Reduces Osteoclast Precursors in Osteoporosis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2010, 21, 1741–1750. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Szymczak-Tomczak, A.; Zawada, A.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Does Drinking Coffee and Tea Affect Bone Metabolism in Patients with Inflammatory Bowel Diseases? Nutrients 2021, 13, 216. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Chyu, J.Y.; Yang, R.-S.; Chen, C.-H.; Shen, C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, Functional Properties and Health-Promoting Potential of Native South American Berries: A Review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Aspera-Werz, R.H.; Chen, T.; Weng, W.; Braun, B.; Histing, T.; Nüssler, A.K. Maqui Berry Extract Prevents Cigarette Smoke Induced Oxidative Stress in Human Osteoblasts in Vitro. EXCLI J. 2021, 20, 281–296. [Google Scholar] [CrossRef]
- Ortiz, T.; Argüelles-Arias, F.; Illanes, M.; García-Montes, J.-M.; Talero, E.; Macías-García, L.; Alcudia, A.; Vázquez-Román, V.; Motilva, V.; De-Miguel, M. Polyphenolic Maqui Extract as a Potential Nutraceutical to Treat TNBS-Induced Crohn’s Disease by the Regulation of Antioxidant and Anti-Inflammatory Pathways. Nutrients 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Do Only Calcium and Vitamin D Matter? Micronutrients in the Diet of Inflammatory Bowel Diseases Patients and the Risk of Osteoporosis. Nutrients 2021, 13, 525. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Dobrowolska, A.; Eder, P.; Krela-Kaźmierczak, I. A Vicious Cycle of Osteosarcopeniain Inflammatory Bowel Diseases—Aetiology, Clinical Implications and Therapeutic Perspectives. Nutrients 2021, 13, 293. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Iki, M. Osteoporosis and smoking. Clin. Calcium 2005, 15, 156–158. [Google Scholar] [PubMed]
- Daniell, H.W. Osteoporosis and Smoking. JAMA 1972, 221, 509. [Google Scholar] [CrossRef]
- Cieplinska, J. Wpływ palenia papierosów na gęstość mineralną i masę tkanki kostnej u mężczyzn. Med. Ogólna Nauki O Zdrowiu 2014, 20. [Google Scholar] [CrossRef]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef] [PubMed]
- Stanisławowski, M.; Kmieć, Z. The participation of RANK, RANKL and OPG in tumor osteolysis. Adv. Hyg. Exp. Med. 2009, 63, 234–241. [Google Scholar]
- Walker, L.M.; Preston, M.R.; Magnay, J.L.; Thomas, P.B.; El Haj, A.J. Nicotinic Regulation of C-Fos and Osteopontin Expression in Human-Derived Osteoblast-like Cells and Human Trabecular Bone Organ Culture. Bone 2001, 28, 603–608. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Abizaid, A.; Rao, Y.; Salas, R.; DiLeone, R.J.; Gündisch, D.; Diano, S.; De Biasi, M.; Horvath, T.L.; Gao, X.-B.; et al. Nicotine Decreases Food Intake through Activation of POMC Neurons. Science 2011, 332, 1330–1332. [Google Scholar] [CrossRef]
- Wong, P.K.K.; Christie, J.J.; Wark, J.D. The Effects of Smoking on Bone Health. Clin. Sci. Lond. Engl. 1979 2007, 113, 233–241. [Google Scholar] [CrossRef]
- Daffner, S.D.; Waugh, S.; Norman, T.L.; Mukherjee, N.; France, J.C. Nicotine Increases Osteoblast Activity of Induced Bone Marrow Stromal Cells in a Dose-Dependent Manner: An in Vitro Cell Culture Experiment. Glob. Spine J. 2012, 2, 153–158. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine Induces Apoptosis in Human Osteoblasts via a Novel Mechanism Driven by H2O2 and Entailing Glyoxalase 1-Dependent MG-H1 Accumulation Leading to TG2-Mediated NF-KB Desensitization: Implication for Smokers-Related Osteoporosis. Free Radic. Biol. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef]
- Theiss, S.M.; Boden, S.D.; Hair, G.; Titus, L.; Morone, M.A.; Ugbo, J. The Effect of Nicotine on Gene Expression during Spine Fusion. Spine 2000, 25, 2588–2594. [Google Scholar] [CrossRef]
- Cusano, N.E. Skeletal Effects of Smoking. Curr. Osteoporos. Rep. 2015, 13, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Salamanna, F.; Veronesi, F.; Torricelli, P.; Nicolini, A.; Benedicenti, S.; Carpi, A.; Giavaresi, G. Role of Obesity, Alcohol and Smoking on Bone Health. Front. Biosci. Elite Ed. 2012, 4, 2586–2606. [Google Scholar] [CrossRef] [PubMed]
- Krall, E.A.; Dawson-Hughes, B. Smoking Increases Bone Loss and Decreases Intestinal Calcium Absorption. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1999, 14, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Krall, E.A.; Dawson-Hughes, B. Smoking and Bone Loss among Postmenopausal Women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1991, 6, 331–338. [Google Scholar] [CrossRef]
- Baron, J.A.; Comi, R.J.; Cryns, V.; Brinck-Johnsen, T.; Mercer, N.G. The Effect of Cigarette Smoking on Adrenal Cortical Hormones. J. Pharmacol. Exp. Ther. 1995, 272, 151–155. [Google Scholar]
- Bachta, A.; Kulig, M.; Tłustochowicz, W. Glucocorticoid-induced osteoporosis. Prog. Med. 2012, 3, 213–217. [Google Scholar]
- Cassidenti, D.L.; Pike, M.C.; Vijod, A.G.; Stanczyk, F.Z.; Lobo, R.A. A Reevaluation of Estrogen Status in Postmenopausal Women Who Smoke. Am. J. Obstet. Gynecol. 1992, 166, 1444–1448. [Google Scholar] [CrossRef]
- Bijelic, R.; Milicevic, S.; Balaban, J. Risk Factors for Osteoporosis in Postmenopausal Women. Med. Arch. Sarajevo Bosnia Herzeg. 2017, 71, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef]
- Lee, D.; Kook, S.-H.; Ji, H.; Lee, S.-A.; Choi, K.-C.; Lee, K.-Y.; Lee, J.-C. N-Acetyl Cysteine Inhibits H2O2-Mediated Reduction in the Mineralization of MC3T3-E1 Cells by down-Regulating Nrf2/HO-1 Pathway. BMB Rep. 2015, 48, 636–641. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Xu, A.-H.; Yang, Y.; Li, J. Role of Nrf2 in Bone Metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Li, L.; Corry, K.A.; Zhang, P.; Yang, Y.; Himes, E.; Mihuti, C.L.; Nelson, C.; Dai, G.; Li, J. Deletion of Nrf2 Reduces Skeletal Mechanical Properties and Decreases Load-Driven Bone Formation. Bone 2015, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Vanni, D.; Pantalone, A.; Salini, V. Cigarette Smoking and Musculoskeletal Disorders. Muscles Ligaments Tendons J. 2013, 3, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Saribal, D.; Hocaoglu-Emre, F.S.; Erdogan, S.; Bahtiyar, N.; Caglar Okur, S.; Mert, M. Inflammatory Cytokines IL-6 and TNF-α in Patients with Hip Fracture. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2019, 30, 1025–1031. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.-L.; Liu, H.; Zeng, Y.-Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of Cigarette Smoking on Immune Responsiveness: Up and down or Upside Down? Oncotarget 2016, 8, 268–284. [Google Scholar] [CrossRef]
- Fujita, T.; Matsui, T.; Nakao, Y.; Shiozawa, S.; Imai, Y. Cytokines and Osteoporosis. Ann. N. Y. Acad. Sci. 1990, 587, 371–375. [Google Scholar] [CrossRef]
- Yadav, P.; Ellinghaus, D.; Rémy, G.; Freitag-Wolf, S.; Cesaro, A.; Degenhardt, F.; Boucher, G.; Delacre, M.; Peyrin-Biroulet, L.; Pichavant, M.; et al. Genetic Factors Interact With Tobacco Smoke to Modify Risk for Inflammatory Bowel Disease in Humans and Mice. Gastroenterology 2017, 153, 550–565. [Google Scholar] [CrossRef]
- Burisch, J.; Munkholm, P. The Epidemiology of Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2015, 50, 942–951. [Google Scholar] [CrossRef]
- Thomas, T.; Chandan, J.S.; Li, V.S.W.; Lai, C.Y.; Tang, W.; Bhala, N.; Kaplan, G.G.; Ng, S.C.; Ghosh, S. Global Smoking Trends in Inflammatory Bowel Disease: A Systematic Review of Inception Cohorts. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Holko, P.; Kawalec, P.; Stawowczyk, E. Prevalence and Drug Treatment Practices of Inflammatory Bowel Diseases in Poland in the Years 2012-2014: An Analysis of Nationwide Databases. Eur. J. Gastroenterol. Hepatol. 2018, 30, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Vegh, Z.; Burisch, J.; Pedersen, N.; Kaimakliotis, I.; Duricova, D.; Bortlik, M.; Avnstrøm, S.; Kofod Vinding, K.; Olsen, J.; Nielsen, K.R.; et al. Incidence and Initial Disease Course of Inflammatory Bowel Diseases in 2011 in Europe and Australia: Results of the 2011 ECCO-EpiCom Inception Cohort. J. Crohns Colitis 2014, 8, 1506–1515. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet Lond. Engl. 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Manninen, P.; Karvonen, A.-L.; Huhtala, H.; Rasmussen, M.; Collin, P. The Epidemiology of Inflammatory Bowel Diseases in Finland. Scand. J. Gastroenterol. 2010, 45, 1063–1067. [Google Scholar] [CrossRef]
- Jussila, A.; Virta, L.J.; Salomaa, V.; Mäki, J.; Jula, A.; Färkkilä, M.A. High and Increasing Prevalence of Inflammatory Bowel Disease in Finland with a Clear North-South Difference. J. Crohns Colitis 2013, 7, e256–e262. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, L.; Mester, G.; Erdélyi, Z.; Balogh, M.; Szipócs, I.; Kamarás, G.; Lakatos, P.L. Epidemiology of inflammatory bowel diseases in Veszprém county of Western Hungary between 1977 and 2001. Orv. Hetil. 2003, 144, 1819–1827. [Google Scholar]
- Lakatos, L.; Lakatos, P.L. Is the Incidence and Prevalence of Inflammatory Bowel Diseases Increasing in Eastern Europe? Postgrad. Med. J. 2006, 82, 332–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, G.-R.; Lyons, M.; Plevris, N.; Jenkinson, P.W.; Bisset, C.; Burgess, C.; Din, S.; Fulforth, J.; Henderson, P.; Ho, G.-T.; et al. IBD Prevalence in Lothian, Scotland, Derived by Capture–Recapture Methodology. Gut 2019, 68, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Bartram, S.A.; Peaston, R.T.; Rawlings, D.J.; Walshaw, D.; Francis, R.M.; Thompson, N.P. Mutifactorial Analysis of Risk Factors for Reduced Bone Mineral Density in Patients with Crohn’s Disease. World J. Gastroenterol. 2006, 12, 5680–5686. [Google Scholar] [CrossRef] [PubMed]
- Pasvol, T.J.; Horsfall, L.; Bloom, S.; Segal, A.W.; Sabin, C.; Field, N.; Rait, G. Incidence and Prevalence of Inflammatory Bowel Disease in UK Primary Care: A Population-Based Cohort Study. BMJ Open 2020, 10, e036584. [Google Scholar] [CrossRef]
- Hein, R.; Köster, I.; Bollschweiler, E.; Schubert, I. Prevalence of Inflammatory Bowel Disease: Estimates for 2010 and Trends in Germany from a Large Insurance-Based Regional Cohort. Scand. J. Gastroenterol. 2014, 49, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Dignass, A.U.; Mann, K.; Goebell, H. Reduced Bone Mineral Density and Unbalanced Bone Metabolism in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 1998, 4, 268–275. [Google Scholar] [CrossRef]
- Ott, C.; Obermeier, F.; Thieler, S.; Kemptner, D.; Bauer, A.; Schölmerich, J.; Rogler, G.; Timmer, A. The Incidence of Inflammatory Bowel Disease in a Rural Region of Southern Germany: A Prospective Population-Based Study. Eur. J. Gastroenterol. Hepatol. 2008, 20, 917–923. [Google Scholar] [CrossRef]
- Príkazka, M.; Letkovicová, M.; Matejíckova, V. Crohns Disease in Slovakia: Prevalence, Socioeconomic and Psychological Analysis. Eur. J. Epidemiol. 1998, 14, 49–53. [Google Scholar] [CrossRef]
- Miznerova, E.; Hlavaty, T.; Koller, T.; Toth, J.; Holociova, K.; Huorka, M.; Killinger, Z.; Payer, J. The Prevalence and Risk Factors for Osteoporosis in Patients with Inflammatory Bowel Disease. Bratisl. Lek. Listy 2013, 114, 439–445. [Google Scholar] [CrossRef]
- Hlavaty, T.; Toth, J.; Koller, T.; Krajcovicova, A.; Oravcova, S.; Zelinkova, Z.; Huorka, M. Smoking, Breastfeeding, Physical Inactivity, Contact with Animals, and Size of the Family Influence the Risk of Inflammatory Bowel Disease: A Slovak Case–Control Study. United Eur. Gastroenterol. J. 2013, 1, 109–119. [Google Scholar] [CrossRef]
- Spekhorst, L.M.; Imhann, F.; Festen, E.A.; van Bodegraven, A.A.; de Boer, N.K.; Bouma, G.; Fidder, H.H.; D’Haens, G.; Hoentjen, F.; Hommes, D.W.; et al. Cohort Profile: Design and First Results of the Dutch IBD Biobank: A Prospective, Nationwide Biobank of Patients with Inflammatory Bowel Disease. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- Van Schaik, F.D.M.; Verhagen, M.A.M.T.; Siersema, P.D.; Oldenburg, B. High Prevalence of Low Bone Mineral Density in Patients with Inflammatory Bowel Disease in the Setting of a Peripheral Dutch Hospital. J. Crohns Colitis 2008, 2, 208–213. [Google Scholar] [CrossRef]
- van der Heide, F.; Wassenaar, M.; van der Linde, K.; Spoelstra, P.; Kleibeuker, J.H.; Dijkstra, G. Effects of Active and Passive Smoking on Crohn’s Disease and Ulcerative Colitis in a Cohort from a Regional Hospital. Eur. J. Gastroenterol. Hepatol. 2011, 23, 255–261. [Google Scholar] [CrossRef]
- Brunet, E.; Roig-Ramos, C.; Vela, E.; Clèries, M.; Melcarne, L.; Villòria, A.; Pontes, C.; Calvet, X. Prevalence, Incidence and Mortality of Inflammatory Bowel Disease in Catalonia. A Population-Based Analysis. Ann. Med. 2018, 50, 613–619. [Google Scholar] [CrossRef]
- Miranda-Bautista, J.; Verdejo, C.; Díaz-Redondo, A.; Bretón, I.; Bellón, J.M.; Pérez-Valderas, M.D.; Caballero-Marcos, A.; de Dios-Lascuevas, M.; González-Río, E.; García-Sánchez, C.; et al. Metabolic Bone Disease in Patients Diagnosed with Inflammatory Bowel Disease from Spain. Ther. Adv. Gastroenterol. 2019, 12, 1756284819862152. [Google Scholar] [CrossRef]
- Garrido, A.; Martínez, M.J.; Ortega, J.A.; Lobato, A.; Rodríguez, M.J.; Guerrero, F.J. Epidemiology of Chronic Inflammatory Bowel Disease in the Northern Area of Huelva. Rev. Espanola Enfermedades Dig. Organo Of. Soc. Espanola Patol. Dig. 2004, 96, 687–691. [Google Scholar] [CrossRef]
- Fernández, A.; Hernández, V.; Martínez-Ares, D.; Sanromán, L.; de Castro, M.L.; Pineda, J.R.; Carmona, A.; González-Portela, C.; Salgado, C.; Martínez-Cadilla, J.; et al. Incidence and Phenotype at Diagnosis of Inflammatory Bowel Disease. Results in Spain of the EpiCom Study. Gastroenterol. Hepatol. 2015, 38, 534–540. [Google Scholar] [CrossRef] [PubMed]
- The Impact of Inflammatory Bowel Disease in Canada 2012 Final Report and Recommendation; Crohn’s and Colitis Fundation of Canada: Toronto, ON, Canada, 2012.
- Targownik, L.E.; Leslie, W.D.; Carr, R.; Clara, I.; Miller, N.; Rogala, L.; Graff, L.A.; Walker, J.R.; Bernstein, C.N. Longitudinal Change in Bone Mineral Density in a Population-Based Cohort of Patients with Inflammatory Bowel Disease. Calcif. Tissue Int. 2012, 91, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Chong, C.A.; Chong, R.Y. National Estimates of the Burden of Inflammatory Bowel Disease among Racial and Ethnic Groups in the United States. J. Crohns Colitis 2014, 8, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Waszak, K.; Kucharski, M.A.; Dobrowolska, A.; Eder, P. Prevalence of Osteoporosis and Osteopenia in a Population of Patients with Inflammatory Bowel Diseases from the Wielkopolska Region. Pol. Arch. Intern. Med. 2018, 128, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Dziekiewicz, M.; Kowalska-Duplaga, K.; Baranowska-Nowak, M.; Neścioruk, M.; Kuźniarski, S.; Banasiuk, M.; Banaszkiewicz, A. Awareness of Smoking in Adolescents with Inflammatory Bowel Disease. Ann. Agric. Environ. Med. 2020, 27, 61–65. [Google Scholar] [CrossRef]
- Need, A.G.; Kemp, A.; Giles, N.; Morris, H.A.; Horowitz, M.; Nordin, B.E.C. Relationships between Intestinal Calcium Absorption, Serum Vitamin D Metabolites and Smoking in Postmenopausal Women. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2002, 13, 83–88. [Google Scholar] [CrossRef]
- Kopiczko, A.; Gryko, K.; Łopuszańska-Dawid, M. Bone Mineral Density, Hand Grip Strength, Smoking Status and Physical Activity in Polish Young Men. Homo Int. Z. Vgl. Forsch. Am, Menschen 2018, 69, 209–216. [Google Scholar] [CrossRef]
- Hijazi, N.; Alourfi, Z. Prevalence and Factors Associated with Low Bone Mass and Osteoporosis in Syrian Postmenopausal Women. Mathews J. Case Rep. 2019, 4. [Google Scholar] [CrossRef]
- Shen, G.S.; Li, Y.; Zhao, G.; Zhou, H.B.; Xie, Z.G.; Xu, W.; Chen, H.N.; Dong, Q.R.; Xu, Y.J. Cigarette Smoking and Risk of Hip Fracture in Women: A Meta-Analysis of Prospective Cohort Studies. Injury 2015, 46, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Thorin, M.H.; Wihlborg, A.; Åkesson, K.; Gerdhem, P. Smoking, Smoking Cessation, and Fracture Risk in Elderly Women Followed for 10 Years. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2016, 27, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kleisiaris, F.C.; Plaiti, E.M.; Papathanasiou, V.I.; Papaioannou, P.; Kastrinakis, I.; Diakantoni, S.; Fradelos, E.; Kourkouta, L. Smoking Is Associated with Osteoporosis Development in Primary Care Population. Am. J. Nurs. Sci. 2015, 4, 96–101. [Google Scholar]
- Mędrela-Kuder, E.; Szymura, K. Selected Anti-Health Behaviours among Women with Osteoporosis. Rocz. Panstw. Zakl. Hig. 2018, 69, 397–403. [Google Scholar] [CrossRef]
- Strozyk, D.; Gress, T.M.; Breitling, L.P. Smoking and Bone Mineral Density: Comprehensive Analyses of the Third National Health and Nutrition Examination Survey (NHANES III). Arch. Osteoporos. 2018, 13, 16. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Zhao, P.; Liu, B.; Yuan, Z.-C. Effect of Cigarette Smoking on Risk of Hip Fracture in Men: A Meta-Analysis of 14 Prospective Cohort Studies. PLoS ONE 2016, 11, e0168990. [Google Scholar] [CrossRef]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastián, V.P.; Álvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef]
- Allais, L.; Kerckhof, F.-M.; Verschuere, S.; Bracke, K.R.; De Smet, R.; Laukens, D.; Van den Abbeele, P.; De Vos, M.; Boon, N.; Brusselle, G.G.; et al. Chronic Cigarette Smoke Exposure Induces Microbial and Inflammatory Shifts and Mucin Changes in the Murine Gut. Environ. Microbiol. 2016, 18, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Hunsballe, J.M.; Rittig, S.; Pedersen, E.B.; Djurhuus, J.C. Smokeless Nicotinergic Stimulation of Vasopressin Secretion in Patients with Persisting Nocturnal Enuresis and Controls. Scand. J. Urol. Nephrol. 2001, 35, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Li, Y.; Wang, H.; Wu, R.; Zhu, W.; Zhang, W.; Cao, L.; Gu, L.; Gong, J.; Li, N.; et al. Cigarette Smoking Is Associated with Intestinal Barrier Dysfunction in the Small Intestine but Not in the Large Intestine of Mice. J. Crohns Colitis 2014, 8, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, S.; Bracke, K.R.; Demoor, T.; Plantinga, M.; Verbrugghe, P.; Ferdinande, L.; Lambrecht, B.N.; Brusselle, G.G.G.; Cuvelier, C.A. Cigarette Smoking Alters Epithelial Apoptosis and Immune Composition in Murine GALT. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet Lond. Engl. 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet Lond. Engl. 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Krela-Kazmierczak, I.; Szymczak-Tomczak, A.; Tomczak, M.; Lykowska-Szuber, L.; Eder, P.; Kucharski, M.A.; Stawczyk-Eder, K.; Waszak, K.; Karczewski, J.; Dobrowolska, A. Is There a Relation between Vitamin D, Interleukin-17, and Bone Mineral Density in Patients with Inflammatory Bowel Disease? Arch. Med. Sci. 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Ueno, A.; Jijon, H.; Traves, S.; Chan, R.; Ford, K.; Beck, P.L.; Iacucci, M.; Fort Gasia, M.; Barkema, H.W.; Panaccione, R.; et al. Opposing Effects of Smoking in Ulcerative Colitis and Crohn’s Disease May Be Explained by Differential Effects on Dendritic Cells. Inflamm. Bowel Dis. 2014, 20, 800–810. [Google Scholar] [CrossRef]
- Kikuchi, H.; Itoh, J.; Fukuda, S. Chronic Nicotine Stimulation Modulates the Immune Response of Mucosal T Cells to Th1-Dominant Pattern via NAChR by Upregulation of Th1-Specific Transcriptional Factor. Neurosci. Lett. 2008, 432, 217–221. [Google Scholar] [CrossRef]
- Lee, G.; Jung, K.-H.; Shin, D.; Lee, C.; Kim, W.; Lee, S.; Kim, J.; Bae, H. Cigarette Smoking Triggers Colitis by IFN-Γ+ CD4+ T Cells. Front. Immunol. 2017, 8, 1344. [Google Scholar] [CrossRef]
- Vrablicova, Z.; Soltys, K.; Krajcovicova, A.; Stuchlikova, K.; Sturdik, I.; Koller, T.; Huorka, M.; Payer, J.; Killinger, Z.; Jackuliak, P.; et al. Impact of Smoking Cigarette on the MRNA Expression of Cytokines in Mucosa of Inflammatory Bowel Disease. Physiol. Res. 2019, 68, S183–S192. [Google Scholar] [CrossRef]
- Biedermann, L.; Brülisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking Cessation Alters Intestinal Microbiota: Insights from Quantitative Investigations on Human Fecal Samples Using FISH. Inflamm. Bowel Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef]
- Wasielica-Berger, J. Osteoporosis in inflammatory bowel diseases. Gastroenterol. Klin. Postępy Stand. 2015, 7, 96–101. [Google Scholar]
- Silvennoinen, J.A.; Lehtola, J.K.; Niemelä, S.E. Smoking Is a Risk Factor for Osteoporosis in Women with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 1996, 31, 367–371. [Google Scholar] [CrossRef]
- Chong, C.; Rahman, A.; Loonat, K.; Sagar, R.C.; Selinger, C.P. Current Smoking Habits in British IBD Patients in the Age of E-Cigarettes. BMJ Open Gastroenterol. 2019, 6, e000309. [Google Scholar] [CrossRef] [PubMed]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and Inflammatory Bowel Disease: A Meta-Analysis. Mayo Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Poonsombudlert, K.; Ungprasert, P. Smoking and Risk of Microscopic Colitis: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2019, 25, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Osterman, M.T.; Bewtra, M.; Lewis, J.D. Passive Smoking and Inflammatory Bowel Disease: A Meta-Analysis. Am. J. Gastroenterol. 2008, 103, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, M.; Spence, A.D.; Addley, J.; Allen, P.B. The Role of Smoking and Alcohol Behaviour in the Management of Inflammatory Bowel Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 553–559. [Google Scholar] [CrossRef]
- Abegunde, A.T.; Muhammad, B.H.; Ali, T. Preventive Health Measures in Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 7625–7644. [Google Scholar] [CrossRef] [PubMed]
- Casals-Seoane, F.; Chaparro, M.; Maté, J.; Gisbert, J.P. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflamm. Bowel Dis. 2016, 22, 1929–1936. [Google Scholar] [CrossRef]
- Labarca, G.; Drake, L.; Horta, G.; Jantz, M.A.; Mehta, H.J.; Fernandez-Bussy, S.; Folch, E.; Majid, A.; Picco, M. Association between Inflammatory Bowel Disease and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Lee, S.M.; Eksteen, B.; Seow, C.H.; Barnabe, C.; Panaccione, R.; Kaplan, G.G. Smoking Influences the Need for Surgery in Patients with the Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis Incorporating Disease Duration. BMC Gastroenterol. 2016, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- To, N.; Gracie, D.J.; Ford, A.C. Systematic Review with Meta-Analysis: The Adverse Effects of Tobacco Smoking on the Natural History of Crohn’s Disease. Aliment. Pharmacol. Ther. 2016, 43, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Poniedziałek, B.; Rzymski, P.; Rychlewska-Hańczewska, A.; Adamski, Z.; Wiktorowicz, K. The Effect of Cigarette Smoking on the Clinical Course of Inflammatory Bowel Disease. Gastroenterol. Rev. 2014, 9, 153–159. [Google Scholar] [CrossRef]
- Maaser, C.; Langholz, E.; Gordon, H.; Burisch, J.; Ellul, P.; Ramirez, V.H.; Karakan, T.; Katsanos, K.H.; Krustins, E.; Levine, A.; et al. European Crohn’s and Colitis Organisation Topical Review on Environmental Factors in IBD. J. Crohns Colitis 2017, 11, 905–920. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016--Executive Summary. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2019, 30, 3–44. [Google Scholar] [CrossRef]
| Country | Prevalence of IBD (%) | Ref. | Prevalence of Osteoporosis in IBD (%) | Ref. | Percentage of IBD Smokers (%) | Ref. | Morbidity (n/100000) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Europe | 0.006(CD) 0.010 (UC) | [72] | no data | 24.0–73.0 (CD) 5.4–64.0 (UC) | [73] | 12.7 (CD) 24.3 (UC) | [72] | |
| North America | 0.420 (IBD) 0.040–0.250 (UC) | [74] | no data | 4.0 (CD) 8.6 (UC) | [73] | 0–20.2 (CD) 0–19.2 (UC) | [75] | |
| Australia | 0.030 (for CD) 0.020 (for UC) | [76] | no data | 15.0 (CD) 5.0 (UC) | [77] | 29.3 (CD) 17.4 (UC) | [78] | |
| Finland | 0.120 (for CD) 0.290 (for UC) | [79] | no data | no data | 9.2 (CD) 24.8 (UC) | [80] | ||
| Hungary | 0.050 (for CD) 0.140 (for UC) | [81] | no data | no data | 4.7 (CD) 11.0 (UC) | [82] | ||
| United Kingdom | 0.280 (for CD) 0.430 (for UC) | [83] | 11.6–13.6 (CD) | [84] | 32.2 (CD) | [84] | 10.2 (CD) 15.7 (UC) | [85] |
| Germany | 0.320 (for CD) 0.410 (for UC) | [86] | 15.0 (CD) 7.0 (UC) | [87] | 16.1 (CD) 7.5 (UC) upon diagnosis | [88] | 6.1 (CD) 3.9 (UC) | [88] |
| Slovakia | 0.680 (CD) | [89] | 10.0 (UC) 15.2 (CD) | [90] | 42.0 (CD) 36.0 (UC) upon diagnosis | [91] | no data | |
| Netherlands | 0.230 (CD) 0.280 (UC) | [92] | 28.0 (CD) 11.0 (UC) | [93] | 46.1 (CD) 20.0 (UC) | [94] | 17.2 (CD) 10.5 (UC) | [78] |
| Spain | 0.190 (CD) 0.350 (UC) | [95] | 17.0 (CD) 27.7 (UC) | [96] | 66.7 (CD) 12.5 (UC) | [97] | 10.8 (CD) 9.4 (UC) | [98] |
| Canada | 0.370 (CD) 0.300 (UC) | [99] | 7.0 (IBD) | [100] | no data | 23.8 (CD) 23.1 (UC) | [78] | |
| United States | 0.900 (IBD) | [101] | no data | no data | 6.3 (CD) 8.8 (UC) | [78] | ||
| Poland | 0.160 (IBD) 0.040 (CD) | [75] | 5.8–11.7 (CD) 2.9–3.8 (UC) | [102] | 12.2 (IBD) 8.3 (CD) 18.2 (UC) /children and adolescents/ | [103] | no data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1515. https://doi.org/10.3390/jcm10071515
Ratajczak AE, Szymczak-Tomczak A, Rychter AM, Zawada A, Dobrowolska A, Krela-Kaźmierczak I. Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2021; 10(7):1515. https://doi.org/10.3390/jcm10071515
Chicago/Turabian StyleRatajczak, Alicja Ewa, Aleksandra Szymczak-Tomczak, Anna Maria Rychter, Agnieszka Zawada, Agnieszka Dobrowolska, and Iwona Krela-Kaźmierczak. 2021. "Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases" Journal of Clinical Medicine 10, no. 7: 1515. https://doi.org/10.3390/jcm10071515
APA StyleRatajczak, A. E., Szymczak-Tomczak, A., Rychter, A. M., Zawada, A., Dobrowolska, A., & Krela-Kaźmierczak, I. (2021). Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases. Journal of Clinical Medicine, 10(7), 1515. https://doi.org/10.3390/jcm10071515






