Pomalidomide Plus Low-Dose Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients: Results of the Real-World “POWERFUL” Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Objectives and Relevant Definitions
2.3. Statistical Methods
2.4. Sample Size Determination
3. Results
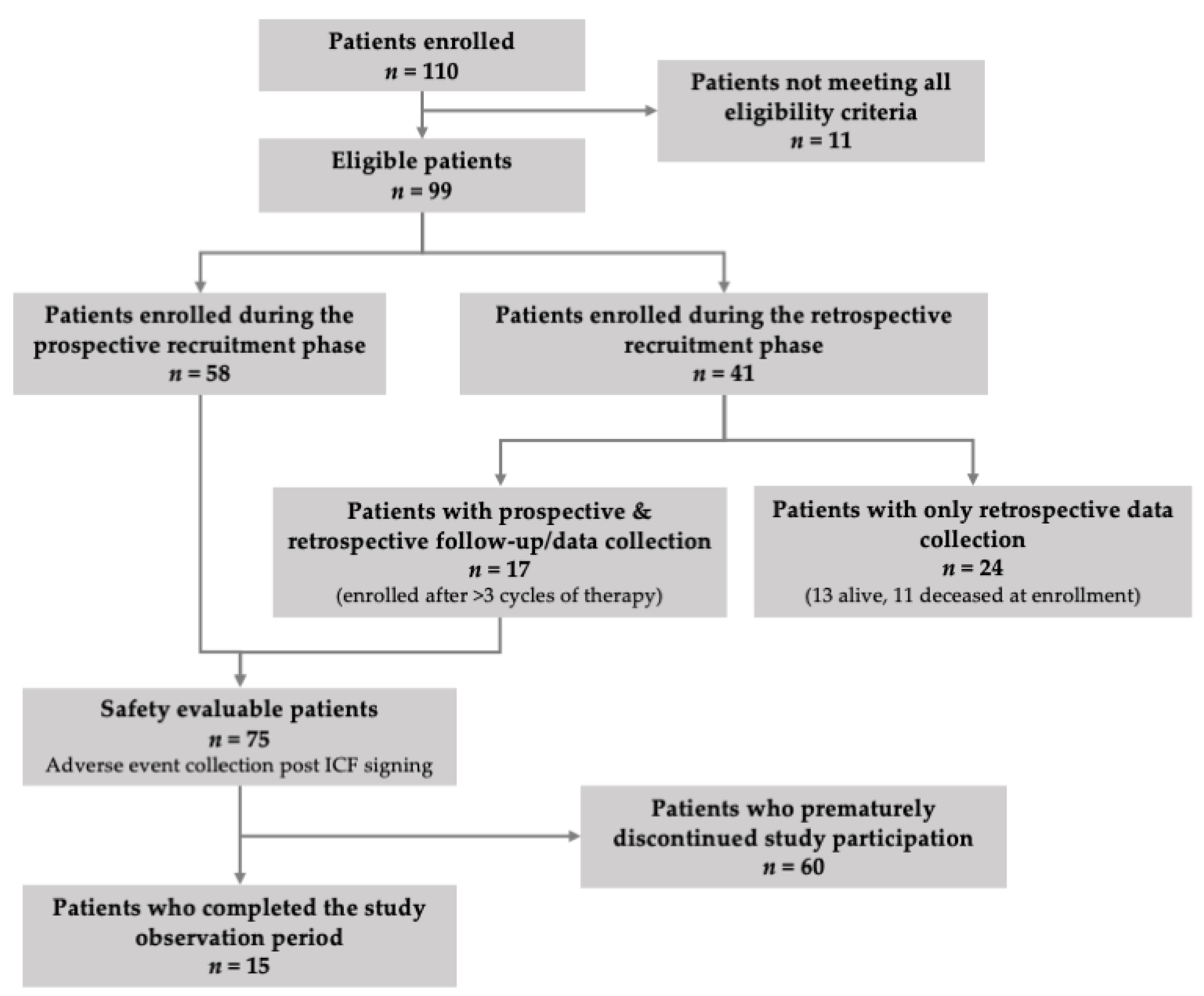
3.1. Patients
3.2. Treatment Characteristics
3.3. Effectiveness
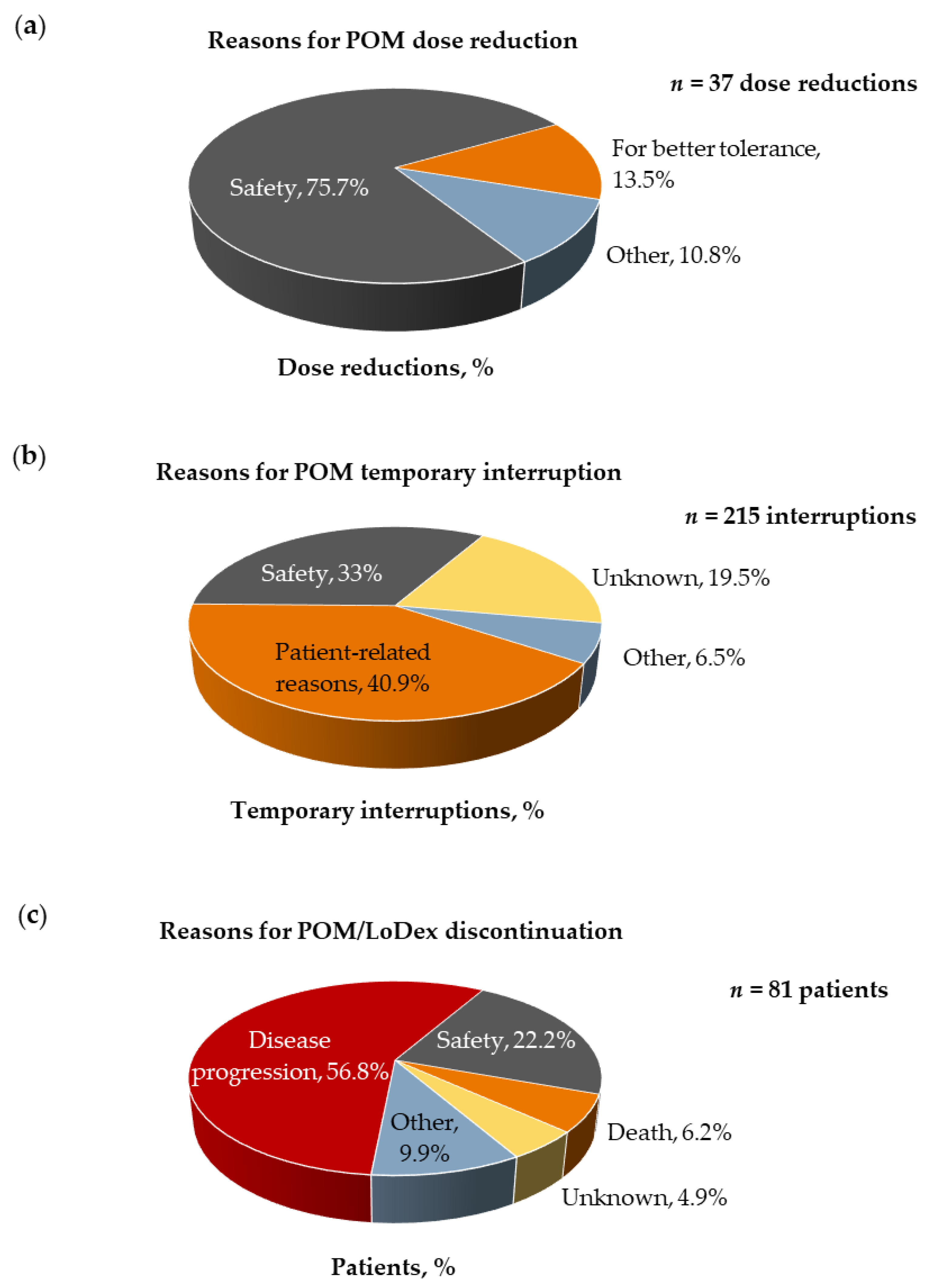
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimopoulos, M.; Moreau, P.; Terpos, E.; Mateos, M.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 995. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Niesvizky, R.; Palumbo, A. Clinical characteristics of patients with relapsed multiple myeloma. Cancer Treat. Rev. 2015, 41, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; San-Miguel, J.F.; Anderson, K.C. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur. J. Haematol. 2010, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Therneau, T.M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Rajkumar, S.V.; Fonseca, R.; Witzig, T.E.; Lust, J.A.; Larson, D.R.; et al. Clinical Course of Patients with Relapsed Multiple Myeloma. Mayo Clin. Proc. 2004, 79, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef]
- Siegel, D.S.; Schiller, G.J.; Song, K.W.; Agajanian, R.; Stockerl-Goldstein, K.; Kaya, H.; Sebag, M.; Samaras, C.; Malek, E.; Talamo, G.; et al. Pomalidomide plus low-dose dexamethasone in relapsed refractory multiple myeloma after lenalidomide treatment failure. Br. J. Haematol. 2019, 188, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Borrello, I. Can we change the disease biology of multiple myeloma? Leuk. Res. 2012, 36, S3–S12. [Google Scholar] [CrossRef][Green Version]
- Terpos, E.; Kanellias, N.; Christoulas, D.; Kastritis, E.; Dimopoulos, M.A. Pomalidomide: A novel drug to treat relapsed and refractory multiple myeloma. Onco. Targets Ther. 2013, 6, 531–538. [Google Scholar] [CrossRef]
- Lopezgirona, A.; Mendy, D.; Ito, T.A.; Miller, K.H.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.E.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Gandhi, A.K.; Kang, J.; Havens, C.G.; Conklin, T.; Ning, Y.; Wu, L.; Ito, T.; Ando, H.; Waldman, M.F.; Thakurta, A.; et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors I karos and A iolos via modulation of the E 3 ubiquitin ligase complex CRL 4 CRBN. Br. J. Haematol. 2014, 164, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Shima, Y.; Raje, N.; Davies, F.E.; Tai, Y.T.; Treon, S.P.; Lin, B.; Schlossman, R.L.; Richardson, P.; et al. Thalidomide and its analogs overcome drug resistance of human multiple myelomacells to conventional therapy. Blood 2000, 96, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Treon, S.P.; Shima, Y.; Hideshima, T.; Podar, K.; Tai, Y.T.; Lin, B.; Lentzsch, S.; Davies, F.E.; Chauhan, D.; et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: Therapeutic applications. Leukemia 2001, 15, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Suwa, T.; Murase, Y.; Tateno, S.; Mizutome, H.; Asatsuma-Okumura, T.; Shimizu, N.; Kishi, T.; Momose, S.; Kizaki, M.; et al. ARID2 is a pomalidomide-dependent CRL4CRBN substrate in multiple myeloma cells. Nat. Chem. Biol. 2020, 16, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Siegel, D.S.; Vij, R.; Hofmeister, C.C.; Baz, R.; Jagannath, S.; Chen, C.; Lonial, S.; Jakubowiak, A.; Bahlis, N.; et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 2014, 123, 1826–1832. [Google Scholar] [CrossRef]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Palumbo, A.; Corradini, P.; Cavo, M.; Delforge, M.; Di Raimondo, F.; Weisel, K.C.; Oriol, A.; Hansson, M.; Vacca, A.; et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood 2016, 128, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Weisel, K.C.; Dimopoulos, M.A.; Baz, R.; Richardson, P.; Delforge, M.; Song, K.W.; Miguel, J.F.S.; Moreau, P.; Goldschmidt, H.; et al. Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and moderate renal impairment: A pooled analysis of three clinical trials. Leuk. Lymphoma 2016, 57, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Weisel, K.; Van De Donk, N.W.; Ramasamy, K.; Gamberi, B.; Streetly, M.; Offidani, M.; Bridoux, F.; De La Rubia, J.; Mateos, M.-V.; et al. Pomalidomide Plus Low-Dose Dexamethasone in Patients With Relapsed/Refractory Multiple Myeloma and Renal Impairment: Results From a Phase II Trial. J. Clin. Oncol. 2018, 36, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Weisel, K.C.; Song, K.W.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Moreau, P.; Banos, A.; Oriol, A.; Garderet, L.; et al. Cytogenetics and long-term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low-dose dexamethasone. Haematologica 2015, 100, 1327–1333. [Google Scholar] [CrossRef]
- Mushtaq, A.; Iftikhar, A.; Hassan, H.; Lakhani, M.; Sagar, F.; Kamal, A.; Zahid, U.; Ali, Z.; Razzaq, F.; Abu Zar, M.; et al. Pomalidomide-Based Regimens for Treatment of Relapsed and Relapsed/Refractory Multiple Myeloma: Systematic Review and Meta-analysis of Phase 2 and 3 Clinical Trials. Clin. Lymphoma Myeloma Leuk. 2019, 19, 447–461. [Google Scholar] [CrossRef]
- Charlinski, G.; Grzasko, N.; Jurczyszyn, A.; Janczarski, M.; Szeremet, A.; Waszczuk-Gajda, A.; Bernatowicz, P.; Swiderska, A.; Guzicka-Kazimierczak, R.; Lech-Maranda, E.; et al. The efficacy and safety of pomalidomide in relapsed/refractory multiple myeloma in a “real-world” study: Polish Myeloma Group experience. Eur. J. Haematol. 2018, 101, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mele, G.; Pastore, D.; Di Renzo, N.; Fragasso, A.; Guarini, A.; Mazza, P.; Musto, P.; Pavone, V.; Tarantini, G.; Curci, P.; et al. Real world Italian experience of pomalidomide plus low-dose dexamethasone in the relapsed and refractory myeloma setting: Extended follow-up of a retrospective multicenter study by the ‘Rete Ematologica Pugliese E Basilicata’. Leuk. Lymphoma 2019, 60, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Maciocia, N.; Melville, A.; Cheesman, S.; Sharpley, F.; Ramasamy, K.; Streetly, M.; Jenner, M.; Benjamin, R.; Schey, S.; Maciocia, P.; et al. Real-world use of pomalidomide and dexamethasone in double refractory multiple myeloma suggests benefit in renal impairment and adverse genetics: A multi-centre UK experience. Br. J. Haematol. 2017, 176, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Kastritis, E.; Roussou, M.; Gavriatopoulou, M.; Kanellias, N.; Migkou, M.; Eleutherakis-Papaiakovou, E.; Ziogas, D.C.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Dialoupi, I.; et al. Impact of last lenalidomide dose, duration, and IMiD-free interval in patients with myeloma treated with pomalidomide/dexamethasone. Blood Adv. 2019, 3, 4095–4103. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Fotiou, D.; Roussou, M.; Migkou, M.; Ziogas, D.C.; Kanellias, N.; Terpos, E.; Dimopoulos, M.A. The addition of IMiDs for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood 2018, 131, 464–467. [Google Scholar] [CrossRef]
- Rychak, E.; Mendy, D.; Shi, T.; Ning, Y.; Leisten, J.; Lu, L.; Miller, K.; Narla, R.K.; Orlowski, R.Z.; Raymon, H.K.; et al. Pomalidomide in combination with dexamethasone results in synergistic anti-tumour responses in pre-clinical models of lenalidomide-resistant multiple myeloma. Br. J. Haematol. 2016, 172, 889–901. [Google Scholar] [CrossRef]
- Ocio, E.M.; Fernández-Lázaro, D.; San-Segundo, L.; López-Corral, L.; Sánchez, L.A.C.; Gutiérrez, N.C.; Garayoa, M.; Paíno, T.; Garcia-Gomez, A.; Delgado, M.; et al. In vivo murine model of acquired resistance in myeloma reveals differential mechanisms for lenalidomide and pomalidomide in combination with dexamethasone. Leukemia 2014, 29, 705–714. [Google Scholar] [CrossRef]
- Shi, C.-X.; Kortüm, K.M.; Zhu, Y.X.; Jedlowski, P.; Bruins, L.; Braggio, E.; Stewart, A.K. Proteasome inhibitors block Ikaros degradation by Lenalidomide in Multiple Myeloma. Haematologica 2015, 100, e315–e317. [Google Scholar] [CrossRef]
- Madan, S.; Lacy, M.Q.; Dispenzieri, A.; Gertz, M.A.; Buadi, F.; Hayman, S.R.; Detweiler-Short, K.; Dingli, D.; Zeldenrust, S.; Lust, J.; et al. Efficacy of retreatment with immunomodulatory drugs (IMiDs) in patients receiving IMiDs for initial therapy of newly diagnosed multiple myeloma. Blood 2011, 118, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Mikhael, J.R.; LaPlant, B.R.; Laumann, K.M.; Kumar, S.; Roy, V.; Dingli, D.; Bergsagel, P.L.; Buadi, F.K.; Rajkumar, S.V.; et al. Pomalidomide–dexamethasone in refractory multiple myeloma: Long-term follow-up of a multi-cohort phase II clinical trial. Leukemia 2017, 32, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Leleu, X.; Palumbo, A.; Moreau, P.; Delforge, M.; Cavo, M.; Ludwig, H.; Morgan, G.J.; Davies, F.E.; Sonneveld, P.; et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia 2014, 28, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Cavo, M.; Offidani, M.; Scaglione, F.; Corso, A.; Di Raimondo, F.; Musto, P.; Petrucci, M.T.; Barosi, G. Management of infectious complications in multiple myeloma patients: Expert panel consensus-based recommendations. Blood Rev. 2019, 34, 84–94. [Google Scholar] [CrossRef]
- Fotiou, D.; Gavriatopoulou, M.; Terpos, E. Multiple Myeloma and Thrombosis: Prophylaxis and Risk Prediction Tools. Cancers 2020, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Delforge, M.; Facon, T.; Einsele, H.; Gay, F.; Moreau, P.; Avet-Loiseau, H.; Boccadoro, M.; Hajek, R.; Mohty, M.; et al. Prevention and management of adverse events of novel agents in multiple myeloma: A consensus of the European Myeloma Network. Leukemia 2018, 32, 1542–1560. [Google Scholar] [CrossRef] [PubMed]

| Patient and Disease Characteristics at Baseline (n = 99) | |
| Males, n (%) (n = 99) | 53 (53.5) |
| Age at baseline (n = 99) | |
| Median (IQR), years | 71.8 (65.8–76.6) |
| >65 years, n (%) | 78 (78.8) |
| >75 years, n (%) | 29 (29.3) |
| Time from initial MM diagnosis to baseline [years; median (IQR)] (n = 99) | 3.8 (2.3–6.6) |
| Medical/surgical history (past/ongoing), n (%) (n = 99) | 87 (87.9) |
| Presence of comorbidities, n (%) | 74 (74.7) |
| Number of comorbidities [median (IQR)] | 1.0 (0.0–3.0) |
| Presence of comorbidities related to MM or its complications, n (%) | 35 (35.4) |
| IgG/IgA MM subtype, n (%) (n = 76) | 50 (65.8)/26 (34.2) |
| ISS Stage I/Stage II/Stage III disease, n (%) (n = 82) | 9 (11.0)/24 (29.3)/49 (59.8) |
| High-risk cytogenetics 1 at baseline or at initial diagnosis, n (%) (n = 57) | 12 (21.1) |
| Relapsed and refractory/relapsed/refractory MM, n (%) (n = 99) | 51 (51.5)/40 (40.4)/8 (8.1) |
| Serum LDH (n = 89) | |
| Median (IQR), U/L | 176 (145–242) |
| >ULN, n (%) | 20 (22.5) |
| Serum creatinine (n = 71) | |
| Median (IQR), mL/min | 65.6 (43.8–93.0) |
| <60 mL/min, n (%) | 31 (43.7) |
| ECOG PS 0/1/2/3, n (%) (n = 99) | 18 (18.2)/46 (46.5)/24 (24.2)/11 (11.1) |
| POM/LoDex initiation in line 3/4/5/6, n (%) (n = 99) | 50 (50.5)/26 (26.3)/17 (17.2)/6 (6.1) |
| Prior MM treatments, n (%) (n = 99) | |
| Steroids | 98 (99.0) |
| IMiDs: any/lenalidomide/thalidomide | 99 (100)/99 (100)/26 (26.3) |
| PIs: any/bortezomib/carfilzomib/ixazomib | 99 (100)/99 (100)/10 (10.1)/8 (8.1) |
| Chemotherapy 2 | 87 (87.9) |
| mAbs | 15 (15.2) |
| Other targeted therapies | 6 (6.1) |
| Autologous stem cell transplantation | 24 (24.2) |
| Refractoriness to prior treatments, n (%) (n = 99) | |
| To IMiDs: any/lenalidomide/thalidomide | 48 (48.5)/48 (48.5)/2 (2.0) |
| To PIs: any/bortezomib/carfilzomib/ixazomib | 42 (42.4)/41 (41.4)/2 (2.0)/3 (3.0) |
| Double refractory to bortezomib and lenalidomide | 33 (33.3) |
| To daratumumab | 5 (5.1) |
| To other agents (including cisplatin, cyclophosphamide, dexamethasone, doxorubicin, etoposide, and melphalan) | 9 (9.1) |
| Concomitant Antimyeloma Therapies Received with POM/LoDex at Any Time, n (%) (n = 99) | ||
| Any antimyeloma agent | 31 (31.3) | |
| Cyclophosphamide | 16 (16.2) | |
| Bortezomib | 8 (8.1) | |
| Daratumumab | 7 (7.1) | |
| Carfilzomib | 1 (1.0) | |
| Concomitant therapies in >5.0% of the patients, n (%)(n = 99) | Prophylaxis | Adverse Events |
| Any concomitant therapy | 81 (81.8) | 24 (24.2) |
| Antithrombotic agents | 56 (56.6) | 1 (1.0) |
| Antivirals for systemic use | 51 (51.5) | 3 (3.0) |
| Antibacterials for systemic use 1 | 45 (45.5) | 10 (10.1) |
| Drugs for acid related disorders | 33 (33.3) | 1 (1.0) |
| Antimycotics for systemic use | 14 (14.1) | 2 (2.0) |
| Antigout preparations 2 | 11 (11.1) | |
| Immunostimulants (filgrastrim) | 10 (10.1) | 8 (8.1) |
| Antianemic preparations 3 | 5 (5.1) | 3 (3.0) |
| Drugs for treatment of bone diseases | 6 (6.1) | |
| Parameter | OR | 95% CI | p-Value | |
| ORR (n = 99) | Sex (male vs. female) | 0.44 | 0.18–1.07 | 0.071 |
| ECOG PS at baseline (<2 vs. ≥2) | 4.55 | 1.54–13.48 | 0.006 | |
| Parameter | HR | 95% CI | p-Value | |
| PFS (n = 84) | Sex (male vs. female) | 2.08 | 1.12–3.89 | 0.021 |
| POM/LoDex initiation after clinical relapse | 1.79 | 0.94–3.42 | 0.076 | |
| Bortezomib in the immediately prior line | 0.55 | 0.28–1.06 | 0.075 | |
| Baseline serum LDH (>ULN vs. ≤ULN) | 2.84 | 1.39–5.81 | 0.004 | |
| TTR (n = 32) | POM/LoDex initiation after clinical relapse | 3.91 | 1.44–10.60 | 0.007 |
| Line of POM/LoDex initiation (3 vs. >3) | 6.65 | 2.14–20.63 | 0.001 | |
| Bortezomib in the immediately prior line | 0.48 | 0.17–1.37 | 0.171 | |
| DoR (n = 32) | Sex (male vs. female) | 9.75 | 1.84–51.66 | 0.007 |
| Age at MM diagnosis (<65 vs. ≥65 years) | 0.31 | 0.06–1.45 | 0.136 |
| Incidence (n = 75) | Non-Serious | Serious | Overall | |||
| nevents | n (%) | nevents | n (%) | nevents | n (%) | |
| AEs | 135 | 45 (60.0) | 83 | 36 (48.0) | 218 | 60 (80.0) |
| AEs related to POM | 42 | 23 (30.7) | 31 | 14 (18.7) | 73 | 32 (42.7) |
| AEs leading to POM discontinuation | 6 | 6 (8.0) | 21 | 15 (20.0) | 27 | 21 (28.0) |
| POM-related AEs leading to POM discontinuation | 4 | 4 (5.3) | 3 | 3 (4.0) | 7 | 7 (9.3) |
| Infections/infestations | 18 | 15 (20.0) | 17 | 15 (20.0) | 35 | 28 (37.3) |
| Infections/infestations related to POM | 4 | 3 (4.0) | 4 | 3 (4.0) | ||
| Thrombosis/deep vein thrombosis | 2 | 1 (1.3) | 1 | 1 (1.3) | 3 | 2 (2.7) |
| Deep vein thrombosis related to POM | 1 | 1 (1.3) | 1 | 1 (1.3) | ||
| nevents | n (%) | |||||
| Grade ≥3 POM-related AEs | 22 | 10 (13.3) | ||||
| Grade ≥3 POM-related hematological toxicities | 14 | 6 (8.0) | ||||
| Neutropenia | 3 | 3 (4.0) | ||||
| Anemia Thrombocytopenia | 4 | 2 (2.7) | ||||
| 4 | 2 (2.7) | |||||
| Platelet count decreased | 2 | 1 (1.3) | ||||
| Neutrophil count decreased | 1 | 1 (1.3) | ||||
| Grade ≥3 POM-related non-hematological toxicities | 8 | 4 (5.3) | ||||
| Acute kidney injury/renal impairment | 2 | 2 (2.7) | ||||
| Back pain | 1 | 1 (1.3) | ||||
| Deep vein thrombosis Device (catheter) related infection Diarrhea | 1 | 1 (1.3) | ||||
| 1 | 1 (1.3) | |||||
| 1 | 1 (1.3) | |||||
| Gastric hemorrhage | 1 | 1 (1.3) | ||||
| Renal impairment | 1 | 1 (1.3) | ||||
| Urinary tract infection | 1 | 1 (1.3) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpos, E.; Repousis, P.; Lalayanni, C.; Hatjiharissi, E.; Assimakopoulou, T.; Vassilopoulos, G.; Pouli, A.; Spanoudakis, E.; Michalis, E.; Pangalis, G.; et al. Pomalidomide Plus Low-Dose Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients: Results of the Real-World “POWERFUL” Study. J. Clin. Med. 2021, 10, 1509. https://doi.org/10.3390/jcm10071509
Terpos E, Repousis P, Lalayanni C, Hatjiharissi E, Assimakopoulou T, Vassilopoulos G, Pouli A, Spanoudakis E, Michalis E, Pangalis G, et al. Pomalidomide Plus Low-Dose Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients: Results of the Real-World “POWERFUL” Study. Journal of Clinical Medicine. 2021; 10(7):1509. https://doi.org/10.3390/jcm10071509
Chicago/Turabian StyleTerpos, Evangelos, Panagiotis Repousis, Chrysavgi Lalayanni, Evdoxia Hatjiharissi, Theodora Assimakopoulou, Georgios Vassilopoulos, Anastasia Pouli, Emmanouil Spanoudakis, Eurydiki Michalis, Gerassimos Pangalis, and et al. 2021. "Pomalidomide Plus Low-Dose Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients: Results of the Real-World “POWERFUL” Study" Journal of Clinical Medicine 10, no. 7: 1509. https://doi.org/10.3390/jcm10071509
APA StyleTerpos, E., Repousis, P., Lalayanni, C., Hatjiharissi, E., Assimakopoulou, T., Vassilopoulos, G., Pouli, A., Spanoudakis, E., Michalis, E., Pangalis, G., Ntanasis-Stathopoulos, I., Poziopoulos, C., Kyrtsonis, M.-C., Pappa, V., Symeonidis, A., Georgopoulos, C., Zikos, P. M., Gavriatopoulou, M., Papadaki, H. A., ... Katodritou, E. (2021). Pomalidomide Plus Low-Dose Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients: Results of the Real-World “POWERFUL” Study. Journal of Clinical Medicine, 10(7), 1509. https://doi.org/10.3390/jcm10071509












