Prognostic and Clinical Value of Cluster Analysis in Idiopathic Pleuroparenchymal Fibroelastosis Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Diagnostic Criteria for Idiopathic PPFE
2.2. Data Collection
2.3. Statistical Analysis
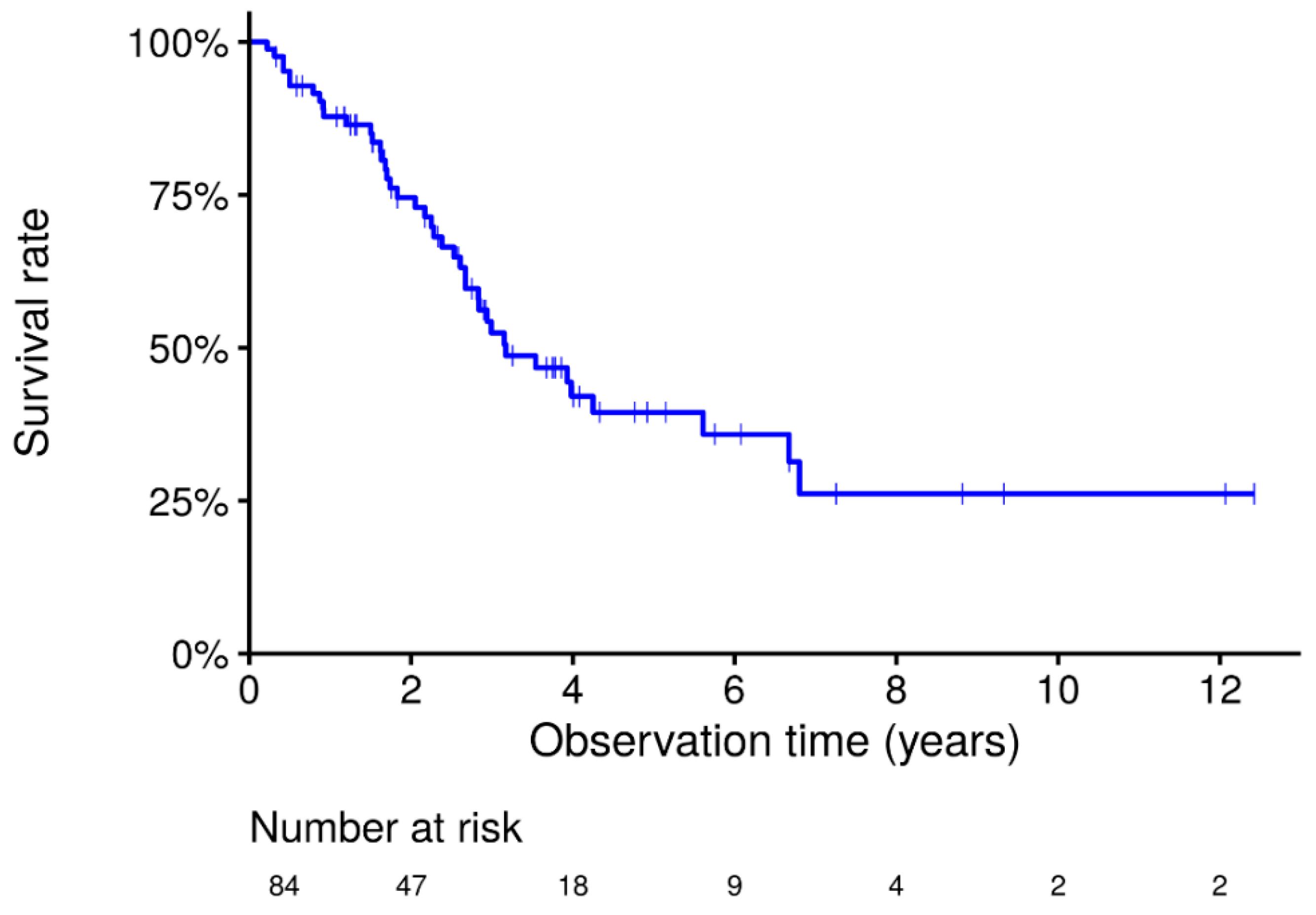
3. Results
3.1. Subject Demographic Characteristics
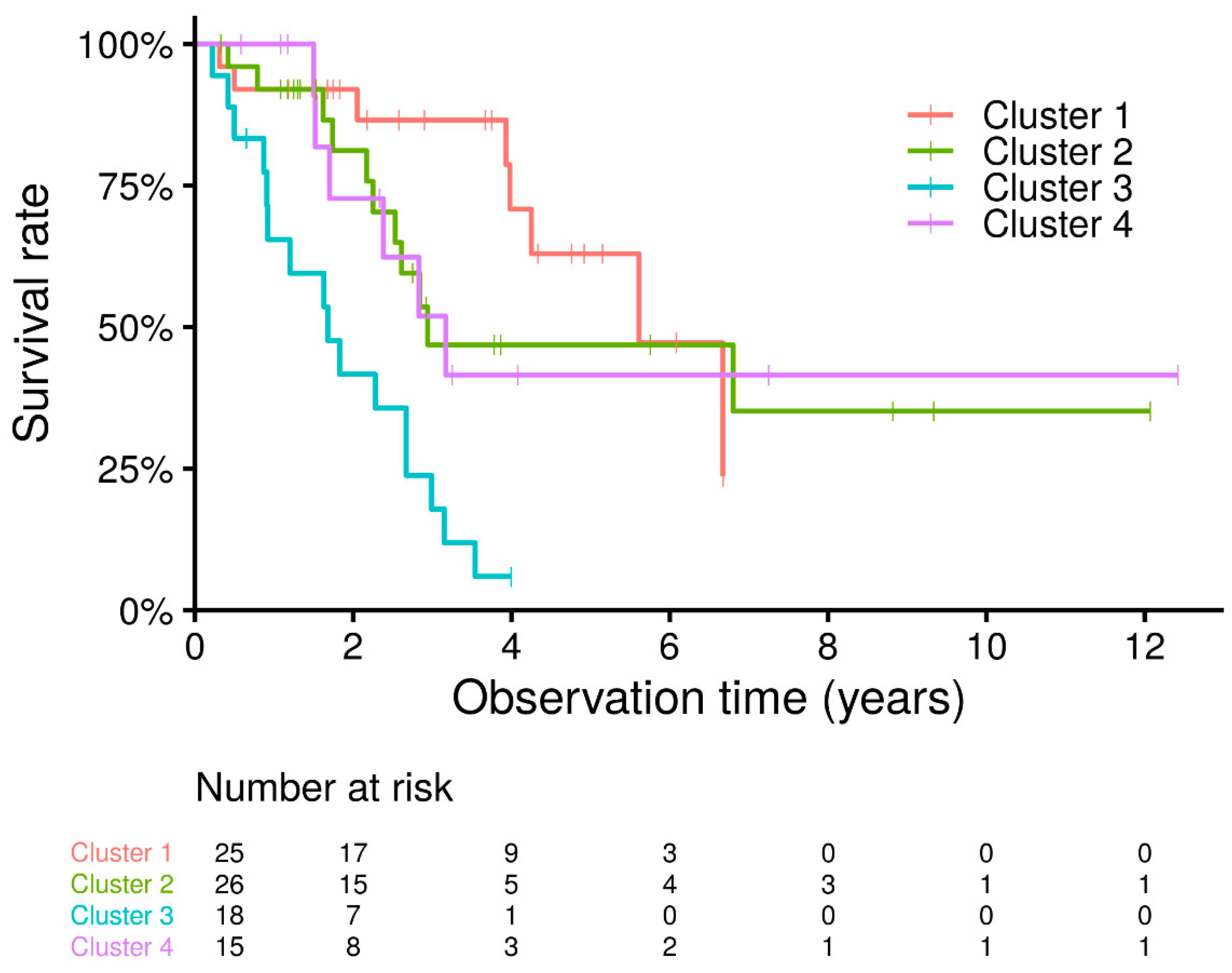
3.2. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travis, W.D.; Costabel, U.; Hansell, D.M.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; Behr, J.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Frankel, S.K.; Cool, C.D.; Lynch, D.A.; Brown, K.K. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004, 126, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nagata, N.; Tsuruta, N.; Kitasato, Y.; Wakamatsu, K.; Yoshimi, M.; Ishii, H.; Hirota, T.; Hamada, N.; Fujita, M.; et al. Heterogeneous clinical features in patients with pulmonary fibrosis showing histology of pleuroparenchymal fibroelastosis. Respir. Investig. 2016, 54, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Adegunsoye, A.; Oldham, J.M.; Chung, J.H.; Montner, S.M.; Lee, C.; Witt, L.J.; Stahlbaum, D.; Bermea, R.S.; Chen, L.W.; Hsu, S.; et al. Phenotypic Clusters Predict Outcomes in a Longitudinal Interstitial Lung Disease Cohort. Chest 2018, 153, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Nakamura, Y.; Satake, Y.; Sumikawa, H.; Johkoh, T.; Colby, T.V.; Yasui, H.; Hozumi, H.; Karayama, M.; Suzuki, Y.; et al. Clinical diagnosis of idiopathic pleuroparenchymal fibroelastosis: A retrospective multicenter study. Respir. Med. 2017, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- La, D.; Livesay, D.R. Predicting functional sites with an automated algorithm suitable for heterogeneous datasets. BMC Bioinform. 2005, 6, 116. [Google Scholar] [CrossRef]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; Du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef]
- Fujisawa, T.; Mori, K.; Mikamo, M.; Ohno, T.; Kataoka, K.; Sugimoto, C.; Kitamura, H.; Enomoto, N.; Egashira, R.; Sumikawa, H.; et al. Nationwide cloud-based integrated database of idiopathic interstitial pneumonias for multidisciplinary discussion. Eur. Respir. J. 2019, 53, 1802243. [Google Scholar] [CrossRef]
- Shioya, M.; Otsuka, M.; Yamada, G.; Umeda, Y.; Ikeda, K.; Nishikiori, H.; Kuronuma, K.; Chiba, H.; Takahashi, H. Poorer prognosis of idiopathic pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis in advanced stage. Can. Respir. J. 2018, 2018, 6043053. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Fujisawa, T.; Sumikawa, H.; Tanaka, T.; Sugimoto, C.; Kono, M.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; et al. Disease course and prognosis of pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis. Respir. Med. 2020, 171, 106078. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yoshimura, K.; Enomoto, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 2018, 8, 14074. [Google Scholar] [CrossRef]
- Khiroya, R.; Macaluso, C.; Montero, M.A.; Wells, A.U.; Chua, F.; Kokosi, M.; Maher, T.M.; Devaraj, A.; Rice, A.; Renzoni, E.A.; et al. Pleuroparenchymal fibroelastosis: A review of histopathologic features and the relationship between histologic parameters and survival. Am. J. Surg. Pathol. 2017, 41, 1683–1689. [Google Scholar] [CrossRef]
- Collard, H.R.; King, T.E., Jr.; Bartelson, B.B.; Vourlekis, J.S.; Schwarz, M.I.; Brown, K.K. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Manali, E.D.; Stathopoulos, G.T.; Kollintza, A.; Kalomenidis, I.; Emili, J.M.; Sotiropoulou, C.; Daniil, Z.; Roussos, C.; Papiris, S.A. The Medical Research Council chronic dyspnea score predicts the survival of patients with idiopathic pulmonary fibrosis. Respir. Med. 2008, 102, 586–592. [Google Scholar] [CrossRef]
- Ishii, H.; Kinoshita, Y.; Kushima, H.; Nagata, N.; Watanabe, K. The similarities and differences between pleuroparenchymal fibroelastosis and idiopathic pulmonary fibrosis. Chronic Respir. Dis. 2019, 16, 1479973119867945. [Google Scholar] [CrossRef]
- Oda, T.; Ogura, T.; Kitamura, H.; Hagiwara, E.; Baba, T.; Enomoto, Y.; Iwasawa, T.; Okudela, K.; Takemura, T.; Sakai, F.; et al. Distinct characteristics of pleuroparenchymal fibroelastosis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Chest 2014, 146, 1248–1255. [Google Scholar] [CrossRef]
- Lee, S.I.; Chae, E.J.; Song, J.S.; Lee, J.H.; Song, J.W. Pleuroparenchymal fibroelastosis in patients with idiopathic pulmonary fibrosis. Respirology 2020, 25, 1046–1052. [Google Scholar] [CrossRef]
- Kono, M.; Fujita, Y.; Takeda, K.; Miyashita, K.; Tsutsumi, A.; Kobayashi, T.; Miki, Y.; Hashimoto, D.; Enomoto, N.; Nakamura, Y.; et al. Clinical significance of lower-lobe interstitial lung disease on high-resolution computed tomography in patients with idiopathic pleuroparenchymal fibroelastosis. Respir. Med. 2019, 154, 122–126. [Google Scholar] [CrossRef]
- Kato, M.; Sasaki, S.; Kurokawa, K.; Nakamura, T.; Yamada, T.; Sasano, H.; Arano, N.; Komura, M.; Ihara, H.; Nagashima, O.; et al. Usual interstitial pneumonia pattern in the lower lung lobes as a prognostic factor in idiopathic pleuroparenchymal fibroelastosis. Respiration 2019, 97, 319–328. [Google Scholar] [CrossRef]
- Chua, F.; Desai, S.R.; Nicholson, A.G.; Devaraj, A.; Renzoni, E.; Rice, A.; Wells, A.U. Pleuroparenchymal fibroelastosis. A review of clinical, radiological, and pathological characteristics. Ann. Am. Thorac. Soc. 2019, 16, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am. J. Respir Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.L.; Tominaga, M.; Hansell, D.M.; Rassl, D.; Parfrey, H.; Guy, S.; Twentyman, O.; Rice, A.; Maher, T.M.; Renzoni, E.A.; et al. Pleuroparenchymal fibroelastosis: A spectrum of histopathological and imaging phenotypes. Eur. Respir. J. 2012, 40, 377–385. [Google Scholar] [CrossRef]
- Watanabe, K.; Ishii, H.; Kiyomi, F.; Terasaki, Y.; Hebisawa, A.; Kawabata, Y.; Johkoh, T.; Sakai, F.; Kondoh, Y.; Inoue, Y.; et al. Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: A proposal. Respir. Investig. 2019, 57, 312–320. [Google Scholar] [CrossRef]
| Number of patients | 84 |
| Year-patients | 245.9 |
| Age | 69.0 [14.8, 18.4] |
| Sex Male/Female, n (%) | 54 (64.3)/30 (35.7) |
| Current or former smoker, n (%) | 31 (36.9) |
| Cough, n (%) | 31 (36.9) |
| Dyspnea, n (%) | 40 (47.6) |
| BMI | 17.3 [14.7, 18.5] |
| Laboratory testing: KL-6, U/mL | 472.0 [361.0, 621.5] |
| SP-D, ng/mL | 180.0 [133.3, 262.5] |
| PaO2, Torr | 80.0 [72.2, 89.0] |
| PaCO2, Torr | 46.6 [41.8, 49.2] |
| Pulmonary function: % FVC, % | 60.5 [47.0, 77.9] |
| FEV1.0/FVC, % | 95.9 [90.1, 100.0] |
| % DLCO, % | 77.7 [68.1, 102.5] |
| RV/TLC, % | 48.2 [42.8, 59.2] |
| Variable | HR | 95% CI lower | 95% CI upper | p-Value |
|---|---|---|---|---|
| Age | 1.035 | 0.991 | 1.079 | 0.117 |
| Sex, Male | 6.594 | 2.484 | 17.505 | <0.001 |
| Dyspnea | 4.480 | 1.616 | 12.421 | 0.004 |
| FVC, % | 0.982 | 0.959 | 1.007 | 0.156 |
| Variable | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | p-Value |
|---|---|---|---|---|---|
| Number of patients | 25 | 26 | 18 | 15 | |
| Year-patients | 83.9 | 84.0 | 32.1 | 45.8 | |
| Age | 69 [61.0, 75.0] | 72.5 [65.0, 79.0] | 71.5 [67.5, 73.8] | 64 [58.0, 69.0] | 0.061 |
| Sex, male | 21 (84.0) | 3 (11.5) | 18 (100) | 12 (80.0) | <0.001 |
| Smoking | 7 (28.0) | 0 (0.0) | 15 (83.3) | 9 (60.0) | <0.001 |
| Cough | 9 (36.0) | 8 (30.8) | 4 (22.2) | 10 (66.7) | 0.06 |
| Dyspnea | 0 (0.0) | 18 (69.2) | 18 (100.0) | 4 (26.7) | <0.001 |
| Fine crackles | 6 (24.0%) | 8 (30.8) | 12 (66.7) | 1 (6.7) | 0.002 |
| BMI | 17.9 [16.1, 19.9] | 15.2 [14.0, 17.3] | 17.6 [14.8, 18.4] | 16.6 [15.1, 18.6] | 0.022 |
| pFVC | 83 [60.5, 90.3] | 52.6 [37.5, 60.2] | 48.7 [40.0, 62.4] | 74.7 [59.1, 78.5] | <0.001 |
| FEV1/FVC | 91.6 [88.9, 97.0] | 96.1 [93.3, 100.0] | 100 [94.6, 100.0] | 97.8 [95.0, 100.0] | 0.02 |
| RV/TLC | 43.8 [37.9, 46.7] | 57.6 [46.9, 62.4] | 54.1 [46.4, 59.5] | 50 [43.5, 53.5] | 0.056 |
| pDLco | 98.1 [92.5, 115.0] | 75.5 [69.2, 86.8] | 68.7 [58.4, 83.2] | 77.7 [68.7, 109.8] | 0.054 |
| Alb | 4 [3.8, 4.1] | 4 [3.8, 4.4] | 3.8 [3.3, 4.1] | 4.2 [4.0, 4.5] | 0.06 |
| LDH | 182.5 [174.8, 209.5] | 214 [188.2, 246.8] | 199 [189.0, 226.8] | 194.5 [162.8, 210.0] | 0.03 |
| KL6 | 392 [331.0, 486.2] | 525 [471.2, 637.8] | 569 [410.0, 929.0] | 389 [358.0, 478.1] | 0.003 |
| SPD | 149.6 [108.5, 209.0] | 173 [133.0, 258.0] | 252 [204.0, 377.0] | 179 [125.0, 223.0] | 0.004 |
| CT: lower lobe | 20 (80.0) | 17 (65.4) | 17 (94.4) | 3 (20.0) | <0.001 |
| CT: UIP like | 12 (48.0) | 12 (46.2) | 13 (72.2) | 3 (20.0) | 0.029 |
| Pneumothorax | 5 (20.0) | 6 (23.1) | 4 (22.2) | 12 (80.0) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, Y.; Mori, K.; Enomoto, Y.; Kono, M.; Sumikawa, H.; Johkoh, T.; Colby, T.V.; Yasui, H.; Hozumi, H.; Karayama, M.; et al. Prognostic and Clinical Value of Cluster Analysis in Idiopathic Pleuroparenchymal Fibroelastosis Phenotypes. J. Clin. Med. 2021, 10, 1498. https://doi.org/10.3390/jcm10071498
Nakamura Y, Mori K, Enomoto Y, Kono M, Sumikawa H, Johkoh T, Colby TV, Yasui H, Hozumi H, Karayama M, et al. Prognostic and Clinical Value of Cluster Analysis in Idiopathic Pleuroparenchymal Fibroelastosis Phenotypes. Journal of Clinical Medicine. 2021; 10(7):1498. https://doi.org/10.3390/jcm10071498
Chicago/Turabian StyleNakamura, Yutaro, Kazutaka Mori, Yasunori Enomoto, Masato Kono, Hiromitsu Sumikawa, Takeshi Johkoh, Thomas V. Colby, Hideki Yasui, Hironao Hozumi, Masato Karayama, and et al. 2021. "Prognostic and Clinical Value of Cluster Analysis in Idiopathic Pleuroparenchymal Fibroelastosis Phenotypes" Journal of Clinical Medicine 10, no. 7: 1498. https://doi.org/10.3390/jcm10071498
APA StyleNakamura, Y., Mori, K., Enomoto, Y., Kono, M., Sumikawa, H., Johkoh, T., Colby, T. V., Yasui, H., Hozumi, H., Karayama, M., Suzuki, Y., Furuhashi, K., Fujisawa, T., Enomoto, N., Inui, N., Kaida, Y., Yokomura, K., Koshimizu, N., Toyoshima, M., ... Suda, T. (2021). Prognostic and Clinical Value of Cluster Analysis in Idiopathic Pleuroparenchymal Fibroelastosis Phenotypes. Journal of Clinical Medicine, 10(7), 1498. https://doi.org/10.3390/jcm10071498






