Increased Circulating Malondialdehyde-Modified Low-Density Lipoprotein Level Is Associated with High-Risk Plaque in Coronary Computed Tomography Angiography in Patients Receiving Statin Therapy
Abstract
1. Introduction
2. Materials and Methods
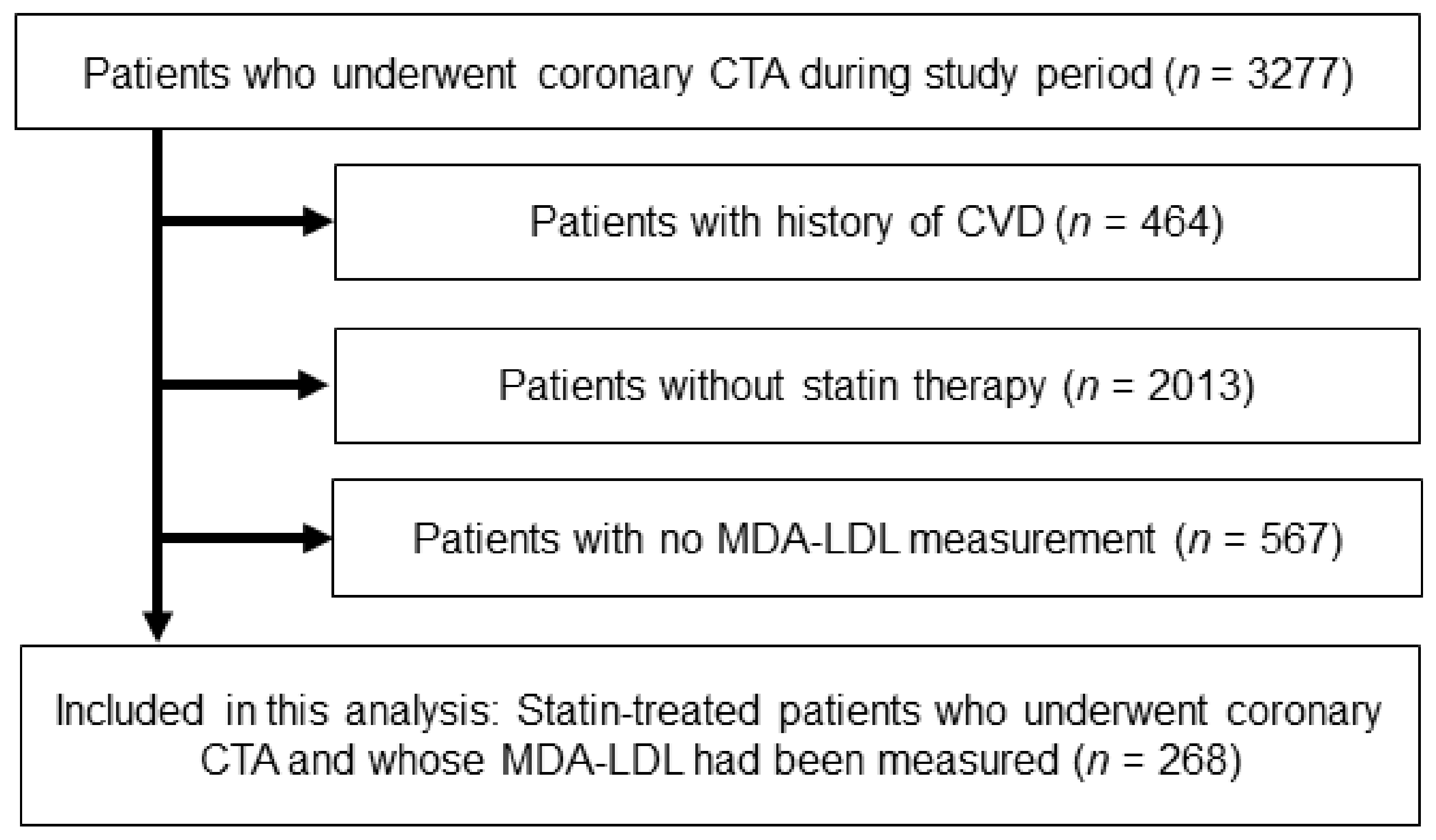
2.1. Study Population and Risk Assessment
2.2. Blood Sampling and the Measurement of MDA-LDL
2.3. Acquisition and Analyses of Coronary CTA Image
2.4. Outcome Data
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
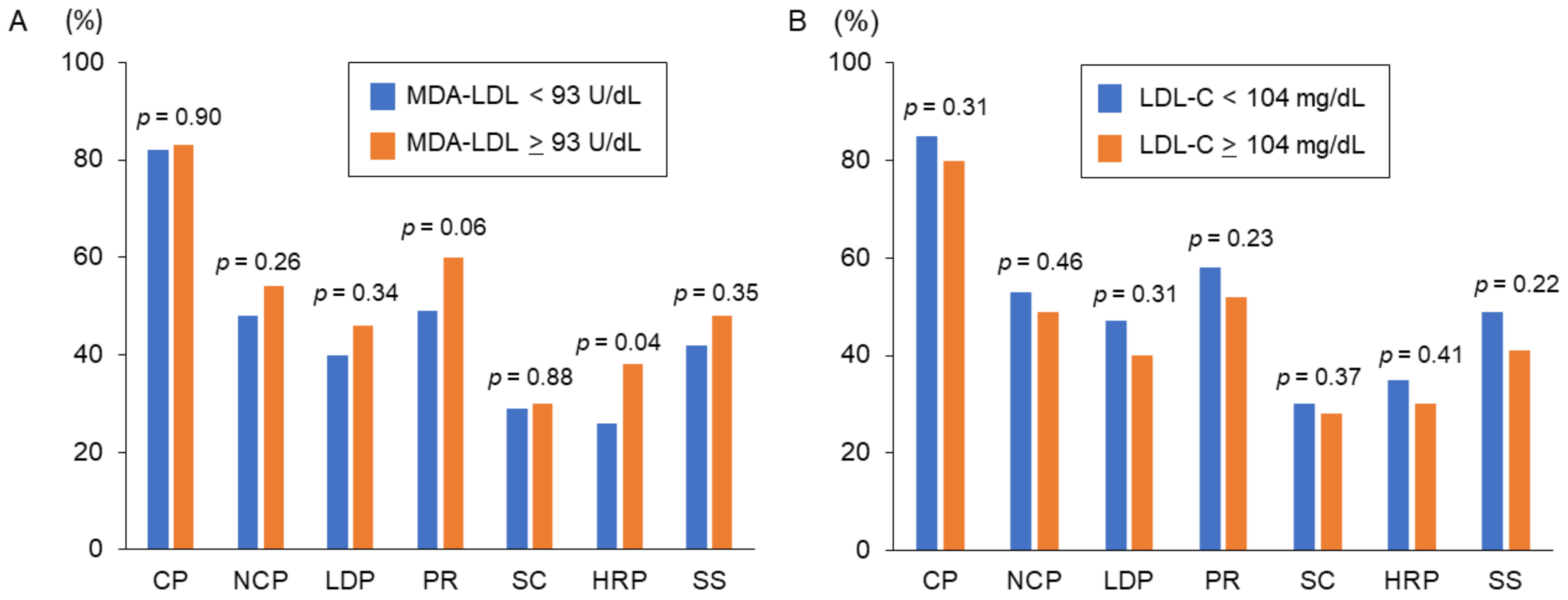
3.2. MDA-LDL and Coronary CTA Findings
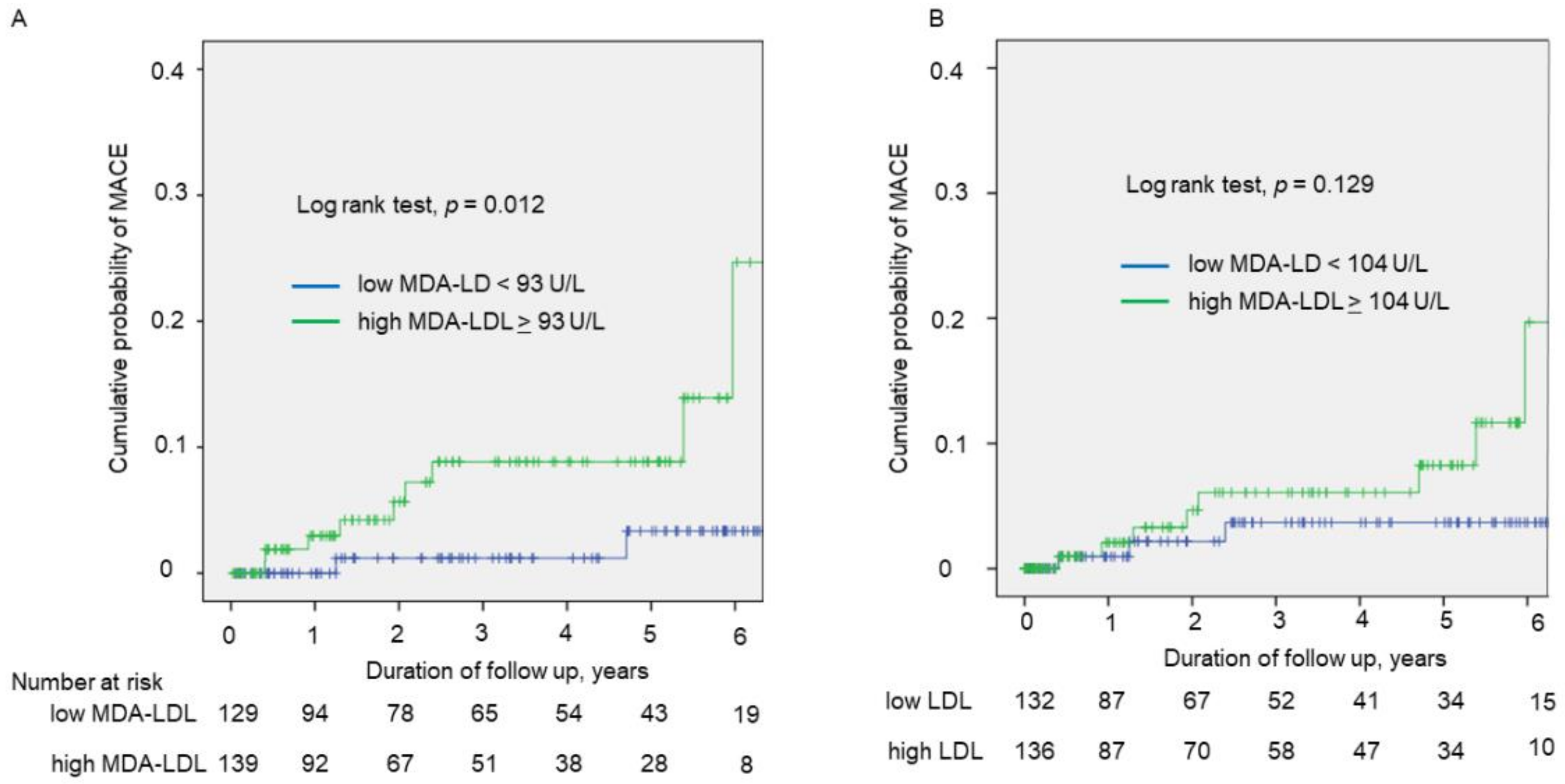
3.3. Prognostic Impact of HRP and MDA-LDL for Cardiovascular Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Mills, E.J.; Rachlis, B.; Wu, P.; Devereaux, P.J.; Arora, P.; Perri, D. Primary prevention of cardiovascular mortality and events with statin treatments: A network meta-analysis involving more than 65,000 patients. J. Am. Coll. Cardiol. 2008, 52, 1769–1781. [Google Scholar] [CrossRef]
- Reith, C.; Armitage, J. Management of residual risk after statin therapy. Atherosclerosis 2016, 245, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Kubo, T.; Okumoto, Y.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; Ino, Y.; Kitabata, H.; Hirata, K.; Imanishi, T.; et al. Circulating malondialdehyde-modified low-density lipoprotein levels are associated with the presence of thin-cap fibroatheromas determined by optical coherence tomography in coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Fujita, H.; Tani, T.; Ohte, N. Malondialdehyde-modified low-density lipoprotein is a predictor of cardiac events in patients with stable angina on lipid-lowering therapy after percutaneous coronary intervention using drug-eluting stent. Atherosclerosis 2015, 239, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Collen, D.; Van de Werf, F. Malondialdehyde-modified LDL as a marker of acute coronary syndromes. JAMA 1999, 281, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Cury, R.C.; Leipsic, J.; Crim, M.T.; Berman, D.S.; Gransar, H.; Budoff, M.J.; Achenbach, S.; Hartaigh, B.O.; Callister, T.Q.; et al. The Coronary Artery Disease-Reporting and Data System (CAD-RADS): Prognostic and Clinical Implications Associated With Standardized Coronary Computed Tomography Angiography Reporting. JACC Cardiovasc. Imaging 2018, 11, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, F.; Sommer, W.H.; Hoffmann, V.; Achenbach, S.; Nikolaou, K.; Conen, D.; Reiser, M.F.; Hoffmann, U.; Becker, C.R. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J. Am. Coll. Cardiol. 2011, 57, 2426–2436. [Google Scholar] [CrossRef]
- Versteylen, M.O.; Kietselaer, B.L.; Dagnelie, P.C.; Joosen, I.A.; Dedic, A.; Raaijmakers, R.H.; Wildberger, J.E.; Nieman, K.; Crijns, H.J.; Niessen, W.J.; et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J. Am. Coll. Cardiol. 2013, 61, 2296–2305. [Google Scholar] [CrossRef]
- Puri, R.; Nicholls, S.J.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Amioka, N.; Miyoshi, T.; Otsuka, H.; Yamada, D.; Takaishi, A.; Ueeda, M.; Hirohata, S.; Ito, H. Serum malondialdehyde-modified low-density lipoprotein levels on admission predict prognosis in patients with acute coronary syndrome undergoing percutaneous coronary intervention. J. Cardiol. 2019, 74, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Miyoshi, T.; Yamauchi, K.; Koyama, Y.; Nakamura, K.; Sato, S.; Kanazawa, S.; Ito, H. Nonalcoholic Hepatic Steatosis Is a Strong Predictor of High-Risk Coronary-Artery Plaques as Determined by Multidetector CT. PLoS ONE 2015, 10, e0131138. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Nakamura, K.; Ito, H. Prognostic Value of Coronary Computed Tomographic Angiography in Patients With Nonalcoholic Fatty Liver Disease. JACC Cardiovasc. Imaging 2020, 13, 1628–1630. [Google Scholar] [CrossRef]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; Bridges, C.R.; Califf, R.M.; Casey, D.E., Jr.; Chavey, W.E., 2nd; Fesmire, F.M.; Hochman, J.S.; Levin, T.N.; et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 123, e426–e579. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Kanter, J.E.; Bornfeldt, K.E. Highlighting Residual Atherosclerotic Cardiovascular Disease Risk. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e1–e9. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Y.; Yin, S.; Chen, N.; Bai, X.; Ke, Q.; Shen, J.; Xia, M. Small dense LDL cholesterol is associated with metabolic syndrome traits independently of obesity and inflammation. Nutr. Metab. 2019, 16, 7. [Google Scholar] [CrossRef]
- Hu, C.; Dandapat, A.; Sun, L.; Chen, J.; Marwali, M.R.; Romeo, F.; Sawamura, T.; Mehta, J.L. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc. Res. 2008, 79, 287–293. [Google Scholar] [CrossRef]
- Kume, N.; Kita, T. Apoptosis of vascular cells by oxidized LDL: Involvement of caspases and LOX-1 and its implication in atherosclerotic plaque rupture. Circ. Res. 2004, 94, 269–270. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Ikenaga, H.; Kurisu, S.; Kono, S.; Sumimoto, Y.; Watanabe, N.; Shimonaga, T.; Higaki, T.; Iwasaki, T.; Mitsuba, N.; Ishibashi, K.; et al. Impact of Malondialdehyde-Modified Low-Density Lipoprotein on Tissue Characteristics in Patients With Stable Coronary Artery Disease- Integrated Backscatter-Intravascular Ultrasound Study. Circ. J. 2016, 80, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Duran, E.K.; Aday, A.W.; Cook, N.R.; Buring, J.E.; Ridker, P.M.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Marwali, M.R.; Hu, C.P.; Mohandas, B.; Dandapat, A.; Deonikar, P.; Chen, J.; Cawich, I.; Sawamura, T.; Kavdia, M.; Mehta, J.L. Modulation of ADP-induced platelet activation by aspirin and pravastatin: Role of lectin-like oxidized low-density lipoprotein receptor-1, nitric oxide, oxidative stress, and inside-out integrin signaling. J. Pharmacol. Exp. Ther. 2007, 322, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Nishikido, T.; Oyama, J.; Keida, T.; Ohira, H.; Node, K. High-dose statin therapy with rosuvastatin reduces small dense LDL and MDA-LDL: The Standard versus high-dose therApy with Rosuvastatin for lipiD lowering (SARD) trial. J. Cardiol. 2016, 67, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, Y.; Zhou, Z.; He, M.; Dai, Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm. Res. 2014, 63, 33–43. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
| All Patients | High MDA-LDL (≥93 U/L) | Low MDA-LDL (<93 U/L) | p-Value | |
|---|---|---|---|---|
| n | 268 | 139 | 129 | |
| Age, year | 67 ± 11 | 67 ± 11 | 68 ± 11 | 0.521 |
| Male sex | 154 (58) | 76 (55) | 78 (61) | 0.338 |
| Body mass index, kg/m2 | 24.6 ± 4.1 | 25.2 ± 3.9 | 24.0 ± 4.2 | 0.016 |
| Hypertension | 203 (76) | 109 (78) | 94 (73) | 0.290 |
| Diabetes mellitus | 122 (46) | 59 (42) | 63 (49) | 0.294 |
| Current Smoker | 70 (26) | 36 (26) | 34 (26) | 0.932 |
| Medications | ||||
| Beta blockers | 71 (27) | 37 (27) | 34 (26) | 0.933 |
| CCBs | 122 (46) | 70 (50) | 52 (40) | 0.099 |
| ACE-Is or ARBs | 135 (50) | 73 (53) | 62 (48) | 0.466 |
| Oral antihyperglycemic drugs | 86 (32) | 45 (32) | 41 (32) | 0.918 |
| Ezetimibe | 15 (6) | 10 (7) | 5 (4) | 0.293 |
| Statin intensity, low/moderate * | 160 (60)/108 (40) | 76 (55)/63 (45) | 84 (65)/45 (35) | 0.082 |
| Statin type, dosage | ||||
| Atorvastatin, 5 mg/10 mg | 18/49 | 5/26 | 13/23 | |
| Fluvastatin, 20 mg/40 mg | 4/1 | 3/1 | 1/0 | |
| Pitavastatin, 1 mg/2 mg/4 mg | 14/28/4 | 6/13/3 | 8/15/1 | |
| Pravastatin, 5 mg/10 mg | 9/36 | 5/21 | 4/15 | |
| Rosuvastatin, 2.5 mg/5 mg/10 mg | 68/24/2 | 30/19/2 | 38/5/0 | |
| Simvastatin, 5 mg/10 mg | 8/3 | 4/1 | 4/2 | |
| Laboratory findings | ||||
| Creatinine, mg/dL | 0.89 ± 0.78 | 0.85 ± 0.64 | 0.93 ± 0.92 | 0.397 |
| eGFR, mL/min/1.73 m2 | 68 ± 18 | 68 ± 17 | 69 ± 19 | 0.665 |
| Total cholesterol, mg/dL | 186 ± 40 | 203 ± 40 | 168 ± 31 | <0.001 |
| LDL cholesterol, mg/dL | 107 ± 32 | 122 ± 33 | 90 ± 22 | <0.001 |
| HDL cholesterol, mg/dL | 58 ± 17 | 57 ± 17 | 60 ± 17 | 0.080 |
| Triglyceride, mg/dL | 121 (88, 171) | 150 (104, 198) | 101 (76, 130) | <0.001 |
| MDA-LDL, U/L | 96 ± 35 | 122 ± 28 | 69 ± 15 | <0.001 |
| HbA1c, % | 6.6 ± 1.3 | 6.6 ± 1.4 | 6.6 ± 1.3 | 0.968 |
| hsCRP, mg/dL | 0.08 (0.05, 0.18) | 0.08 (0.04, 0.16) | 0.08 (0.05, 0.20) | 0.608 |
| Patients achieving LDLcholesterol <70 mg/dL | 24 (9) | 1 (1) | 23 (18) | <0.001 |
| Variables | High-Risk Plaque | Significant Stenosis | ||||
|---|---|---|---|---|---|---|
| Present | Absent | p Value | Present | Absent | p Value | |
| n | 87 | 181 | 119 | 148 | ||
| Age, year | 68 ± 10 | 67 ± 12 | 0.747 | 68 ± 11 | 67 ± 12 | 0.489 |
| Male sex | 64 (74) | 90 (50) | <0.001 | 84 (70) | 70 (47) | <0.001 |
| Body mass index, kg/m2 | 24.7 ± 3.4 | 24.5 ± 4.4 | 0.718 | 24.4 ± 4.1 | 24.7 ± 4.1 | 0.450 |
| Hypertension | 73 (84) | 130 (76) | 0.031 | 93 (78) | 110 (74) | 0.546 |
| Diabetes mellitus | 48 (55) | 74 (41) | 0.028 | 63 (53) | 59 (40) | 0.039 |
| Current smoker | 29 (33) | 41 (23) | 0.062 | 32 (27) | 38 (25) | 0.854 |
| Oral antihyperglycemic drugs | 32 (37) | 54 (30) | 0.254 | 42 (35) | 44 (30) | 0.333 |
| Creatinine, mg/dL | 1.00 ± 0.99 | 0.83 ± 0.65 | 0.087 | 0.91 ± 0.71 | 0.88 ± 0.83 | 0.066 |
| Total cholesterol, mg/dL | 186 ± 45 | 187 ± 38 | 0.834 | 183 ± 41 | 188 ± 38 | 0.300 |
| LDL cholesterol, mg/dL | 107 ± 37 | 107 ± 30 | 0.867 | 106 ± 34 | 107 ± 31 | 0.728 |
| HDL cholesterol, mg/dL | 55 ± 16 | 60 ± 17 | 0.014 | 58 ± 18 | 58 ± 16 | 0.905 |
| Triglyceride, mg/dL | 130 (90, 182) | 117 (85, 170) | 0.206 | 118 (86, 158) | 122 (89, 183) | 0.155 |
| MDA-LDL, U/L | 105 ± 40 | 92 ± 32 | 0.011 | 96 ± 33 | 96 ± 36 | 0.979 |
| HbA1c, % | 6.7 ± 1.3 | 6.5 ± 1.4 | 0.347 | 6.6 ± 1.3 | 6.5 ± 1.4 | 0.122 |
| hsCRP, mg/dL | 0.08 (0.05, 0.16) | 0.08 (0.04, 0.19) | 0.907 | 0.09 (0.05, 0.200) | 0.07 (0.040, 0.160) | 0.132 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds Ratio (95%CI) | p Value | Odds Ratio (95%CI) | p Value | |
| Age, per 1 year | 1.004 (0.981–1.028) | 0.746 | ||
| Male | 2.814 (1.609–4.918) | <0.001 | 2.749 (1.502–1.502) | 0.001 |
| Hypertension | 2.046 (1.060–3.947) | 0.033 | 2.027 (1.022–4.019) | 0.049 |
| Diabetes Mellitus | 1.780 (1.062–2.982) | 0.029 | 1.630 (0.941–2.824) | 0.081 |
| Current smoker | 1.707 (0.970–3.006) | 0.064 | ||
| HDL cholesterol, per 1 mg/dL | 0.980 (0.964–0.996) | 0.015 | 0.994 (0.977–1.012) | 0.531 |
| LDL cholesterol, >104 mg/dL | 0.807 (0.483–1.347) | 0.412 | ||
| Triglyceride *, per 1 index | 1.454 (0.854–2.475) | 0.168 | ||
| MDA-LDL, >93 U/L | 1.722 (1.024–2.897) | 0.041 | 1.883 (1.082–3.279) | 0.025 |
| hsCRP *, per 1 index | 0.983 (0.793–1.219) | 0.878 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Ito, H. Increased Circulating Malondialdehyde-Modified Low-Density Lipoprotein Level Is Associated with High-Risk Plaque in Coronary Computed Tomography Angiography in Patients Receiving Statin Therapy. J. Clin. Med. 2021, 10, 1480. https://doi.org/10.3390/jcm10071480
Ichikawa K, Miyoshi T, Osawa K, Miki T, Ito H. Increased Circulating Malondialdehyde-Modified Low-Density Lipoprotein Level Is Associated with High-Risk Plaque in Coronary Computed Tomography Angiography in Patients Receiving Statin Therapy. Journal of Clinical Medicine. 2021; 10(7):1480. https://doi.org/10.3390/jcm10071480
Chicago/Turabian StyleIchikawa, Keishi, Toru Miyoshi, Kazuhiro Osawa, Takashi Miki, and Hiroshi Ito. 2021. "Increased Circulating Malondialdehyde-Modified Low-Density Lipoprotein Level Is Associated with High-Risk Plaque in Coronary Computed Tomography Angiography in Patients Receiving Statin Therapy" Journal of Clinical Medicine 10, no. 7: 1480. https://doi.org/10.3390/jcm10071480
APA StyleIchikawa, K., Miyoshi, T., Osawa, K., Miki, T., & Ito, H. (2021). Increased Circulating Malondialdehyde-Modified Low-Density Lipoprotein Level Is Associated with High-Risk Plaque in Coronary Computed Tomography Angiography in Patients Receiving Statin Therapy. Journal of Clinical Medicine, 10(7), 1480. https://doi.org/10.3390/jcm10071480






