Derivation and Validation of Essential Predictors and Risk Index for Early Detection of Diabetic Retinopathy Using Electronic Health Records
Abstract
1. Introduction
2. Materials and Methods
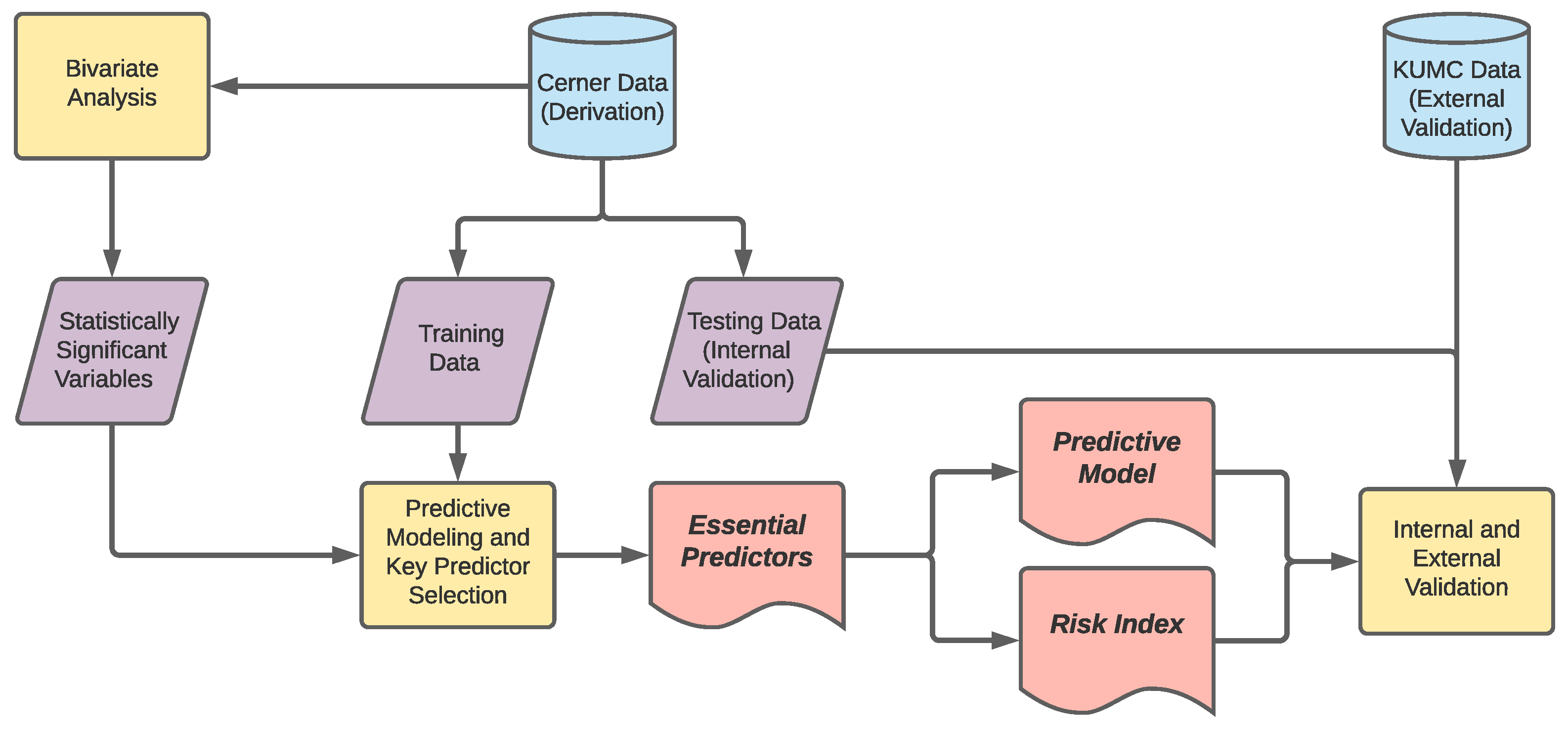
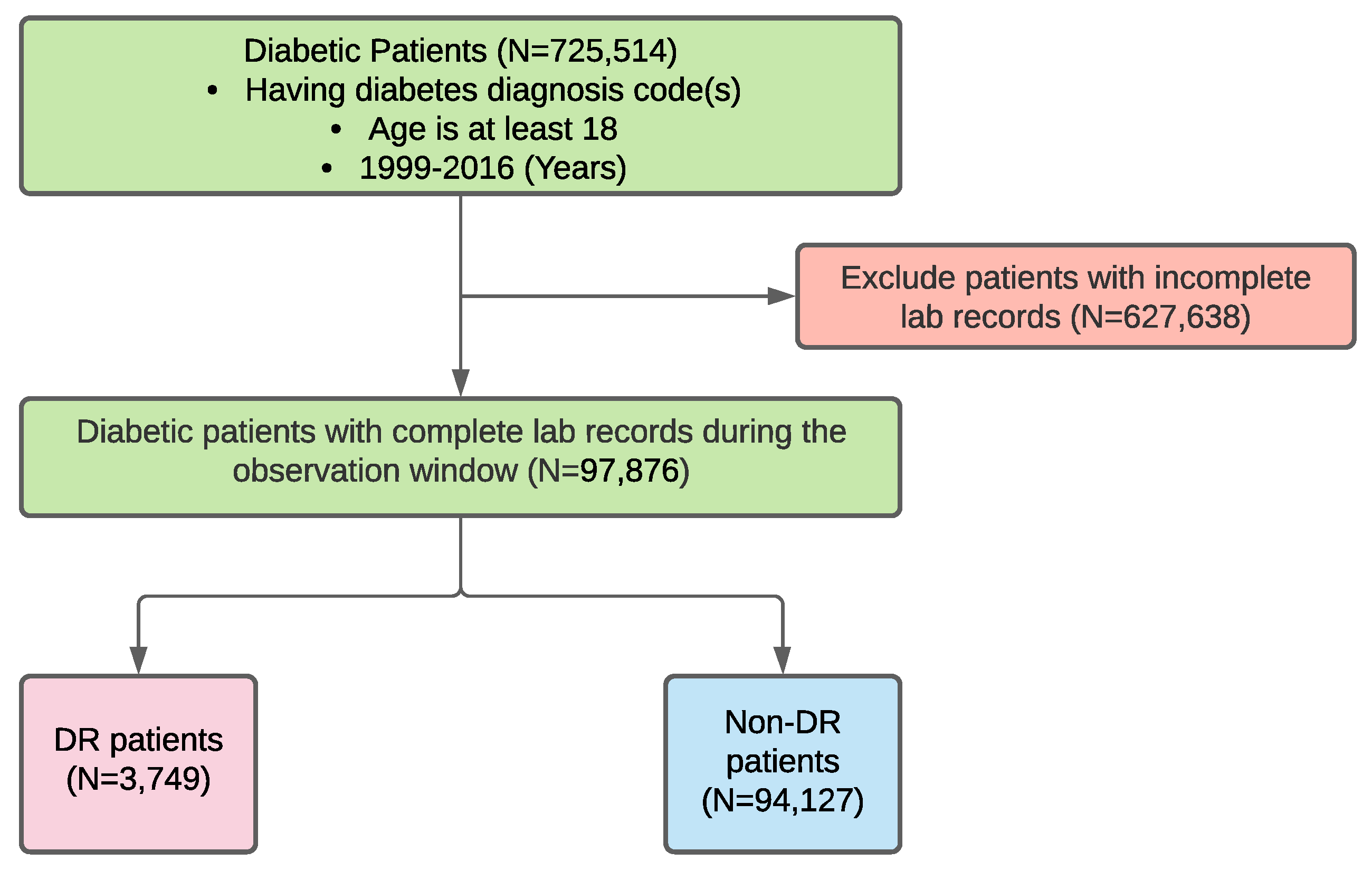
2.1. Data Sources and Data Extraction
2.2. Data Preprocessing
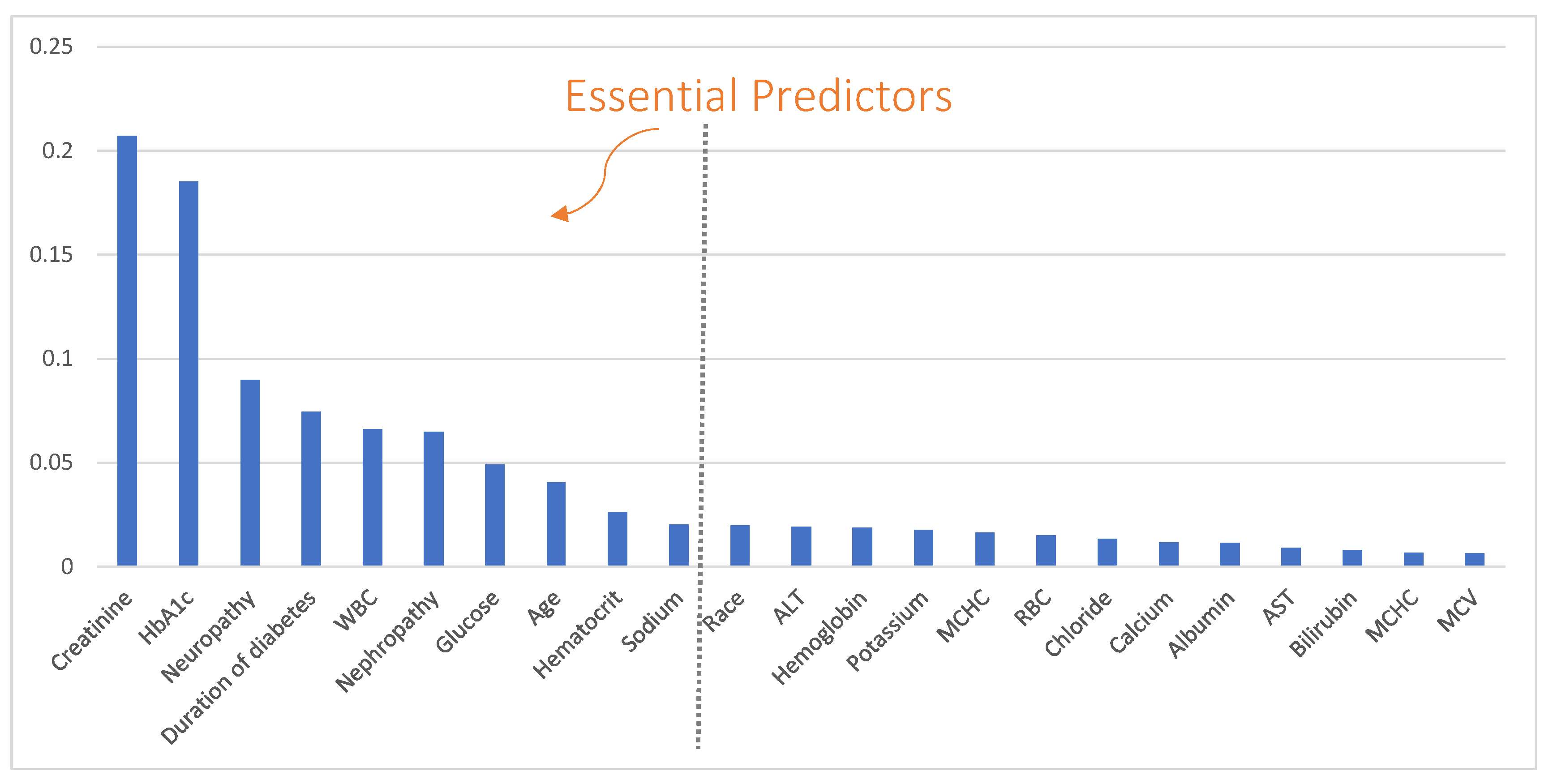
2.3. Essential Predictor Identification and Predictive Modeling
2.4. Risk Index Development
- (i)
- Create a logistic regression based on the specified n predictors, and obtain the predictors’ coefficients , where is the intercept and is the coefficient of ith predictor.
- (ii)
- Break down each numerical predictor into intervals (i.e., levels) and determine the reference level for each predictor based on clinical expertise.
- (iii)
- Calculate the distance from each level to the reference level in terms of regression risk units for each predictor. The distance is defined as , where and are the level values of level j and the reference level of predictor i, respectively. The level value is defined as the middle point for numerical, interval levels and non-negative integers for other types of levels.
- (iv)
- Define a constant B regarding how many regression risk units can be mapped to one point in the scoring system. In this study, we let . In other words, one point in the risk scoring system corresponds to the increased regression risk units associated with a 5-year change in age.
- (v)
- Compute the score for each level of a predictor by rounding to the nearest integer. The risk index is the summation of all predictors’ scores.
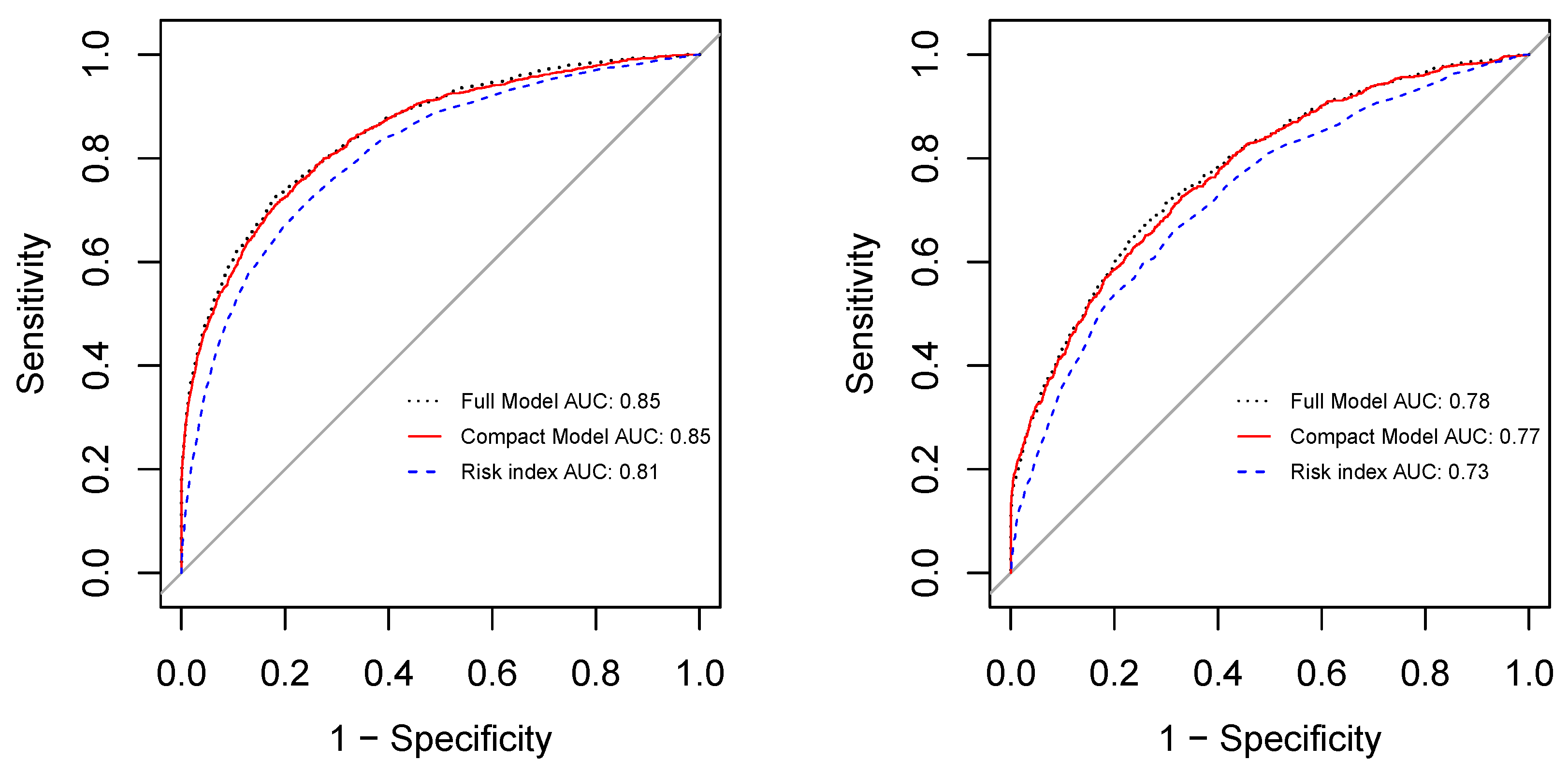
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobrin Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Brown, J.B.; Pedula, K.L.; Summers, K.H. Diabetic retinopathy: Contemporary prevalence in a well-controlled population. Diabetes Care 2003, 26, 2637–2642. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in diabetes. Diabetes Care 2004, 27, s84–s87. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L. American diabetes association. Diabetic retinopathy. Diabetes Care 2002, 25, S90–S93. [Google Scholar]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020; pp. 12–15. [Google Scholar]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef]
- Bellazzi, R.; Zupan, B. Predictive data mining in clinical medicine: Current issues and guidelines. Int. J. Med. Inform. 2008, 77, 81–97. [Google Scholar] [CrossRef]
- Steyerberg, E. A practical Approach to Development, Validation, and Updating; Springer: New York, NY, USA, 2009. [Google Scholar]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for some traditional and novel measures. Epidemiology 2010, 21, 128. [Google Scholar] [CrossRef]
- Moons, K.G.; Kengne, A.P.; Woodward, M.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Grobbee, D.E. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio) marker. Heart 2012, 98, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef]
- Collins, G.S.; Mallett, S.; Omar, O.; Yu, L.M. Developing risk prediction models for type 2 diabetes: A systematic review of methodology and reporting. BMC Med. 2011, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.; Mathur, R.; Dent, T.; Meads, C.; Greenhalgh, T. Risk models and scores for type 2 diabetes: Systematic review. BMJ 2011, 343. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Ehehalt, S.; Dietz, K.; Willasch, A.M.; Neu, A.; Baden-Wuerttemberg, D.G. Prediction model for the incidence and prevalence of type 1 diabetes in childhood and adolescence: Evidence for a cohort-dependent increase within the next two decades in Germany. Pediatr. Diabetes 2012, 13, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, V.; Laakso, M. Genetic screening for the risk of type 2 diabetes: Worthless or valuable? Diabetes Care 2013, 36, S120–S126. [Google Scholar] [CrossRef]
- Walford, G.A.; Porneala, B.C.; Dauriz, M.; Vassy, J.L.; Cheng, S.; Rhee, E.P.; Wang, T.J.; Meigs, J.B.; Gerszten, R.E.; Florez, J.C. Metabolite traits and genetic risk provide complementary information for the prediction of future type 2 diabetes. Diabetes Care 2014, 37, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.E.; Kerr, W.T.; Thames, A.; Li, T.; Xiao, J.; Cohen, M.S. Electronic health record phenotyping improves detection and screening of type 2 diabetes in the general United States population: A cross-sectional, unselected, retrospective study. J. Biomed. Inf. 2016, 60, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Engerman, R.L.; Kern, T.S. Hyperglycemia as a cause of diabetic retinopathy. Metabolism 1986, 35, 20–23. [Google Scholar] [CrossRef]
- Matthews, D.R.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Kohner, E.M. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch. Ophthalmol. 2004, 122, 1631. [Google Scholar] [PubMed]
- Klein, R.; Klein, B. Blood pressure control and diabetic retinopathy. Br. J. Ophthalmol. 2002. [CrossRef]
- Chang, Y.C.; Wu, W.C. Dyslipidemia and diabetic retinopathy. Rev. Diabet. Stud. RDS 2013, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.S.; Busik, J.V. The role of dyslipidemia in diabetic retinopathy. Vis. Res. 2017, 139, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wong, T.Y. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diabetes Rep. 2012, 12, 346–354. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Gregg, E.W.; Geiss, L.S.; Imperatore, G.; Williams, D.E.; Zhang, X.; Albright, A.L.; Cowie, C.C.; Klein, R.; Saaddine, J.B. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the US population: Implications for diabetes diagnostic thresholds. Diabetes Care 2009, 32, 2027–2032. [Google Scholar] [CrossRef]
- Irace, C.; Scarinci, F.; Scorcia, V.; Bruzzichessi, D.; Fiorentino, R.; Randazzo, G.; Scorcia, G.; Gnasso, A. Association among low whole blood viscosity, haematocrit, haemoglobin and diabetic retinopathy in subjects with type 2 diabetes. Br. J. Ophthalmol. 2011, 95, 94–98. [Google Scholar] [CrossRef]
- Davis, M.D.; Fisher, M.R.; Gangnon, R.E.; Barton, F.; Aiello, L.M.; Chew, E.Y.; Ferris, F.; Knatterud, G.L. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report# 18. Investig. Ophthalmol. Vis. Sci. 1998, 39, 233–252. [Google Scholar]
- Conway, B.N.; Miller, R.G.; Klein, R.; Orchard, T.J. Prediction of proliferative diabetic retinopathy with hemoglobin level. Arch. Ophthalmol. 2009, 127, 1494–1499. [Google Scholar] [CrossRef][Green Version]
- Qiao, Q.; Keinänen-Kiukaanniemi, S.; Läärä, E. The relationship between hemoglobin levels and diabetic retinopathy. J. Clin. Epidemiol. 1997, 50, 153–158. [Google Scholar] [CrossRef]
- Yoo, T.K.; Park, E.C. Diabetic retinopathy risk prediction for fundus examination using sparse learning: A cross-sectional study. BMC Med. Inf. Decis. Mak. 2013, 13, 106. [Google Scholar]
- Ogunyemi, O.; Kermah, D. Machine learning approaches for detecting diabetic retinopathy from clinical and public health records. In AMIA Annual Symposium Proceedings; American Medical Informatics Association: Bethesda, MD, USA, 2015; Volume 2015, p. 983. [Google Scholar]
- Ogunyemi, O.; Teklehaimanot, S.; Patty, L.; Moran, E.; George, S. Evaluating predictive modeling’s potential to improve teleretinal screening participation in urban safety net clinics. Stud. Health Technol. Inf. 2013, 192, 162. [Google Scholar]
- Yue, S.; Zhang, J.; Wu, J.; Teng, W.; Liu, L.; Chen, L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int. J. Environ. Res. Public Healthh 2015, 12, 10009–10019. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Ahn, S.J.; Ahn, J.; Park, K.H.; Lee, K. Elevated systemic neutrophil count in diabetic retinopathy and diabetes: A hospital-based cross-sectional study of 30,793 Korean subjects. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7697–7703. [Google Scholar] [CrossRef]
- Piri, S.; Delen, D.; Liu, T.; Zolbanin, H.M. A data analytics approach to building a clinical decision support system for diabetic retinopathy: Developing and deploying a model ensemble. Decis. Support Syst. 2017, 101, 12–27. [Google Scholar] [CrossRef]
- Waitman, L.R.; Warren, J.J.; Manos, E.L.; Connolly, D.W. Expressing Observations from Electronic Medical Record Flowsheets in an i2b2 based Clinical Data Repository to Support Research and Quality Improvement. AMIA Annu. Symp. Proc. AMIA Symp. 2011, 2011, 1454–1463. [Google Scholar]
- Ng, K.; Steinhubl, S.R.; de Filippi, C.; Dey, S.; Stewart, W.F. Early detection of heart failure using electronic health records: Practical implications for time before diagnosis, data diversity, data quantity, and data density. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 649–658. [Google Scholar] [CrossRef]
- Critical Data, M. Secondary Analysis of Electronic Health Records; Springer Nature: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Song, X.; Waitman, L.R.; Hu, Y.; Yu, A.S.; Robins, D.; Liu, M. Robust clinical marker identification for diabetic kidney disease with ensemble feature selection. J. Am. Med. Inf. Assoc. 2019, 26, 242–253. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y. Xgboost: Extreme Gradient Boosting. R Package Version 0.4-2. 2015. Available online: https://cran.r-project.org/web/packages/xgboost/vignettes/xgboost.pdf (accessed on 1 April 2021).
- Siau, K.; Wang, W. Building trust in artificial intelligence, machine learning, and robotics. Cut. Bus. Technol. J. 2018, 31, 47–53. [Google Scholar]
- Krause, J.; Perer, A.; Ng, K. Interacting with predictions: Visual inspection of black-box machine learning models. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems, Jose, CA, USA, 7–12 May 2016; pp. 5686–5697. [Google Scholar]
- Chen, T.; Li, X.; Li, Y.; Xia, E.; Qin, Y.; Liang, S.; Xu, F.; Liang, D.; Zeng, C.; Liu, Z. Prediction and risk stratification of kidney outcomes in IgA nephropathy. Am. J. Kidney Dis. 2019, 74, 300–309. [Google Scholar] [CrossRef]
- van Walraven, C.; Dhalla, I.A.; Bell, C.; Etchells, E.; Stiell, I.G.; Zarnke, K.; Austin, P.C.; Forster, A.J. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ 2010, 182, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino Sr, R.B. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wong, J.; Molyneaux, L.; Constantino, M.; Twigg, S.M.; Yue, D.K. Timing is everything: Age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 2008, 31, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Improving patient compliance with diabetic retinopathy screening and treatment. Community Eye Health 2015, 28, 68. [Google Scholar]

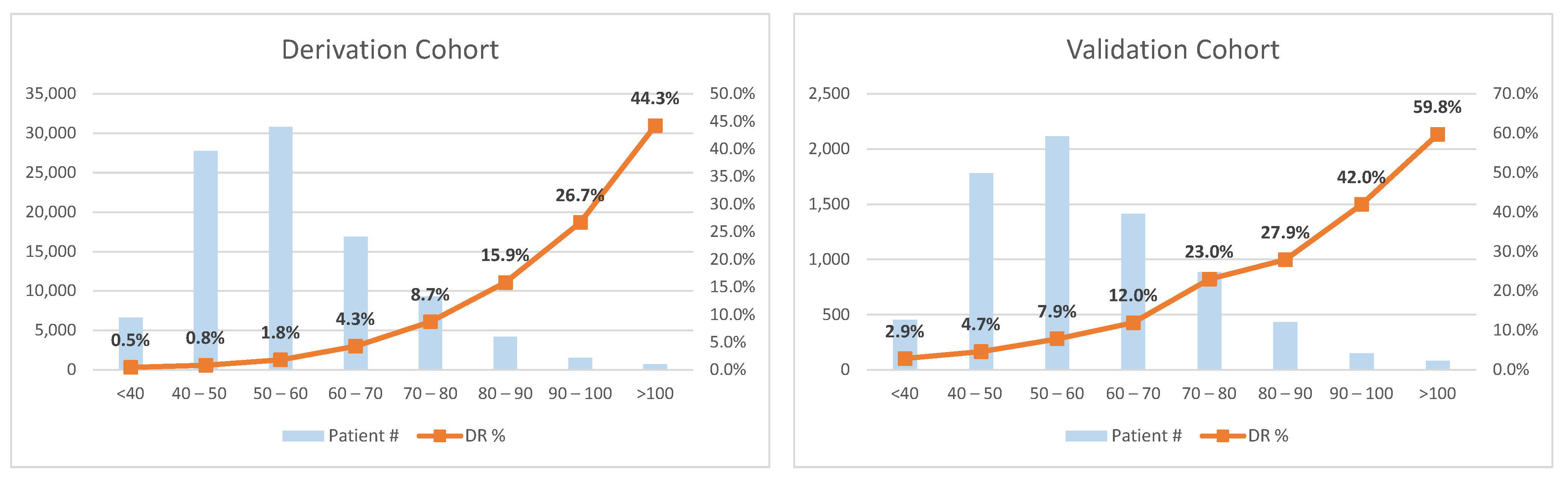
| ICD Version | ICD Code | Code Description |
|---|---|---|
| ICD-9-CM | 250.x | Diabetes mellitus |
| 362.0x | Diabetic retinopathy | |
| 250.4x | nephropathy | |
| 250.6x | neuropathy | |
| ICD-10-CM | E10.x | Type 1 diabetes mellitus |
| E11.x | Type 2 diabetes mellitus | |
| E10.31x–E10.35x | Type 1 diabetes mellitus with diabetic retinopathy | |
| E11.31x–E11.35x | Type 2 diabetes mellitus with diabetic retinopathy | |
| E10.21 | Type 1 diabetes mellitus with diabetic nephropathy | |
| E11.21 | Type 2 diabetes mellitus with diabetic nephropathy | |
| E10.40 | Type 1 diabetes mellitus with diabetic neuropathy | |
| E11.40 | Type 2 diabetes mellitus with diabetic neuropathy |
| Demog. & DM | Cerner Derivation Data | KUMC Validation Data | ||||
|---|---|---|---|---|---|---|
| # (DR#) | OR (95% CI) | p-Value | # (DR#) | OR (95% CI) | p-Value | |
| Age | <0.001 | 0.011 | ||||
| 6036 (75) | Reference | – | 179 (7) | Reference | – | |
| 18–34 | 2723 (135) | 4.15 (3.12–5.54) | <0.001 | 261 (37) | 4.06 (1.87–10.14) | 0.001 |
| 35–49 | 13,247 (620) | 3.90 (3.09–5.01) | <0.001 | 908 (102) | 3.11 (1.52–7.48) | 0.005 |
| 50–64 | 33,554 (1585) | 3.94 (3.14–5.02) | <0.001 | 2851 (371) | 3.68 (1.85–8.71) | 0.001 |
| 65–74 | 25,118 (854) | 2.80 (2.22–3.58) | <0.001 | 2024 (238) | 3.27 (1.64–7.78) | 0.002 |
| 75–84 | 17,198 (480) | 2.28 (1.80–2.94) | <0.001 | 1094 (114) | 2.85 (1.41–6.86) | 0.008 |
| Gender | ||||||
| Female | 53,396 (2034) | Reference | – | 3703 (439) | Reference | – |
| Male | 44,480 (1715) | 1.01 (0.95–1.08) | 0.707 | 3614 (430) | 1.00 (0.87–1.16) | 0.955 |
| Race | <0.001 | <0.001 | ||||
| Black | 17,993 (1363) | Reference | – | 1572 (254) | Reference | – |
| White | 72,488 (2126) | 0.37 (0.34–0.40) | <0.001 | 5005 (499) | 0.57 (0.48–0.68) | <0.001 |
| Other | 7395 (260) | 0.44 (0.39–0.51) | <0.001 | 1360 (116) | 740 (0.76–1.22) | 0.768 |
| Duration (years) | <0.001 | <0.001 | ||||
| 0–1 | 36,536 (775) | Reference | – | 2285 (179) | Reference | – |
| 1–2 | 26,359 (843) | 1.52 (1.38–1.68) | <0.001 | 1729 (196) | 1.50 (1.22–1.86) | <0.001 |
| 2–3 | 13,560 (723) | 2.60 (2.34–2.88) | <0.001 | 1054 (135) | 1.73 (1.36–2.19) | <0.001 |
| 3–4 | 8696 (518) | 2.92 (2.61–3.27) | <0.001 | 719 (79) | 1.45 (1.09–1.91) | <0.001 |
| >4 | 12,725 (890) | 3.47 (3.14–3.83) | <0.001 | 1530 (280) | 2.64 (2.16–3.22) | <0.001 |
| Nephropathy | ||||||
| No | 92,153 (2711) | Reference | – | 6239 (617) | Reference | – |
| Yes | 5723, (1038) | 7.31 (6.76–7.90) | <0.001 | 1078 (252) | 2.78 (2.36–3.27) | <0.001 |
| Neuropathy | ||||||
| No | 88,657 (2507) | Reference | – | 5986 (559) | Reference | – |
| Yes | 9219 (1242) | 5.35 (4.98–5.75) | <0.001 | 1331 (310) | 2.95 (2.53–3.44) | <0.001 |
| Lab results | Case avg (SD) | Control avg (SD) | p-value | Case avg (SD) | Control avg (SD) | p-value |
| HbA1c | 8.36 (2.03) | 7.14 (1.51) | <0.001 | 8.20 (2.07) | 6.95 (1.45) | <0.001 |
| Creatinine | 1.94 (1.81) | 1.07 (0.46) | <0.001 | 1.56 (1.45) | 1.10 (0.47) | <0.001 |
| Glucose | 174.83 (61.95) | 142.89 (46.24) | <0.001 | 170.74 (53.15) | 143.78 (40.21) | <0.001 |
| Hemoglobin | 12.08 (1.65) | 13.03 (1.70) | <0.001 | 12.40 (1.91) | 12.86 (1.90) | <0.001 |
| Hematocrit | 36.24 (4.72) | 38.97 (4.75) | <0.001 | 37.36 (5.60) | 38.69 (5.58) | <0.001 |
| Calcium | 9.12 (0.49) | 9.26 (0.44) | <0.001 | 9.27 (0.49) | 9.37 (0.46) | <0.001 |
| Triglycerides | 155.56 (89.85) | 154.40 (84.71) | 0.436 | 165.96 (94.59) | 155.80 (86.11) | 0.003 |
| Potassium | 4.33 (0.38) | 4.24 (0.35) | <0.001 | 4.20 (0.37) | 4.14 (0.32) | <0.001 |
| Chloride | 103.00 (3.45) | 103.19 (2.85) | 0.001 | 103.11 (3.07) | 103.30 (2.69) | 0.082 |
| MCH | 29.57 (2.00) | 29.95 (1.94) | <0.001 | 29.50 (2.13) | 29.83 (2.07) | 0.001 |
| Sodium | 138.49 (2.39) | 138.83 (2.47) | <0.001 | 137.03 (2.10) | 137.31 (2.19) | <0.001 |
| MCHC | 33.32 (0.92) | 33.43 (0.96) | <0.001 | 33.19 (0.80) | 33.24 (0.78) | 0.084 |
| MCV | 88.74 (5.31) | 89.53 (5.00) | <0.001 | 88.84 (5.56) | 89.67 (5.34) | <0.001 |
| Albumin | 3.64 (0.55) | 3.86 (0.46) | <0.001 | 3.80 (0.48) | 3.95 (0.46) | <0.001 |
| Bilirubin | 0.58 (0.27) | 0.61 (0.28) | <0.001 | 0.60 (0.28) | 0.64 (0.30) | 0.651 |
| Anion Gap | 9.55 (2.69) | 9.47 (2.56) | 0.058 | 8.05 (1.83) | 7.88 (1.73) | 0.011 |
| AST | 24.45 (9.79) | 24.88 (10.08) | 0.009 | 22.80 (10.28) | 24.11 (10.30) | <0.001 |
| ALT | 25.18 (12.24) | 27.86 (14.47) | <0.001 | 22.34 (12.23) | 25.23 (14.00) | <0.001 |
| RBC | 4.10 (0.58) | 4.37 (0.56) | <0.001 | 4.23 (0.66) | 4.34 (0.65) | <0.001 |
| WBC | 7.97 (2.24) | 8.13 (2.21) | <0.001 | 8.02 (2.35) | 8.17 (2.27) | 0.074 |
| Variable | Coefficients () | OR (95% CI) | p-Value | Levels | Level Values () | Points |
|---|---|---|---|---|---|---|
| Age | −0.0187 | 0.98 (0.97–0.99) | <0.001 | 18–34 | 26 | 12 |
| 35–49 | 42 | 9 | ||||
| 50–64 | 57 | 6 | ||||
| 65–74 | 69.5 | 4 | ||||
| 75–84 | 79.5 | 2 | ||||
| ≥85 | 87.5 | 0 | ||||
| Creatinine | 0.8601 | 2.36 (2.24–2.50) | <0.001 | <0.5 | 0.41 | 0 |
| 0.5–1 | 0.75 | 3 | ||||
| 1–1.5 | 1.25 | 8 | ||||
| 1.5–2 | 1.75 | 12 | ||||
| >2 | 2.68 | 21 | ||||
| HbA1c | 0.2877 | 1.33 (1.29–1.37) | <0.001 | <6 | 5 | 0 |
| 6–8 | 7 | 6 | ||||
| 8–10 | 9 | 12 | ||||
| 10–12 | 11 | 18 | ||||
| >12 | 14 | 28 | ||||
| Neuropathy | 0.9229 | 2.52 (2.27–2.78) | <0.001 | No | 0 | 0 |
| Yes | 1 | 10 | ||||
| Duration of diabetes | 0.1455 | 1.16 (1.13–1.18) | <0.001 | <1 | 0.5 | 0 |
| 1–2 | 1.5 | 2 | ||||
| 2–3 | 2.5 | 3 | ||||
| 3–4 | 3.5 | 5 | ||||
| >4 | 9.2 | 14 | ||||
| WBC | −0.1077 | 0.90 (0.88–0.92) | <0.001 | <4 | 3.5 | 17 |
| 4–6 | 5 | 15 | ||||
| 6–8 | 7 | 13 | ||||
| 8–12 | 10 | 9 | ||||
| >12 | 18.2 | 0 | ||||
| Nephropathy | 0.5598 | 1.75 (1.55–1.98) | <0.001 | No | 0 | 0 |
| Yes | 1 | 6 | ||||
| Glucose | 0.0059 | 1.01 (1.00–1.01) | <0.001 | <60 | 53 | 0 |
| 60–80 | 70 | 1 | ||||
| 80–100 | 90 | 2 | ||||
| 100–200 | 150 | 6 | ||||
| >200 | 364 | 20 | ||||
| Hematocrit | −0.0609 | 0.94 (0.93—0.95) | <0.001 | <30 | 25.7 | 19 |
| 30–35 | 32.5 | 15 | ||||
| 35–40 | 37.5 | 11 | ||||
| 40–50 | 45 | 7 | ||||
| >50 | 55 | 0 | ||||
| Sodium | 0.0822 | 1.09 (1.07—1.11) | <0.001 | <136 | 131.5 | 0 |
| 136–144 | 140 | 7 | ||||
| >144 | 146.5 | 13 |
| Models and AUCs | Age Groups | ||||||
|---|---|---|---|---|---|---|---|
| 18–34 | 35–49 | 50–64 | 65–74 | 75–84 | ≥85 | ||
| Compact Model | Internal AUC | 0.92 | 0.92 | 0.86 | 0.81 | 0.77 | 0.72 |
| External AUC | 0.86 | 0.82 | 0.79 | 0.73 | 0.70 | 0.82 | |
| Risk Index | Internal AUC | 0.89 | 0.88 | 0.83 | 0.78 | 0.74 | 0.65 |
| External AUC | 0.85 | 0.83 | 0.74 | 0.69 | 0.64 | 0.72 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Miao, Z.; Liu, T.; Liu, M.; Grdinovac, K.; Song, X.; Liang, Y.; Delen, D.; Paiva, W. Derivation and Validation of Essential Predictors and Risk Index for Early Detection of Diabetic Retinopathy Using Electronic Health Records. J. Clin. Med. 2021, 10, 1473. https://doi.org/10.3390/jcm10071473
Wang R, Miao Z, Liu T, Liu M, Grdinovac K, Song X, Liang Y, Delen D, Paiva W. Derivation and Validation of Essential Predictors and Risk Index for Early Detection of Diabetic Retinopathy Using Electronic Health Records. Journal of Clinical Medicine. 2021; 10(7):1473. https://doi.org/10.3390/jcm10071473
Chicago/Turabian StyleWang, Ru, Zhuqi Miao, Tieming Liu, Mei Liu, Kristine Grdinovac, Xing Song, Ye Liang, Dursun Delen, and William Paiva. 2021. "Derivation and Validation of Essential Predictors and Risk Index for Early Detection of Diabetic Retinopathy Using Electronic Health Records" Journal of Clinical Medicine 10, no. 7: 1473. https://doi.org/10.3390/jcm10071473
APA StyleWang, R., Miao, Z., Liu, T., Liu, M., Grdinovac, K., Song, X., Liang, Y., Delen, D., & Paiva, W. (2021). Derivation and Validation of Essential Predictors and Risk Index for Early Detection of Diabetic Retinopathy Using Electronic Health Records. Journal of Clinical Medicine, 10(7), 1473. https://doi.org/10.3390/jcm10071473







