The Prognostic Value of Pain Phenotyping in Relation to Treatment Outcomes in Patients with Axial Spondyloarthritis Treated in Clinical Practice: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Treatment
2.3. Data Collection
2.4. PainDETECT Questionnaire
2.5. Patient-Reported Outcome Measures (PROMs) and Clinical Examination
2.6. Inflammatory Bowel Disease (IBD) Related Questionnaires
2.7. Eye Examination
2.8. Outcome Responder Criteria
2.9. Involvement of Patient Research Partners
2.10. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. BASDAI Responses and Changes in Core Outcome Domains
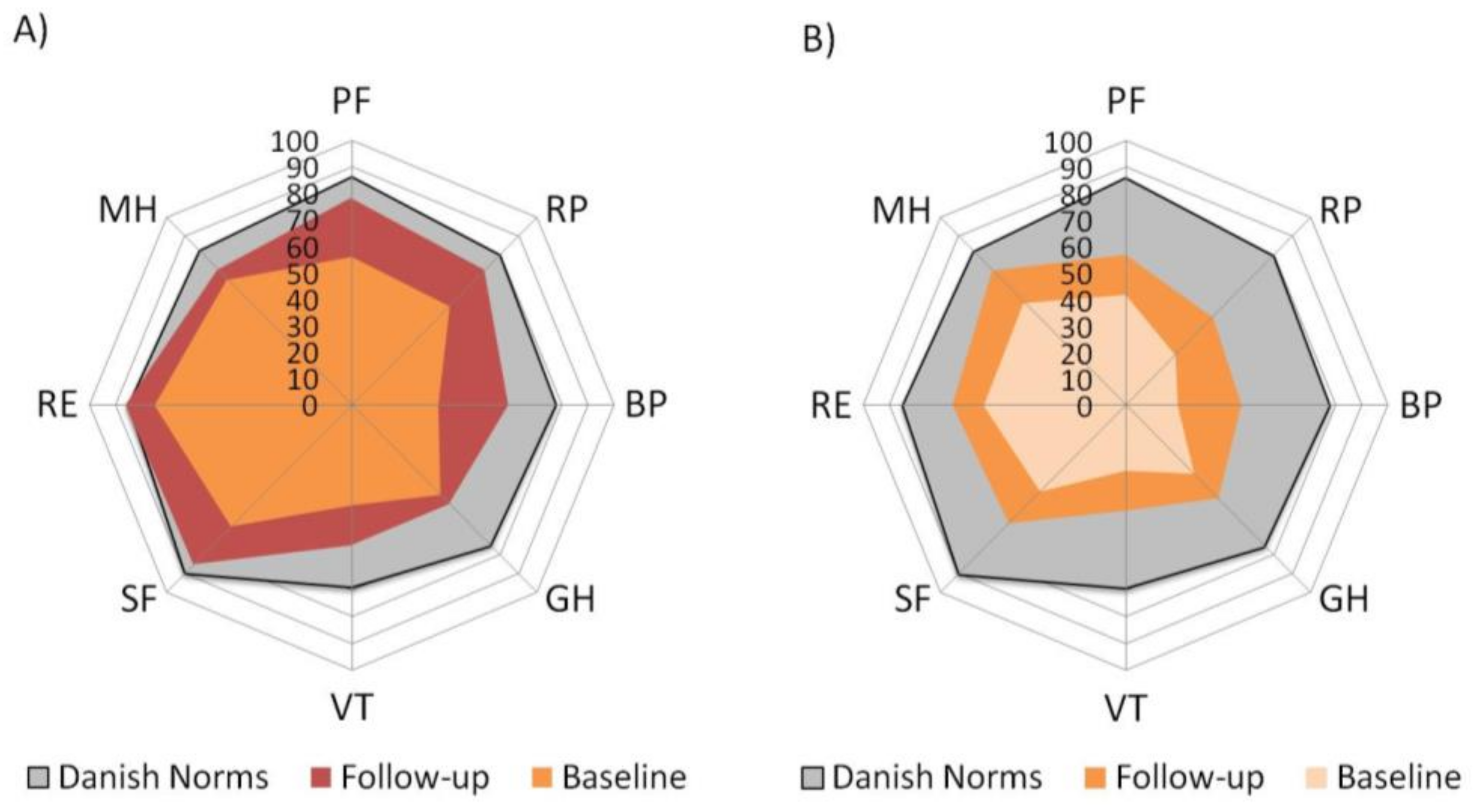
3.3. Health-Related Quality of Life
3.4. Associations between Baseline HBI and SCCAI and Variables Reflecting Disease Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieper, J.; Braun, J.; Dougados, M.; Baeten, D. Axial spondyloarthritis. Nat. Rev. Dis. Primers 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Landewé, R.; van der Heijde, D.; Listing, J.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Collantes-Estevez, E.; Davis, J.; Dijkmans, B.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 2009, 68, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Bidad, K.; Gracey, E.; Hemington, K.S.; Mapplebeck, J.C.S.; Davis, K.D.; Inman, R.D. Pain in ankylosing spondylitis: A neuro-immune collaboration. Nat. Rev. Rheumatol. 2017, 13, 410–420. [Google Scholar] [CrossRef]
- Krabbe, S.; Glintborg, B.; Ostergaard, M.; Hetland, M.L. Extremely poor patient-reported outcomes are associated with lack of clinical response and decreased retention rate of tumour necrosis factor inhibitor treatment in patients with axial spondyloarthritis. Scand. J. Rheumatol. 2019, 48, 128–132. [Google Scholar] [CrossRef]
- Wach, J.; Letroublon, M.C.; Coury, F.; Tebib, J.G. Fibromyalgia in Spondyloarthritis: Effect on Disease Activity Assessment in Clinical Practice. J. Rheumatol. 2016, 43, 2056–2063. [Google Scholar] [CrossRef]
- Levy, O.; Segal, R.; Maslakov, I.; Markov, A.; Tishler, M.; Amit-Vazina, M. The impact of concomitant fibromyalgia on visual analogue scales of pain, fatigue and function in patients with various rheumatic disorders. Clin. Exp. Rheumatol. 2016, 34, S120–S124. [Google Scholar]
- Gok, K.; Cengiz, G.; Erol, K.; Ozgocmen, S. Neuropathic Pain Component in Axial Spondyloarthritis and the Influence on Disease Burden. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2018, 24, 324–327. [Google Scholar] [CrossRef]
- Geler-Kulcu, D.; Batibay, S.; Ozturk, G.; Mesci, N. The association of neuropathic pain and disease activity, functional level, and quality of life in patients with ankylosing spondylitis: A cross-sectional study. Turk. J. Med. Sci. 2018, 48, 257–265. [Google Scholar] [CrossRef]
- Andreasen, R.A.; Kristensen, L.E.; Ellingsen, T.; Christensen, R.; Baraliakos, X.; Wied, J.; Aalykke, C.; Ulstrup, T.; Schiottz-Christensen, B.; Horn, H.C.; et al. Clinical characteristics of importance to outcome in patients with axial spondyloarthritis: Protocol for a prospective descriptive and exploratory cohort study. BMJ Open 2017, 7, e015536. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tolle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.S.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Braun, J.; van den Berg, R.; Baraliakos, X.; Boehm, H.; Burgos-Vargas, R.; Collantes-Estevez, E.; Dagfinrud, H.; Dijkmans, B.; Dougados, M.; Emery, P.; et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 2011, 70, 896–904. [Google Scholar] [CrossRef]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Hojgaard, P.; Ellegaard, K.; Nielsen, S.M.; Christensen, R.; Guldberg-Moller, J.; Ballegaard, C.; Dreyer, L.; Mease, P.; de Wit, M.; Skov, L.; et al. Pain Mechanisms and Ultrasonic Inflammatory Activity as Prognostic Factors in Patients with Psoriatic Arthritis: A Prospective Cohort Study. Arthritis Care Res. 2019, 71, 798–810. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Calin, A.; Garrett, S.; Whitelock, H.; Kennedy, L.G.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the Bath Ankylosing Spondylitis Functional Index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar]
- Zochling, J.; Braun, J. Assessments in ankylosing spondylitis. Best Pract. Res. Clin. Rheumatol. 2007, 21, 699–712. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E., Jr.; Lu, J.F.; Sherbourne, C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care 1994, 32, 40–66. [Google Scholar] [CrossRef]
- McHorney, C.A.; Ware, J.E., Jr.; Raczek, A.E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care 1993, 31, 247–263. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Bjorner, J.B.; Thunedborg, K.; Kristensen, T.S.; Modvig, J.; Bech, P. The Danish SF-36 Health Survey: Translation and preliminary validity studies. J. Clin. Epidemiol. 1998, 51, 991–999. [Google Scholar] [CrossRef]
- Strand, V.; Crawford, B.; Singh, J.; Choy, E.; Smolen, J.S.; Khanna, D. Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann. Rheum. Dis. 2009, 68, 1800–1804. [Google Scholar] [CrossRef]
- Jenkinson, T.R.; Mallorie, P.A.; Whitelock, H.C.; Kennedy, L.G.; Garrett, S.L.; Calin, A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J. Rheumatol. 1994, 21, 1694–1698. [Google Scholar]
- Maksymowych, W.P.; Mallon, C.; Morrow, S.; Shojania, K.; Olszynski, W.P.; Wong, R.L.; Sampalis, J.; Conner-Spady, B. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann. Rheum. Dis. 2009, 68, 948–953. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- de Wit, M.P.T.; Berlo, S.E.; Aanerud, G.J.; Aletaha, D.; Bijlsma, J.W.; Croucher, L.; Da Silva, J.A.P.; Glüsing, B.; Gossec, L.; Hewlett, S.; et al. European League Against Rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann. Rheum. Dis. 2011, 70, 722–726. [Google Scholar] [CrossRef]
- Rifbjerg-Madsen, S.; Christensen, A.W.; Christensen, R.; Hetland, M.L.; Bliddal, H.; Kristensen, L.E.; Danneskiold-Samsoe, B.; Amris, K. Pain and pain mechanisms in patients with inflammatory arthritis: A Danish nationwide cross-sectional DANBIO registry survey. PLoS ONE 2017, 12, e0180014. [Google Scholar] [CrossRef]
- Rencber, N.; Saglam, G.; Huner, B.; Kuru, O. Presence of Fibromyalgia Syndrome and Its Relationship with Clinical Parameters in Patients with Axial Spondyloarthritis. Pain Physician 2019, 22, E579–E585. [Google Scholar] [PubMed]
- Glintborg, B.; Hojgaard, P.; Lund Hetland, M.; Steen Krogh, N.; Kollerup, G.; Jensen, J.; Chrysidis, S.; Jensen Hansen, I.M.; Holland-Fischer, M.; Hojland Hansen, T.; et al. Impact of tobacco smoking on response to tumour necrosis factor-alpha inhibitor treatment in patients with ankylosing spondylitis: Results from the Danish nationwide DANBIO registry. Rheumatology 2016, 55, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ornbjerg, L.M.; Brahe, C.H.; Askling, J.; Ciurea, A.; Mann, H.; Onen, F.; Kristianslund, E.K.; Nordstrom, D.; Santos, M.J.; Codreanu, C.; et al. Treatment response and drug retention rates in 24 195 biologic-naive patients with axial spondyloarthritis initiating TNFi treatment: Routine care data from 12 registries in the EuroSpA collaboration. Ann. Rheum. Dis. 2019, 78, 1536–1544. [Google Scholar] [CrossRef] [PubMed]

| Demographics | NcP (n = 20) | MP (n = 29) | p-Value |
|---|---|---|---|
| Males, no. (%) | 12 (60) | 13 (45) | 0.387 a |
| Age, years, median (Q1; Q3) | 49 (40; 58) | 43 (32; 51) | 0.106 b |
| PDQ score, median (Q1; Q3) | 7 (4.5; 9) | 19 (16; 21) | <0.001 b |
| BMI, kg/m2, median (Q1; Q3) | 26.1 (24.4; 28.6) | 26.6 (24.2; 30.5) | 0.483 b |
| Smoking (current), no. (%) | 9 (45) | 9 (31) | 0.375 a |
| AxSpA symptom duration, months, median (Q1; Q3) | 109 (22; 229) | 50 (27; 84) | 0.489 b |
| Radiographic axSpA, no. (%) | 10 (50) | 6 (21) | 0.061 a |
| Higher education, no. (%) | 13 (65) | 15 (52) | 0.394 a |
| Peripheral joint involvement, no. (%) | 11 (55) | 13 (45) | 0.567 a |
| Comorbidity | |||
| CCI, median (Q1; Q3) | 1 (0; 2) | 1 (0; 1) | 0.705 b |
| Mental disorder (depression, anxiety) past/present, no. (%) | 2 (10) | 10 (34) | 0.089 a |
| Medication | |||
| NSAIDs daily use, no. (%) | 18 (90) | 24 (83) | 0.685 a |
| MTX use, no. (%) | 7 (35) | 9 (31) | 1.000 a |
| MTX dose (mg/week), median (Q1; Q3) | 0 (0; 7.5) | 0 (0; 15.0) | 0.942 b |
| SSZ use, no. (%) | 2 (10) | 3 (10) | 1.000 a |
| bDMARD I, no. (%) | 19 (95) | 25 (76) | 0.636 a |
| Glucocorticoid use, no. (%) | 1 (5) | 0 (0) | 0.408 a |
| Glucocorticoid dose (mg/day) median (Q1; Q3) | 0 (0; 0) | 0 (0; 0) | 0.229 b |
| Extra-articular manifestations * | |||
| Uveitis, no. (%) | 5 (25) | 4 (14) | 0.456 a |
| IBD, no. (%) | 0 (0) | 2 (7) | 0.507 a |
| Psoriasis, no. (%) | 4 (20) | 10 (34) | 0.345 a |
| Dactylitis, no. (%) | 7 (35) | 12 (41) | 0.769 a |
| Achilles-enthesitis, no. (%) | 13 (65) | 23 (79) | 0.331 a |
| Nephrolithiasis, no. (%) | 4 (20) | 6 (21) | 1.000 a |
| Patient-reported outcome measures (PROMs) | |||
| BASDAI (0–10), median (Q1; Q3) | 57 (46; 70) | 77 (64; 85) | 0.002 b |
| BASFI (0–10), median (Q1; Q3) | 44 (33; 53) | 67 (58; 77) | <0.001 b |
| VAS pain (0–10), median (Q1; Q3) | 65 (53; 79) | 77 (69; 84) | 0.026 b |
| VAS fatigue (0–10), median (Q1; Q3) | 72 (55; 83) | 81 (74; 86) | 0.060 b |
| VAS global (0–10), median (Q1; Q3) | 73 (61; 87) | 83 (74; 92) | 0.033 b |
| SF36-MCS (0–100), median (Q1; Q3) | 47.6 (37.8; 52.0) | 41.3 (36.2; 49.9) | 0.382 b |
| SF36-PCS (0–100), median (Q1; Q3) | 38.0 (35.0; 44.8) | 30.3 (28.5; 36.5) | 0.002 b |
| HBI (0–16+), median (Q1; Q3) | 5 (4; 6) | 4 (4; 6) | 0.491 b |
| SCCAI (0–19), median (Q1; Q3) | 4 (3; 5) | 4 (3; 4) | 0.892 b |
| Clinical examination | NcP (n = 20) | MP (n = 29) | p-value |
| BASMI (0–10), median (Q1; Q3) | 20 (15; 35) | 20 (10; 30) | 0.892 b |
| Tender joint count; TJC (0–44), median (Q1; Q3) | 2 (1; 4) | 6 (2; 8) | 0.020 b |
| Swollen joint count; SJC (0–44), median (Q1; Q3) | 0 (0; 0) | 0 (0; 0) | 0.965 b |
| Fibromyalgia, no. (%) | 1 (5) | 5 (17) | 0.199 a |
| Tender point count; TPC (0–18), median (Q1; Q3) | 2 (0; 3) | 5 (2; 9) | 0.023 b |
| SPARCC enthesitis index (0–16), median (Q1; Q3) | 2 (0; 3) | 2 (1; 6) | 0.098 b |
| ASDAS-CRP, median (Q1; Q3) | 2.2 (1.3; 2.9) | 2.9 (1,8; 3.6) | 0.063 b |
| VAS physician (0–rwe10), median (Q1; Q3) | 54 (44; 63) | 62 (44; 72) | 0.213 b |
| Paraclinical assessment | |||
| HLA-B27, no. (%) | 15 (75) | 17 (59) | 0.213 a |
| CRP (mg/L), median (Q1; Q3) | 8.7 (2.5; 14.0) | 6 (1; 12) | 0.450 b |
| P-calprotectin (μg/mL), median (Q1; Q3) | 325 (234.9; 365) | 195 (153; 324) | 0.035 b |
| F-calprotectin (<50 × 10−6), median (Q1; Q3) | 34 (13; 77) | 16 (14; 41) | 0.212 b |
| Outcome | NcP (n = 20) | MP (n = 29) | OR, Crude (95% CI) | OR, Adjusted * (95% CI) | p-Values Crude/Adjusted * |
|---|---|---|---|---|---|
| BASDAI50 responders, no. (%) | 8 (40) | 8 (28) | 1.75 (0.52 to 5.87) | 1.23 (0.33 to 4.6) | 0.362/0.221 |
| ΔBASDAI ≥ 2, no. (%) | 11 (55) | 12 (41) | 1.73 (0.55 to 5.47) | 0.94 (0.24 to 3.67) | 0.348/0.09 |
| ΔASDAS-CRP ≥ 1.1, no. (%) | 12 (60) | 12 (41) | 2.13 (0.67 to 6.78) | 1.22 (0.36 to 4.20) | 0.200/0.362 |
| ΔASDAS-CRP ≥ 2.0, no (%) | 6 (30) | 8 (28) | 1.13 (0.32 to 3.95) | 0.81 (0.20 to 3.17) | 0.854/0.203 |
| Outcome | NcP (n = 20) | MP (n = 29) | Contrast between Groups (95% CI) | p-Value | |
| ΔBASDAI, score | −2.7 (−3.9 to −1.5) | −2.2 (−3.1 to −1.2) | 0.5 (−1.07 to 2.18) | 0.496 | |
| %BASDAI change, % | −40 (−56 to −23) | −32 (−46 to −18) | 8.0 (−16 to 30) | 0.522 | |
| ΔASDAS-CRP, score | −1.2 (−1.6 to −0.9) | −1.6 (−1.9 to 1.3) | −0.40 (−0.83 to 0.1) | 0.088 | |
| Health-related quality of life | |||||
| ΔSF36-PCS (0–100) | 8.5 (3.8 to 13.2) | 4.4 (0.4 to 8.4) | −4.1 (−10.7 to 2.37) | 0.206 | |
| ΔSF36-MCS (0–100) | 8.1 (3.9 to 12.3) | 4.4 (0.8 to 8.0) | −3.70 (−9.2 to 1.8) | 0.185 | |
| Core domains | |||||
| ΔPF (BASFI 0–10) | −2.1 (−3.2 to −0.9) | −1.6 (−2.5 to −0.6) | 0.50 (−1.1 to 2.1) | 0.532 | |
| ΔPain (VAS 0–10) | −3.3 (−4.6 to −2.0) | −2.3 (−3.4 to −1.2) | 1.0 (−0.8 to 2.8) | 0.255 | |
| ΔSM (BASMI 0–10) | −0.7 (−1.0 to −0.3) | −1.3 (−1.6 to −0.9) | −0.6 (−1.1 to −0.4) | 0.035 | |
| ΔSS (VAS 0–10) | −4.0 (−5.3 to −2.7) | −2.9 (−4.0 to 1.8) | 1.1 (−0.7 to 2.8) | 0.215 | |
| ΔPGA (VAS 0–10) | −2.9 (−4.1 to −1.6) | −2.6 (−3.6 to −1.6) | 0.3 (−1.4 to 1.9) | 0.754 | |
| ΔSwollen joint count (0–44) | −0.5 (−0.7 to −0.4) | −0.6 (−0.7 to 0.5) | −0.1 (−0.2 to 0.1) | 0.400 | |
| ΔEnthesitis Index (0–18) | −1.5 (−2.3 to −0.8) | −1.7 (−2.3 to −1.0) | −0.2 (−1.2 to 0.9) | 0.734 | |
| ΔAPR (CRP, mg/L) | −9.6 (−24.1 to 5.1) | −2.0 (−14.1 to 10.1) | 7.6 (−11.4 to 26.5) | 0.429 | |
| ΔFatigue (VAS 0–10) | −1.9 (−3.2 to −0.6) | −1.8 (−3.0 to −7.2) | −0.1 (−1.7 to 1.8) | 0.937 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreasen, R.A.; Kristensen, L.E.; Egstrup, K.; Baraliakos, X.; Strand, V.; Horn, H.C.; Wied, J.; Schiøttz-Christensen, B.; Aalykke, C.; Jensen Hansen, I.M.; et al. The Prognostic Value of Pain Phenotyping in Relation to Treatment Outcomes in Patients with Axial Spondyloarthritis Treated in Clinical Practice: A Prospective Cohort Study. J. Clin. Med. 2021, 10, 1469. https://doi.org/10.3390/jcm10071469
Andreasen RA, Kristensen LE, Egstrup K, Baraliakos X, Strand V, Horn HC, Wied J, Schiøttz-Christensen B, Aalykke C, Jensen Hansen IM, et al. The Prognostic Value of Pain Phenotyping in Relation to Treatment Outcomes in Patients with Axial Spondyloarthritis Treated in Clinical Practice: A Prospective Cohort Study. Journal of Clinical Medicine. 2021; 10(7):1469. https://doi.org/10.3390/jcm10071469
Chicago/Turabian StyleAndreasen, Rikke Asmussen, Lars Erik Kristensen, Kenneth Egstrup, Xenofon Baraliakos, Vibeke Strand, Hans Christian Horn, Jimmi Wied, Berit Schiøttz-Christensen, Claus Aalykke, Inger Marie Jensen Hansen, and et al. 2021. "The Prognostic Value of Pain Phenotyping in Relation to Treatment Outcomes in Patients with Axial Spondyloarthritis Treated in Clinical Practice: A Prospective Cohort Study" Journal of Clinical Medicine 10, no. 7: 1469. https://doi.org/10.3390/jcm10071469
APA StyleAndreasen, R. A., Kristensen, L. E., Egstrup, K., Baraliakos, X., Strand, V., Horn, H. C., Wied, J., Schiøttz-Christensen, B., Aalykke, C., Jensen Hansen, I. M., Ellingsen, T., & Christensen, R. (2021). The Prognostic Value of Pain Phenotyping in Relation to Treatment Outcomes in Patients with Axial Spondyloarthritis Treated in Clinical Practice: A Prospective Cohort Study. Journal of Clinical Medicine, 10(7), 1469. https://doi.org/10.3390/jcm10071469







