Replication of Previous Findings? Comparing Gray Matter Volumes in Transgender Individuals with Gender Incongruence and Cisgender Individuals
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Data Acquisition
2.3. Data Preprocessing
2.4. ROI Selection and Definition
2.5. Data Analysis
3. Results
3.1. Participants
3.2. GMV Differences
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; ISBN1 978-0-89042-554-1. ISBN2 978-0-89042-555-8. [Google Scholar]
- Heylens, G.; Elaut, E.; Kreukels, B.P.C.; Paap, M.C.S.; Cerwenka, S.; Richter-Appelt, H.; Cohen-Kettenis, P.T.; Haraldsen, I.R.; De Cuypere, G. Psychiatric characteristics in transsexual individuals: Multicentre study in four European countries. Br. J. Psychiatry 2014, 204, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Safer, J.D.; Tangpricha, V. Care of the Transgender Patient. Ann. Intern. Med. 2019, 171, ITC1. [Google Scholar] [CrossRef] [PubMed]
- Valentine, S.E.; Shipherd, J.C. A systematic review of social stress and mental health among transgender and gender non-conforming people in the United States. Clin. Psychol. Rev. 2018, 66, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.M.; Drescher, J.; Krueger, R.B.; Atalla, E.; Cochran, S.D.; First, M.B.; Cohen-Kettenis, P.T.; Arango-de Montis, I.; Parish, S.J.; Cottler, S.; et al. Disorders related to sexuality and gender identity in the ICD-11: Revising the ICD-10 classification based on current scientific evidence, best clinical practices, and human rights considerations. World Psychiatry 2016, 15, 205–221. [Google Scholar] [CrossRef]
- Van Caenegem, E.; Wierckx, K.; Elaut, E.; Buysse, A.; Dewaele, A.; Van Nieuwerburgh, F.; De Cuypere, G.; T’Sjoen, G. Prevalence of Gender Nonconformity in Flanders, Belgium. Arch. Sex. Behav. 2015, 44, 1281–1287. [Google Scholar] [CrossRef]
- Kuyper, L.; Wijsen, C. Gender Identities and Gender Dysphoria in the Netherlands. Arch. Sex. Behav. 2014, 43, 377–385. [Google Scholar] [CrossRef]
- Arcelus, J.; Bouman, W.P.; Van Den Noortgate, W.; Claes, L.; Witcomb, G.; Fernandez-Aranda, F. Systematic Review and Meta-Analysis of Prevalence Studies in Transsexualism. Eur. Psychiatry 2015, 30, 807–815. [Google Scholar] [CrossRef]
- Luders, E.; Sánchez, F.J.; Gaser, C.; Toga, A.W.; Narr, K.L.; Hamilton, L.S.; Vilain, E. Regional gray matter variation in male-to-female transsexualism. Neuroimage 2009, 46, 904–907. [Google Scholar] [CrossRef]
- Savic, I.; Arver, S. Sex Dimorphism of the Brain in Male-to-Female Transsexuals. Cereb. Cortex 2011, 21, 2525–2533. [Google Scholar] [CrossRef]
- Spizzirri, G.; Duran, F.L.S.; Chaim-Avancini, T.M.; Serpa, M.H.; Cavallet, M.; Pereira, C.M.A.; Santos, P.P.; Squarzoni, P.; da Costa, N.A.; Busatto, G.F.; et al. Grey and white matter volumes either in treatment-naïve or hormone-treated transgender women: A voxel-based morphometry study. Sci. Rep. 2018, 8, 736. [Google Scholar] [CrossRef]
- Zhou, J.-N.; Hofman, M.A.; Gooren, L.J.G.; Swaab, D.F. A sex difference in the human brain and its relation to transsexuality. Nature 1995, 378, 68–70. [Google Scholar] [CrossRef]
- Garcia-Falgueras, A.; Swaab, D.F. A sex difference in the hypothalamic uncinate nucleus: Relationship to gender identity. Brain 2008, 131, 3132–3146. [Google Scholar] [CrossRef]
- Swaab, D. Sexual differentiation of the human brain: Relevance for gender identity, transsexualism and sexual orientation. Gynecol. Endocrinol. 2004, 19, 301–312. [Google Scholar] [CrossRef]
- Simon, L.; Kozák, L.R.; Simon, V.; Czobor, P.; Unoka, Z.; Szabó, Á.; Csukly, G. Regional Grey Matter Structure Differences between Transsexuals and Healthy Controls—A Voxel Based Morphometry Study. PLoS ONE 2013, 8, e83947. [Google Scholar] [CrossRef]
- Mueller, S.C.; De Cuypere, G.; T’Sjoen, G. Transgender research in the 21st century: A selective critical review from a neurocognitive perspective. Am. J. Psychiatry 2017, 174, 1155–1162. [Google Scholar] [CrossRef]
- Hoekzema, E.; Schagen, S.E.E.; Kreukels, B.P.C.; Veltman, D.J.; Cohen-Kettenis, P.T.; Delemarre-van de Waal, H.; Bakker, J. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology 2015, 55, 59–71. [Google Scholar] [CrossRef]
- Manzouri, A.; Kosidou, K.; Savic, I. Anatomical and Functional Findings in Female-to-Male Transsexuals: Testing a New Hypothesis. Cereb. Cortex 2017, 27, 998–1010. [Google Scholar] [CrossRef]
- Burke, S.M.; Manzouri, A.H.; Savic, I. Structural connections in the brain in relation to gender identity and sexual orientation. Sci. Rep. 2017, 7, 17954. [Google Scholar] [CrossRef]
- Wallien, M.S.C.; Zucker, K.J.; Steensma, T.D.; Cohen-Kettenis, P.T. 2D:4D finger-length ratios in children and adults with gender identity disorder. Horm. Behav. 2008, 54, 450–454. [Google Scholar] [CrossRef]
- Guillamon, A.; Junque, C.; Gómez-Gil, E. A Review of the Status of Brain Structure Research in Transsexualism. Arch. Sex. Behav. 2016, 45, 1615–1648. [Google Scholar] [CrossRef]
- Clemens, B.; Derntl, B.; Smith, E.; Junger, J.; Neulen, J.; Mingoia, G.; Schneider, F.; Abel, T.; Bzdok, D.; Habel, U. Predictive Pattern Classification Can Distinguish Gender Identity Subtypes from Behavior and Brain Imaging. Cereb. Cortex 2020, 30, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.; Berman, Z.; Tavor, I.; Wexler, N.; Gaber, O.; Stein, Y.; Shefi, N.; Pool, J.; Urchs, S.; Margulies, D.S.; et al. Sex beyond the genitalia: The human brain mosaic. Proc. Natl. Acad. Sci. USA 2015, 112, 15468–15473. [Google Scholar] [CrossRef] [PubMed]
- Clemens, B.; Junger, J.; Pauly, K.; Neulen, J.; Neuschaefer-Rube, C.; Frölich, D.; Mingoia, G.; Derntl, B.; Habel, U. Male-to-female gender dysphoria: Gender-specific differences in resting-state networks. Brain Behav. 2017, 7, e00691. [Google Scholar] [CrossRef] [PubMed]
- Junger, J.; Pauly, K.; Bröhr, S.; Birkholz, P.; Neuschaefer-Rube, C.; Kohler, C.; Schneider, F.; Derntl, B.; Habel, U. Sex matters: Neural correlates of voice gender perception. Neuroimage 2013, 79, 275–287. [Google Scholar] [CrossRef]
- Smith, E.; Junger, J.; Pauly, K.; Kellermann, T.; Neulen, J.; Neuschaefer-Rube, C.; Derntl, B.; Habel, U. Gender incongruence and the brain—Behavioral and neural correlates of voice gender perception in transgender people. Horm. Behav. 2018, 105, 11–21. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Zaudig, M.; Fydrich, T. Strukturiertes Klinisches Interview für DSM-IV (SKID-I und SKID-II); Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Mietchen, D. Computational morphometry for detecting changes in brain structure due to development, aging, learning, disease and evolution. Front. Neuroinform. 2009, 3, 1–12. [Google Scholar] [CrossRef]
- Honea, R.; Crow, T.J.; Passingham, D.; Mackay, C.E. Regional Deficits in Brain Volume in Schizophrenia: A Meta-Analysis of Voxel-Based Morphometry Studies. Am. J. Psychiatry 2005, 162, 2233–2245. [Google Scholar] [CrossRef]
- White Hughto, J.M.; Reisner, S.L.; Pachankis, J.E. Transgender stigma and health: A critical review of stigma determinants, mechanisms, and interventions. Soc. Sci. Med. 2015, 147, 222–231. [Google Scholar] [CrossRef]
- Pol, H.E.H.; Cohen-Kettenis, P.T.; Van Haren, N.E.M.; Peper, J.S.; Brans, R.G.H.; Cahn, W.; Schnack, H.G.; Gooren, L.J.G.; Kahn, R.S. Changing your sex changes your brain: Influences of testosterone and estrogen on adult human brain structure. Eur. J. Endocrinol. 2006, 155, S107–S114. [Google Scholar] [CrossRef]
- Flint, C.; Förster, K.; Koser, S.A.; Konrad, C.; Zwitserlood, P.; Berger, K.; Hermesdorf, M.; Kircher, T.; Nenadic, I.; Krug, A.; et al. Biological sex classification with structural MRI data shows increased misclassification in transgender women. Neuropsychopharmacology 2020, 45, 1758–1765. [Google Scholar] [CrossRef]
- Manzouri, A.; Savic, I. Possible Neurobiological Underpinnings of Homosexuality and Gender Dysphoria. Cereb. Cortex 2019, 29, 2084–2101. [Google Scholar] [CrossRef]
- Mueller, S.C.; Landré, L.; Wierckx, K.; T’Sjoen, G. A Structural Magnetic Resonance Imaging Study in Transgender Persons on Cross-Sex Hormone Therapy. Neuroendocrinology 2017, 105, 123–130. [Google Scholar] [CrossRef]
- Sorouri Khorashad, B.; Khazai, B.; Talaei, A.; Acar, F.; Hudson, A.R.; Borji, N.; Saberi, H.; Aminzadeh, B.; Mueller, S.C. Neuroanatomy of transgender persons in a Non-Western population and improving reliability in clinical neuroimaging. J. Neurosci. Res. 2020, 98, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Zubiaurre-Elorza, L.; Junque, C.; Gomez-Gil, E.; Segovia, S.; Carrillo, B.; Rametti, G.; Guillamon, A. Cortical Thickness in Untreated Transsexuals. Cereb. Cortex 2013, 23, 2855–2862. [Google Scholar] [CrossRef]
- Kranz, G.S.; Zhang, B.B.B.; Handschuh, P.; Ritter, V.; Lanzenberger, R. Gender-affirming hormone treatment—A unique approach to study the effects of sex hormones on brain structure and function. Cortex 2020, 129, 68–79. [Google Scholar] [CrossRef]
- Bingel, U.; Quante, M.; Knab, R.; Bromm, B.; Weiller, C.; Büchel, C. Subcortical structures involved in pain processing: Evidence from single-trial fMRI. Pain 2002, 99, 313–321. [Google Scholar] [CrossRef]
- Derbyshire, S.W.G.; Whalley, M.G.; Stenger, V.A.; Oakley, D.A. Cerebral activation during hypnotically induced and imagined pain. Neuroimage 2004, 23, 392–401. [Google Scholar] [CrossRef]
- Hui, K.K.; Liu, J.; Chen, A.J.W.; Makris, N.; Kennedy, D.N.; Moore, C.; Gollub, R.L.; Rosen, B.R.; Kwong, K.K. Acupuncture Modulates the Limbic System and Subcortical Gray Structures of the Human Brain—Direct Evidence by fMRI. Neuroimage 1998, 7, S441. [Google Scholar] [CrossRef]
- Coghill, R.C.; Gilron, I.; Iadarola, M.J. Hemispheric lateralization of somatosensory processing. J. Neurophysiol. 2001, 85, 2602–2612. [Google Scholar] [CrossRef]
- Bingel, U.; Gläscher, J.; Weiller, C.; Büchel, C. Somatotopic Representation of Nociceptive Information in the Putamen: An Event-related fMRI Study. Cereb. Cortex 2004, 14, 1340–1345. [Google Scholar] [CrossRef][Green Version]
- Maillard, L.; Ishii, K.; Bushara, K.; Waldvogel, D.; Schulman, A.E.; Hallett, M. Mapping the basal ganglia: fMRI evidence for somatotopic representation of face, hand, and foot. Neurology 2000, 55, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fontan, A.; Cignetti, F.; Nazarian, B.; Anton, J.-L.; Vaugoyeau, M.; Assaiante, C. How does the body representation system develop in the human brain? Dev. Cogn. Neurosci. 2017, 24, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Vocks, S.; Schulte, D.; Busch, M.; Grönemeyer, D.; Herpertz, S.; Suchan, B. Changes in neuronal correlates of body image processing by means of cognitive-behavioural body image therapy for eating disorders: A randomized controlled fMRI study. Psychol. Med. 2011, 41, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Mohr, H.M.; Zimmermann, J.; Röder, C.; Lenz, C.; Overbeck, G.; Grabhorn, R. Separating two components of body image in anorexia nervosa using fMRI. Psychol. Med. 2010, 40, 1519–1529. [Google Scholar] [CrossRef]
- Petkova, V.I.; Björnsdotter, M.; Gentile, G.; Jonsson, T.; Li, T.Q.; Ehrsson, H.H. From part-to whole-body ownership in the multisensory brain. Curr. Biol. 2011, 21, 1118–1122. [Google Scholar] [CrossRef]
- Fisher, A.D.; Castellini, G.; Ristori, J.; Casale, H.; Cassioli, E.; Sensi, C.; Fanni, E.; Amato, A.M.L.; Bettini, E.; Mosconi, M.; et al. Cross-Sex Hormone Treatment and Psychobiological Changes in Transsexual Persons: Two-Year Follow-Up Data. J. Clin. Endocrinol. Metab. 2016, 101, 4260–4269. [Google Scholar] [CrossRef]
- Raznahan, A.; Lee, Y.; Stidd, R.; Long, R.; Greenstein, D.; Clasen, L.; Addington, A.; Gogtay, N.; Rapoport, J.L.; Giedd, J.N. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. USA 2010, 107, 16988–16993. [Google Scholar] [CrossRef]
- Fernández, R.; Guillamon, A.; Cortés-Cortés, J.; Gómez-Gil, E.; Jácome, A.; Esteva, I.; Almaraz, M.C.; Mora, M.; Aranda, G.; Pásaro, E. Molecular basis of Gender Dysphoria: Androgen and estrogen receptor interaction. Psychoneuroendocrinology 2018, 98, 161–167. [Google Scholar] [CrossRef]
- Fisher, A.D.; Ristori, J.; Castellini, G.; Cocchetti, C.; Cassioli, E.; Orsolini, S.; Sensi, C.; Romani, A.; Mazzoli, F.; Cipriani, A.; et al. Neural Correlates of Gender Face Perception in Transgender People. J. Clin. Med. 2020, 9, 1731. [Google Scholar] [CrossRef]
- Hölig, C.; Föcker, J.; Best, A.; Röder, B.; Büchel, C. Activation in the angular gyrus and in the pSTS is modulated by face primes during voice recognition. Hum. Brain Mapp. 2017, 38, 2553–2565. [Google Scholar] [CrossRef]
- Allison, T.; Puce, A.; McCarthy, G. Social perception from visual cues: Role of the STS region. Trends Cogn. Sci. 2000, 4, 267–278. [Google Scholar] [CrossRef]
- Binder, J.R.; Desai, R.H. The neurobiology of semantic memory. Trends Cogn. Sci. 2011, 15, 527–536. [Google Scholar] [CrossRef]
- Abé, C.; Johansson, E.; Allzén, E.; Savic, I. Sexual Orientation Related Differences in Cortical Thickness in Male Individuals. PLoS ONE 2014, 9, e114721. [Google Scholar] [CrossRef]
- Ponseti, J.; Siebner, H.R.; Klöppel, S.; Wolff, S.; Granert, O.; Jansen, O.; Mehdorn, H.M.; Bosinski, H.A. Homosexual Women Have Less Grey Matter in Perirhinal Cortex than Heterosexual Women. PLoS ONE 2007, 2, e762. [Google Scholar] [CrossRef]
- Witelson, S.F.; Kigar, D.L.; Scamvougeras, A.; Kideckel, D.M.; Buck, B.; Stanchev, P.L.; Bronskill, M.; Black, S. Corpus Callosum Anatomy in Right-Handed Homosexual and Heterosexual Men. Arch. Sex. Behav. 2008, 37, 857–863. [Google Scholar] [CrossRef]
- Votinov, M.; Goerlich, K.S.; Puiu, A.A.; Smith, E.; Nickl-Jockschat, T.; Derntl, B.; Habel, U. Brain structure changes associated with sexual orientation. Sci. Rep. 2021, 11, 5078. [Google Scholar] [CrossRef]
- Maguen, S.; Shipherd, J.C. Suicide risk among transgender individuals. Psychol. Sex. 2010, 1, 34–43. [Google Scholar] [CrossRef]
- Narang, P.; Sarai, S.K.; Aldrin, S.; Lippmann, S. Suicide Among Transgender and Gender-Nonconforming People. Prim. Care Companion CNS Disord. 2018, 20. [Google Scholar] [CrossRef]
- Baldinger-Melich, P.; Urquijo Castro, M.F.; Seiger, R.; Ruef, A.; Dwyer, D.B.; Kranz, G.S.; Klöbl, M.; Kambeitz, J.; Kaufmann, U.; Windischberger, C.; et al. Sex Matters: A Multivariate Pattern Analysis of Sex- and Gender-Related Neuroanatomical Differences in Cis- and Transgender Individuals Using Structural Magnetic Resonance Imaging. Cereb. Cortex 2020, 30, 1345–1356. [Google Scholar] [CrossRef]
- Van de Grift, T.C.; Elaut, E.; Cerwenka, S.C.; Cohen-Kettenis, P.T.; Kreukels, B.P.C. Surgical Satisfaction, Quality of Life, and Their Association After Gender-Affirming Surgery: A Follow-up Study. J. Sex Marital Ther. 2018, 44, 138–148. [Google Scholar] [CrossRef]

| CW (n = 25) | CM (n = 24) | TM (n = 33) | TW (n = 33) | |
|---|---|---|---|---|
| Age | 31 (11) | 33 (11) | 24 (7) | 33 (13) |
| Years of education | 15 (3) | 15 (3) | 14 (3) | 14 (3) |
| TIV | 1460 (115) | 1605 (97) | 1412 (107) | 1567 (102) |
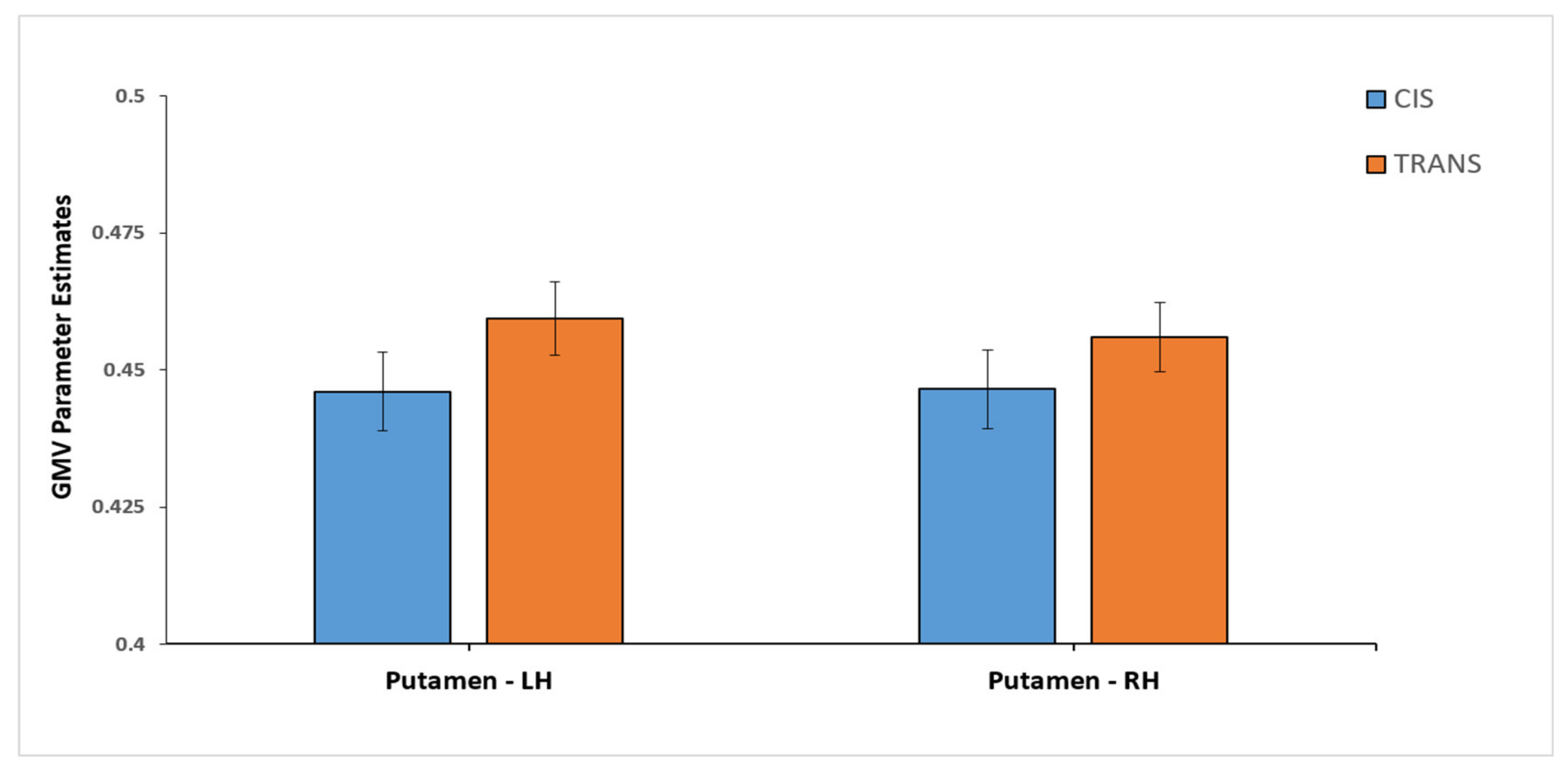
| CIS (n = 49) | TRANS (n = 66) | |
|---|---|---|
| Putamen (L) * | 0.446 (0.05) | 0.4593 (0.055) |
| Putamen (R) * | 0.4465 (0.05) | 0.4560 (0.051) |
| Precentral Gyrus (L) | 0.3341 (0.041) | 0.3338 (0.04) |
| Precentral Gyrus (R) | 0.3232 (0.038) | 0.3180 (0.038) |
| Thalamus | 0.3699 (0.045) | 0.3677 (0.042) |
| AG (L) | 0.3904 (0.058) | 0.3821 (0.059) |
| AG (R) | 0.3491 (0.052) | 0.3425 (0.045) |
| Cerebellum (L) | 0.4048 (0.037) | 0.4023 (0.033) |
| Cerebellum (R) | 0.4105 (0.039) | 0.4086 (0.035) |
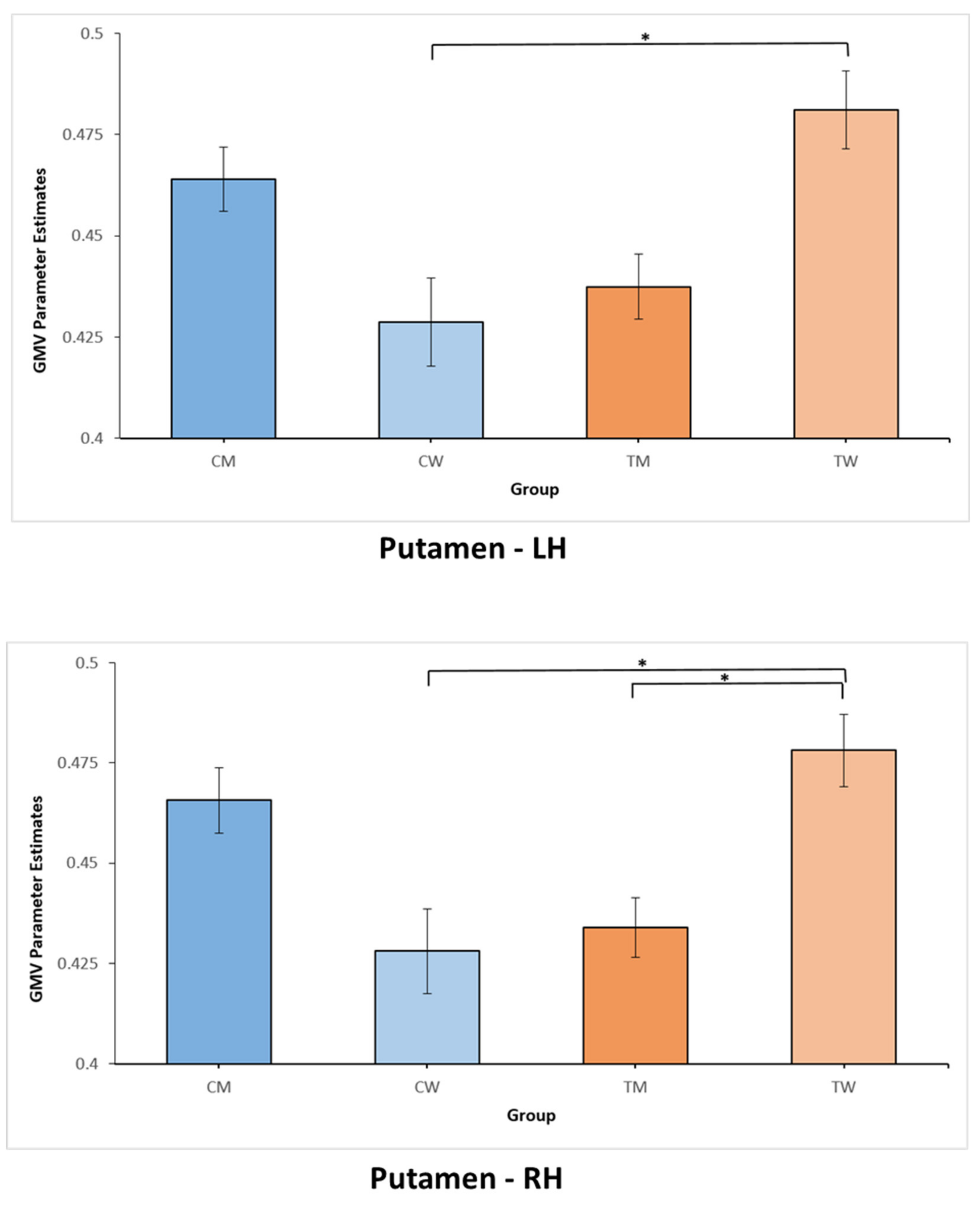
| CW (n = 25) | CM (n = 24) | TM (n = 33) | TW (n = 33) | |
|---|---|---|---|---|
| Putamen (L) (a) | 0.4287 (0.055) | 0.464 (0.039) | 0.4374 (0.046) | 0.4812 (0.054) |
| Putamen (R) (a)&(b) | 0.4280 (0.053) | 0.4657 (0.04) | 0.4339 (0.042) | 0.4781 (0.051) |
| Precentral Gyrus (L) | 0.3176 (0.036) | 0.3513 (0.04) | 0.3305 (0.029) | 0.3371 (0.049) |
| Precentral Gyrus (R) | 0.31 (0.039) | 0.3369 (0.033) | 0.317 (0.034) | 0.3189 (0.041) |
| Thalamus | 0.3622 (0.038) | 0.378 (0.05) | 0.3679 (0.042) | 0.3675 (0.042) |
| AG (L) | 0.3727 (0.05) | 0.4088 (0.062) | 0.3661 (0.05) | 0.3982 (0.064) |
| AG (R) | 0.3263 (0.045) | 0.373 (0.05) | 0.3353 (0.045) | 0.3497 (0.045) |
| Cerebellum (L) | 0.3945 (0.036) | 0.4155 (0.036) | 0.3964 (0.034) | 0.4081 (0.032) |
| Cerebellum (R) | 0.3987 (0.036) | 0.4227 (0.038) | 0.4021 (0.036) | 0.4152 (0.034) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemens, B.; Votinov, M.; Puiu, A.A.; Schüppen, A.; Hüpen, P.; Neulen, J.; Derntl, B.; Habel, U. Replication of Previous Findings? Comparing Gray Matter Volumes in Transgender Individuals with Gender Incongruence and Cisgender Individuals. J. Clin. Med. 2021, 10, 1454. https://doi.org/10.3390/jcm10071454
Clemens B, Votinov M, Puiu AA, Schüppen A, Hüpen P, Neulen J, Derntl B, Habel U. Replication of Previous Findings? Comparing Gray Matter Volumes in Transgender Individuals with Gender Incongruence and Cisgender Individuals. Journal of Clinical Medicine. 2021; 10(7):1454. https://doi.org/10.3390/jcm10071454
Chicago/Turabian StyleClemens, Benjamin, Mikhail Votinov, Andrei Alexandru Puiu, Andre Schüppen, Philippa Hüpen, Josef Neulen, Birgit Derntl, and Ute Habel. 2021. "Replication of Previous Findings? Comparing Gray Matter Volumes in Transgender Individuals with Gender Incongruence and Cisgender Individuals" Journal of Clinical Medicine 10, no. 7: 1454. https://doi.org/10.3390/jcm10071454
APA StyleClemens, B., Votinov, M., Puiu, A. A., Schüppen, A., Hüpen, P., Neulen, J., Derntl, B., & Habel, U. (2021). Replication of Previous Findings? Comparing Gray Matter Volumes in Transgender Individuals with Gender Incongruence and Cisgender Individuals. Journal of Clinical Medicine, 10(7), 1454. https://doi.org/10.3390/jcm10071454






