Changes in Back Pain Scores after Bariatric Surgery in Obese Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Inclusion Criteria
- (1)
- Randomized controlled trials (RCT) and observational studies of any bariatric surgery (bariatrics, gastric bypass, gastric sleeve, Roux-en-Y, RYGB, sleeve gastrectomy, or adjustable gastric band).
- (2)
- Studies which enrolled adult obese patients with a BMI > 40 kg/m2, or a BMI ≥ 35kg/m2 with comorbidities, undergoing primary bariatric surgery.
- (3)
- Studies which reported the pain intensity change of the lower back with the Numeric Rating Pain Scale (NPS) or Visual Analogue Scale (VAS) before and after bariatric surgery.
- (4)
- Studies which reported a follow-up of at least 3 months.
2.3. Exclusion Criteria
2.4. Types of Outcomes Measures
- (1)
- Primary outcome: the change in the pain intensity score of the LBP before and after bariatric surgery, as measured in VAS or NPS, and the change in BMI before and after bariatric surgery.
- (2)
- Secondary outcomes: the change in disc space height, back-specific disability questionnaires (Roland–Morris score, Oswestry Disability Index (ODI), and Waddell Disability Index) and pain pressure threshold (PPT) before and after bariatric surgery; the association between the back-specific disability questionnaires (Roland–Morris score or Oswestry Disability Index (ODI) or Waddell Disability Index) and BMI changes following bariatric surgery for severely obese patients.
2.5. Selection of Studies
2.6. Data Extraction
2.7. Risk Bias Assessment
2.8. Statistical Analysis
2.9. Quality Assessment
3. Results
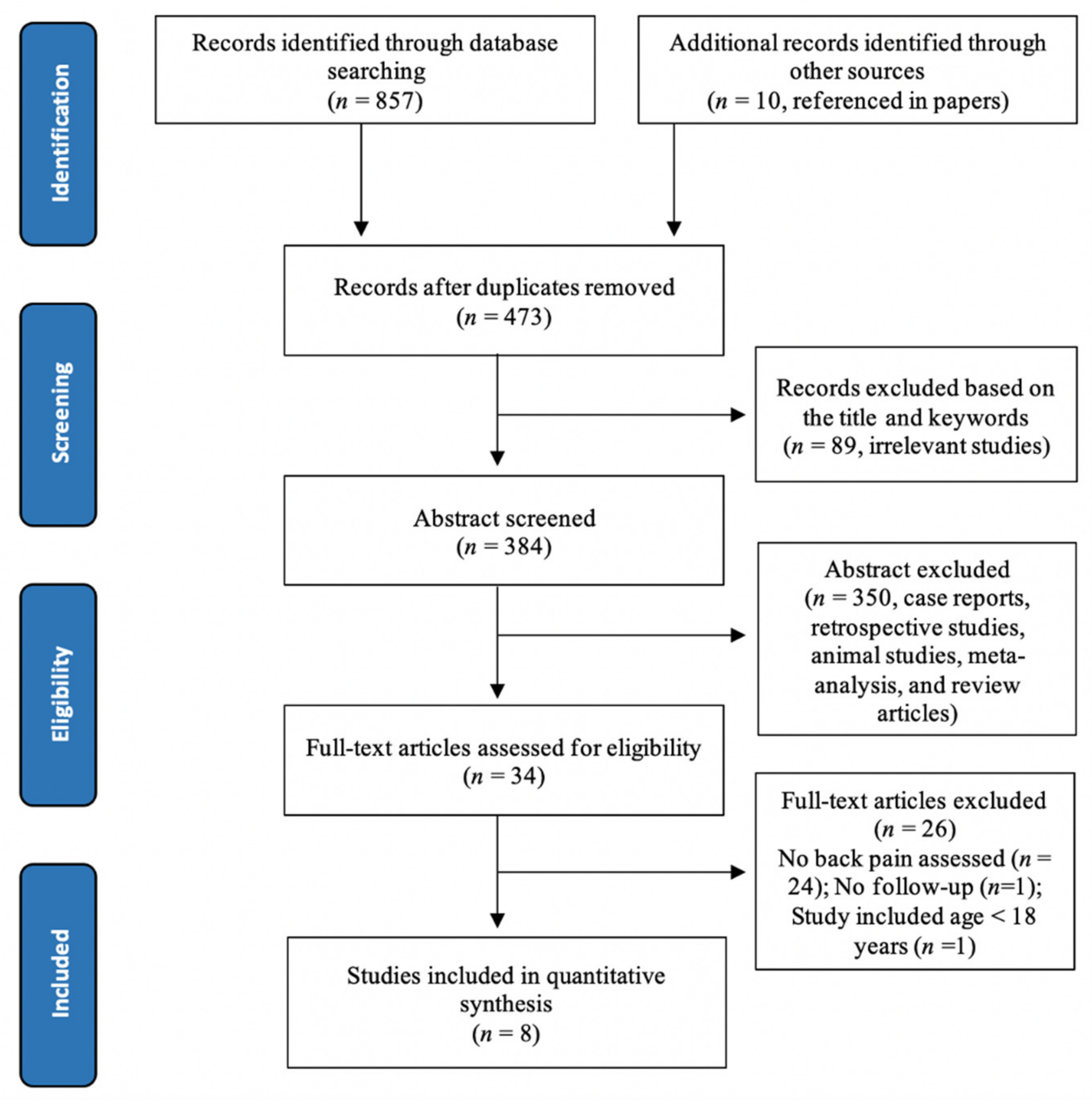
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
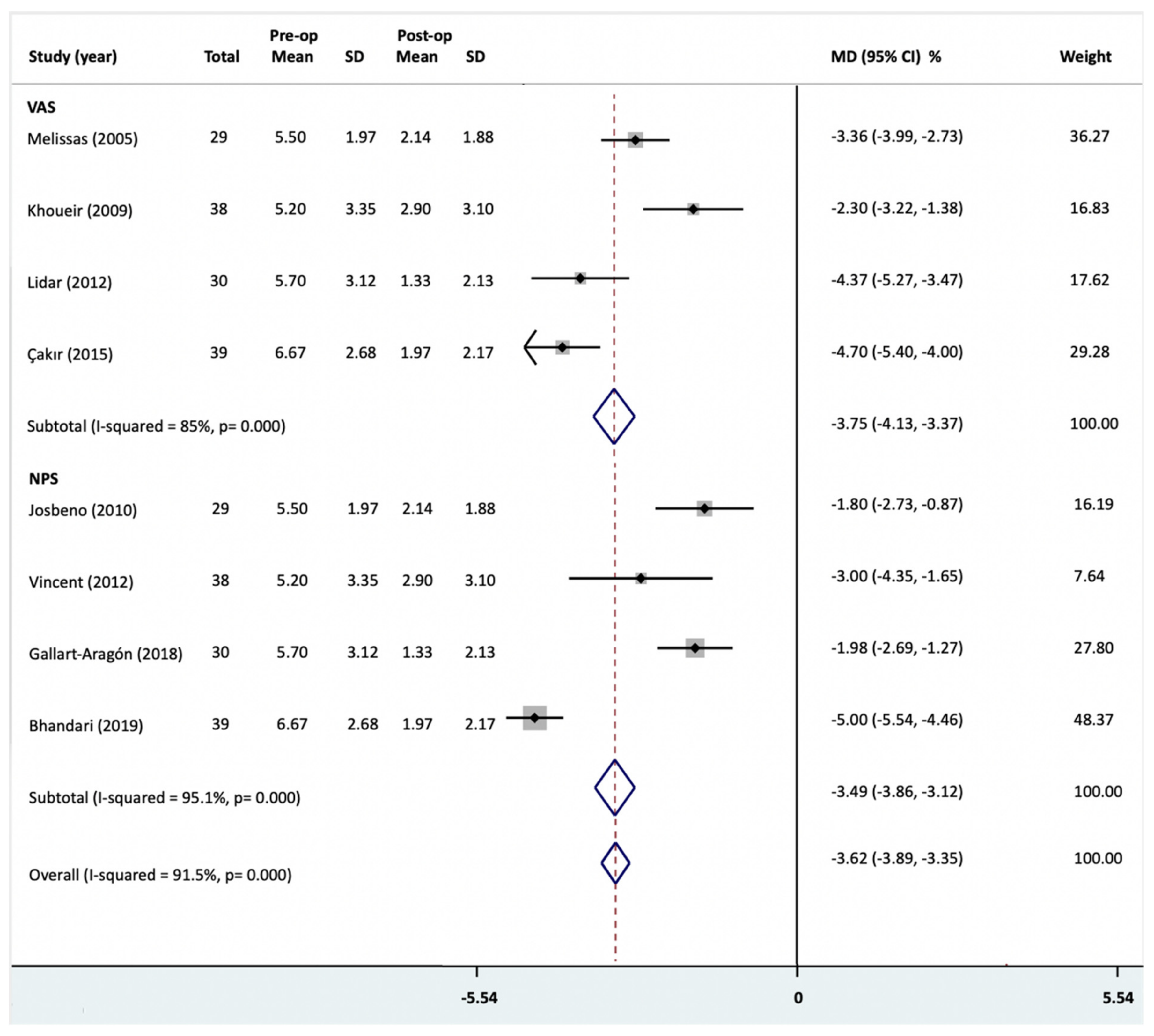
3.4. Back Pain Intensity
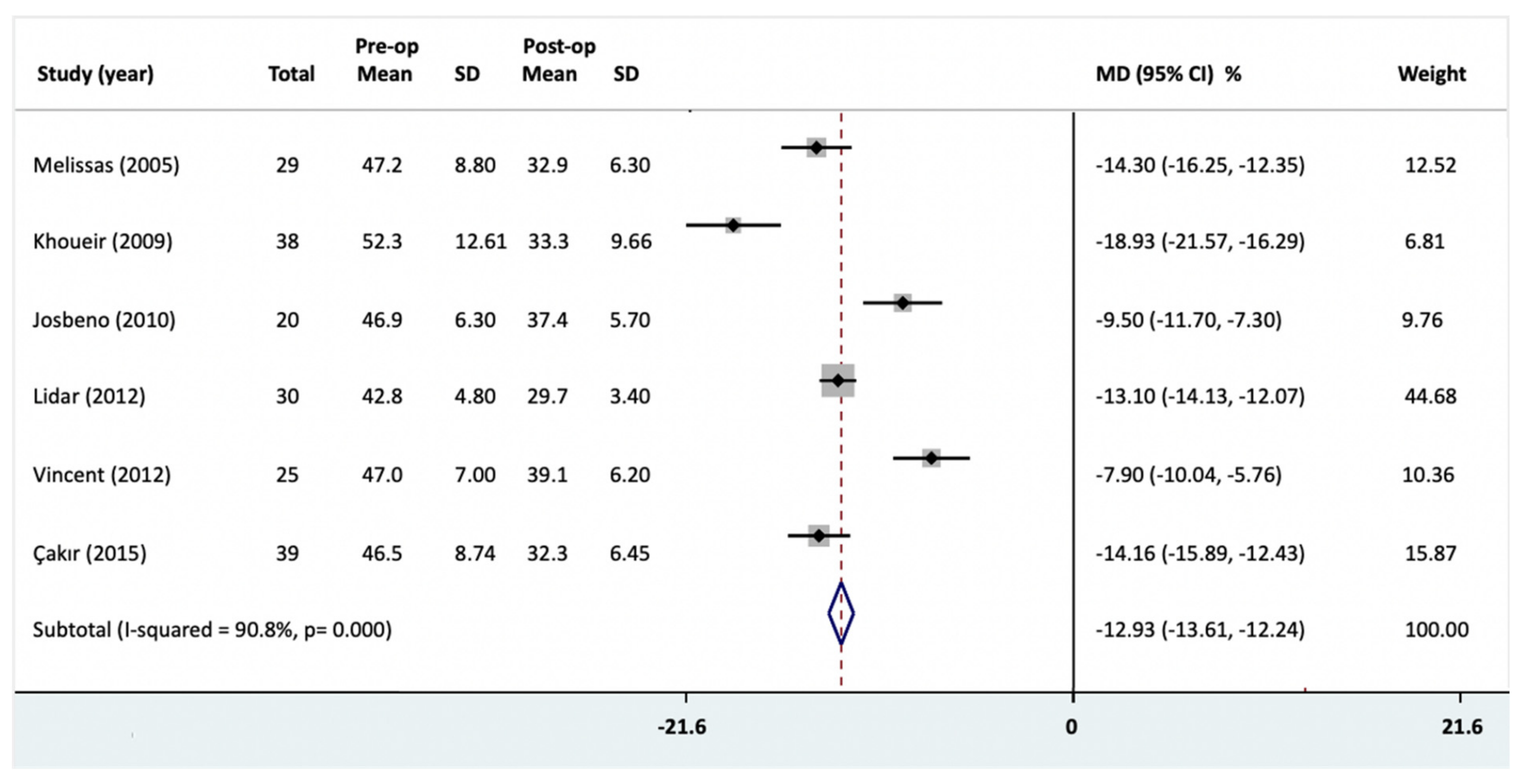
3.5. BMI Change
3.6. The Association of Mean Pain Change with BMI Change
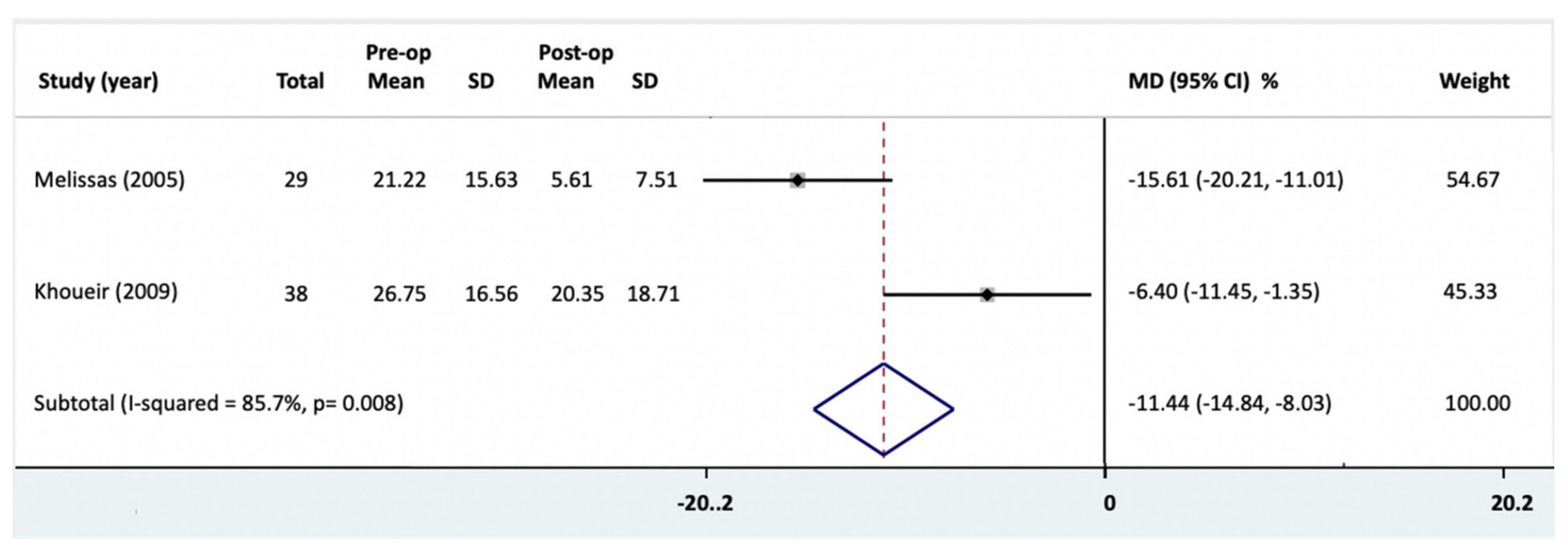
3.7. Disability Change
3.8. Other Clinical Outcomes
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight: Fact Sheet; WHO Media Cent; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Goodwin, P.J.; Stambolic, V. Impact of the obesity epidemic on cancer. Annu. Rev. Med. 2015, 66, 281–296. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Atchison, J.W.; Vincent, H.K. Obesity and low back pain: Relationships and treatment. Pain Manag. 2012, 2, 79–86. [Google Scholar] [CrossRef]
- Shiri, R.; Karppinen, J.; Leino-Arjas, P.; Solovieva, S.; Viikari-Juntura, E. The association between obesity and low back pain: A meta-analysis. Am. J. Epidemiol. 2009, 171, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Liuke, M.; Solovieva, S.; Lamminen, A.; Luoma, K.; Leino-Arjas, P.; Luukkonen, R.; Riihimäki, H. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes. 2005, 29, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Chan, D.; Luk, K.D.K.; Cheung, K.M.C. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: A population-based study. Arthritis Rheum. 2012, 64, 1488–1496. [Google Scholar] [CrossRef]
- Urquhart, D.M.; Berry, P.; Wluka, A.E.; Strauss, B.J.; Wang, Y.; Proietto, J.; Jones, G.; Dixon, J.B.; Cicuttini, F.M. 2011 young investigator award winner. Spine 2011, 36, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, D.M.; Kurniadi, I.; Triangto, K.; Wang, Y.; Wluka, A.E.; O’Sullivan, R.; Jones, G.; Cicuttini, F.M. Obesity is associated with reduced disc height in the lumbar spine but not at the lumbosacral junction. Spine 2014, 39, E962–E966. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K. Surgical treatment of obesity—Weighing the facts. N. Engl. J. Med. 2009, 361, 520–521. [Google Scholar] [CrossRef]
- Arterburn, D.; Wellman, R.; Emiliano, A.; Smith, S.R.; Odegaard, A.O.; Murali, S.; Williams, N.; Coleman, K.J.; Courcoulas, A.; Coley, R.Y.; et al. Comparative effectiveness and safety of bariatric procedures for weight loss. Ann. Intern. Med. 2018, 169, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, A.F.; Helvie, P.; Patel, A.A. Bariatric surgery and low back pain: A systematic literature review. Glob. Spine J. 2019, 10, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, I.; Currie, A.C.; Newton, R.C.; Albon, L.; Slater, G.; Hawkins, W.; Pring, C. A meta-analysis of the impact of bariatric surgery on back pain. Obes. Surg. 2020, 30, 3201–3207. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connel, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000. [Google Scholar]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: The quorom statement. Lancet 1999, 354, 1896–1900. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiologya proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.J.; Norris, S.L. Grade guidelines: 3. rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Bhandari, M.; Mathur, W.; Kosta, S.; Salvi, P.; Fobi, M. Assessment of functional ability of nonambulatory patients with obesity: After and before bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 2087–2093. [Google Scholar] [CrossRef]
- Khoueir, P.; Black, M.H.; Crookes, P.F.; Kaufman, H.S.; Katkhouda, N.; Wang, M.Y. Prospective assessment of axial back pain symptoms before and after bariatric weight reduction surgery. Spine J. 2009, 9, 454–463. [Google Scholar] [CrossRef]
- Vincent, H.K.; Ben-David, K.; Conrad, B.P.; Lamb, K.M.; Seay, A.N.; Vincent, K.R. Rapid changes in gait, musculoskeletal pain, and quality of life after bariatric surgery. Surg. Obes. Relat. Dis. 2012, 8, 346–354. [Google Scholar] [CrossRef]
- Çakır, T.; Oruç, M.T.; Aslaner, A.; Duygun, F.; Yardımcı, E.C.; Mayir, B.; Bülbüller, N. The effects of laparoscopic sleeve gastrectomy on head, neck, shoulder, low back and knee pain of female patients. Int. J. Clin. Exp. Med. 2015, 8, 2668–2673. [Google Scholar]
- Gallart-Aragón, T.; Fernández-Lao, C.; Galiano-Castillo, N.; Cantarero-Villanueva, I.; Lozano-Lozano, M.; Arroyo-Morales, M. Improvements in health-related quality of life and pain: A cohort study in obese patients after laparoscopic sleeve gastrectomy. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 53–57. [Google Scholar] [CrossRef]
- Josbeno, D.A.; Jakicic, J.M.; Hergenroeder, A.; Eid, G.M. Physical activity and physical function changes in obese individuals after gastric bypass surgery. Surg. Obes. Relat. Dis. 2010, 6, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Lidar, Z.; Behrbalk, E.; Regev, G.J.; Salame, K.; Keynan, O.; Schweiger, C.; Appelbaum, L.; Levy, Y.; Keidar, A. Intervertebral disc height changes after weight reduction in morbidly obese patients and its effect on quality of life and radicular and low back pain. Spine 2012, 37, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Melissas, J.; Kontakis, G.; Volakakis, E.; Tsepetis, T.; Alegakis, A.; Hadjipavlou, A. The effect of surgical weight reduction on functional status in morbidly obese patients with low back pain. Obes. Surg. 2005, 15, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.; Klarenbach, S.; Wiebe, N.; Birch, D.; Karmali, S.; Manns, B.; Hazel, M.; Sharma, A.M.; Tonelli, M. Bariatric surgery: A systematic review and network meta-analysis of randomized trials. Obes. Rev. 2011, 12, 602–621. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Boyd, D.; Chew, C.; MacDonald, N.; Dennis, A. Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 2017, 118, 424–429. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef]
- Podichetty, V.K. The aging spine: The role of inflammatory mediators in intervertebral disc degeneration. Cell. Mol. Boil. 2007, 53, 4–18. [Google Scholar]
- Vismara, L.; Menegoni, F.; Zaina, F.; Galli, M.; Negrini, S.; Capodaglio, P. Effect of obesity and low back pain on spinal mobility: A cross sectional study in women. J. Neuroeng. Rehabil. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
| Author_Year | Population | Outcomes | Follow-Up (Months) | Study Design | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. Total | Intervention | N. Female | Mean Age (Years) | Initial BMI (kg/m2) | Post-Op BMI (kg/m2) | LBP | Pre-Op LBP | Post-Op LBP | Disability | |||
| Melissas_2005 | 29 | VBG | 23 | 37.5 | 47.2 ± 8.8 | 32.9 ± 6.3 | VAS | 5.5 ± 1.97 | 2.14 ± 1.88 | ODI/RMD/WDI | 24 | Cohort study |
| Khoueir_2009 | 38 | RYGB or DS or SG | 30 | 48.4 | 52.3 ± 12.61 | 38.32 ± 9.66 | VAS | 5.2 ± 3.35 | 2.9 ± 3.1 | Yes/ODI | 12 | Cohort study |
| Josbeno_2010 | 20 | LRYGB | 18 | 41.6 | 46.9 ± 6.3 | 37.4 ± 5.7 | NPS | 3.5 ± 1.8 | 1.7 ± 2.63 | 3 | Cohort study | |
| Lidar_2012 | 30 | LAGB or SG or LRYGB or DS | 15 | 49 | 42.8 ± 4.8 | 29.7 ± 3.4 | VAS | 5.7 ± 3.12 | 1.33 ± 2.13 | 12 | Cohort study | |
| Vincent_2012 | 25 | LRYGB or LAGB | 20 | 41 | 47 ± 7 | 39.1 | NPS | 5.5 ± 4 | 2.5 ± 3.7 | 3 | Cohort study | |
| Çakır_2015 | 39 | SG | 39 | 37.7 | 46.49 | 32.3 | VAS | 6.67 ± 2.68 | 1.97 ± 2.17 | 6 | Cohort study | |
| Gallart-Aragón_2018 | 72 | SG | 47 | 45.36 | - | - | NPS | 3.95 ± 3.73 | 1.97 ± 2.95 | 6 | Cohort study | |
| Bhandari_2019 | 45 | SG or one-anastomosis gastric bypass or LRYGB | 34 | 54.7 | 54.2 ± 8.6 | - | NPS | 7.3 ± 1.4 | 2.3 ± 1.4 | RMD | 12 | Cohort study |
| Author | Year | Country | Surgical Procedures | Selection (/4) | Comparability (/2) | Outcome (/3) | Total (/9) |
|---|---|---|---|---|---|---|---|
| Melissas | 2005 | Greece | VBG | 4 | 0 | 3 | 7 |
| Khoueir | 2009 | USA | RYGB or DS or SG | 4 | 0 | 3 | 7 |
| Josbeno | 2010 | USA | LRYGB | 4 | 0 | 3 | 7 |
| Lidar | 2012 | Israel | LAGB or SG or LRYGB or DS | 4 | 0 | 3 | 7 |
| Vincent | 2012 | USA | LRYGB or LAGB | 4 | 1 | 3 | 8 |
| Çakır | 2015 | Turkey | SG | 4 | 0 | 3 | 7 |
| Gallart-Aragón | 2018 | Spain | SG | 4 | 0 | 3 | 7 |
| Bhandari | 2019 | India | SG or one-anastomosis gastric bypass or LRYGB | 4 | 0 | 3 | 7 |
| Outcome | Test | Statistical Model | Homogeneity | Begg’s P | Level of Quality | |||
|---|---|---|---|---|---|---|---|---|
| MD/r | 95% CI | p Value | p Value | I2 (%) | ||||
| NPS | Meta-analysis | −3.49 | −3.86, −3.12 a | - | 0.000 ***,b | 95.1 c | 1.000 | L 1,2,3 |
| Sensitivity analysis | - | |||||||
| Publication date | - | −0.68, 1.06 | - | 94.8 | - | |||
| Follow-up period | −0.24, 0.86 | 76.1 | ||||||
| Number of patients | −0.19, 0.20 | 96.7 | ||||||
| Meta-regression with BMI | - | |||||||
| VAS | Meta-analysis | −3.75 | −4.13, −3.37 a | - | 0.000 ***,b | 85 c | 0.12 | L 1,2,3 |
| Sensitivity analysis | - | - | ||||||
| Publication date | - | −2.46, 1.68 | - | 60.8 | - | |||
| Follow-up period | −3.52, 3.97 | 90 | ||||||
| Number of patients | −3.14, 3.29 | 92.4 | ||||||
| Meta-regression with BMI | 1.87 | −1.83, 5.57 | 0.162 | - | ||||
| ODI | Meta-analysis | −11.44 | −14.84, −8.03 a | - | 0.008 **,b | 85.7 c | 1.000 | M 1,2,3 |
| Sensitivity analysis | ||||||||
| Publication date | - | |||||||
| Follow-up period | ||||||||
| Number of patients | ||||||||
| Meta-regression with BMI | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koremans, F.W.; Chen, X.; Das, A.; Diwan, A.D. Changes in Back Pain Scores after Bariatric Surgery in Obese Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1443. https://doi.org/10.3390/jcm10071443
Koremans FW, Chen X, Das A, Diwan AD. Changes in Back Pain Scores after Bariatric Surgery in Obese Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(7):1443. https://doi.org/10.3390/jcm10071443
Chicago/Turabian StyleKoremans, Froukje W., Xiaolong Chen, Abhirup Das, and Ashish D. Diwan. 2021. "Changes in Back Pain Scores after Bariatric Surgery in Obese Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 7: 1443. https://doi.org/10.3390/jcm10071443
APA StyleKoremans, F. W., Chen, X., Das, A., & Diwan, A. D. (2021). Changes in Back Pain Scores after Bariatric Surgery in Obese Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(7), 1443. https://doi.org/10.3390/jcm10071443








