Optimal Skeletal Muscle Mass Index Cut-Off Values for Presarcopenia Evaluated by Computed Tomography against Dual-Energy X-ray Absorptiometry in Patients with Chronic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluation of SMM and Diagnosis of Presarcopenia
2.3. Clinical and Laboratory Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: A systematic review and meta-analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Moctezuma-Velazquez, C.; Duarte-Rojo, A.; Ebadi, M.; Ghosh, S.; Rose, C.; Montano-Loza, A.J. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol. Int. 2018, 12, 377–386. [Google Scholar] [CrossRef]
- Takada, H.; Amemiya, F.; Yasumura, T.; Yoda, H.; Okuwaki, T.; Imagawa, N.; Shimamura, N.; Tanaka, K.; Kadokura, M.; Maekawa, S.; et al. Relationship between presarcopenia and event occurrence in patients with primary hepatocellular carcinoma. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2016, 46, 743–751. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef]
- Van Vugt, J.L.A.; Levolger, S.; de Bruin, R.W.F.; van Rosmalen, J.; Metselaar, H.J.; IJzermans, J.N.M. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am. J. Transpl. 2016, 16, 2277–2292. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease: Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Lindqvist, C.; Brismar, T.B.; Majeed, A.; Wahlin, S. Assessment of muscle mass depletion in chronic liver disease; dual-energy x-ray absorptiometry compared with computed tomography. Nutrition 2019, 61, 93–98. [Google Scholar] [CrossRef]
- Giusto, M.; Lattanzi, B.; Albanese, C.; Galtieri, A.; Farcomeni, A.; Giannelli, V.; Lucidi, C.; Di Martino, M.; Catalano, C.; Merli, M. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur. J. Gastroenterol. Hepatol. 2015, 27, 328–334. [Google Scholar] [CrossRef]
- Georgiou, A.; Papatheodoridis, G.V.; Alexopoulou, A.; Deutsch, M.; Vlachogiannakos, I.; Ioannidou, P.; Papageorgiou, M.V.; Papadopoulos, N.; Yannakoulia, M.; Kontogianni, M.D. Validation of cutoffs for skeletal muscle mass index based on computed tomography analysis against dual energy X-ray absorptiometry in patients with cirrhosis: The KIRRHOS study. Ann. Gastroenterol. 2020, 33, 80–86. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Tsuji, K.; Itobayashi, E.; Kariyama, K.; Ishikawa, T.; Tajiri, K.; Ochi, H.; et al. Albumin-bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan Society of Hepatology: A comparison with the liver damage and Child-Pugh classifications. Liver Cancer 2017, 6, 204–215. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.S.; Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 28 December 2020).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A. Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017, 23, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Okajima, H.; Uemoto, S. Proposal for new selection criteria considering pre-transplant muscularity and visceral adiposity in living donor liver transplantation. J. Cachexia Sarcopenia Muscle 2018, 9, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Fujimoto, Y.; Ogawa, K.; Mori, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014, 20, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Rosa-caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex. Differ. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef]
- Kim, M.; Shinkai, S. Prevalence of muscle weakness based on different diagnostic criteria in community-dwelling older adults: A comparison of grip strength dynamometers. Geriatr. Gerontol. Int. 2017, 17, 2089–2095. [Google Scholar] [CrossRef]
- Graffy, P.M.; Liu, J.; Pickhardt, P.J.; Burns, J.E.; Yao, J.; Summers, R.M. Deep learning-based muscle segmentation and quantifcation at abdominal CT: Application to a longitudinal adult screening cohort for sarcopenia assessment. Br. J. Radiol. 2019, 92, 20190237. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ma, J.; Li, L.; Zhu, X.-d. Severe muscle loss during radical chemoradiotherapy for non-metastatic nasopharyngeal carcinoma predicts poor survival. Cancer Med. 2019, 8, 6604–6613. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Saeki, H.; Hu, Q.; Tsuda, Y.; Zaitsu, Y.; Hisamatsu, Y.; Ando, K.; Kimura, Y.; Oki, E.; Mori, M. Skeletal muscle loss after esophagectomy is an independent risk factor for patients with esophageal cancer. Ann. Surg. Oncol. 2020, 27, 492–498. [Google Scholar] [CrossRef]
- Tsukagoshi, M.; Yokobori, T.; Yajima, T.; Maeno, T.; Shimizu, K.; Mogi, A.; Araki, K.; Harimoto, N.; Shirabe, K.; Kaira, K. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Medicine 2020, 99, 7. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef]
- Looijaard, S.M.; te Lintel Hekkert, M.L.; Wüst, R.C.; Otten, R.H.; Meskers, C.G.; Maier, A.B. Pathophysiological mechanisms explaining poor clinical outcome of older cancer patients with low skeletal muscle mass. Acta Physiol. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshida, D.; Honda, T.; Hata, J.; Shibata, M.; Hirakawa, Y.; Furuta, Y.; Kishimoto, H.; Ohara, T.; Kitazono, T.; et al. Prevalence and mortality of sarcopenia in a community-dwelling older Japanese population: The Hisayama Study. J. Epidemiol. 2020, JE20190289. [Google Scholar] [CrossRef]
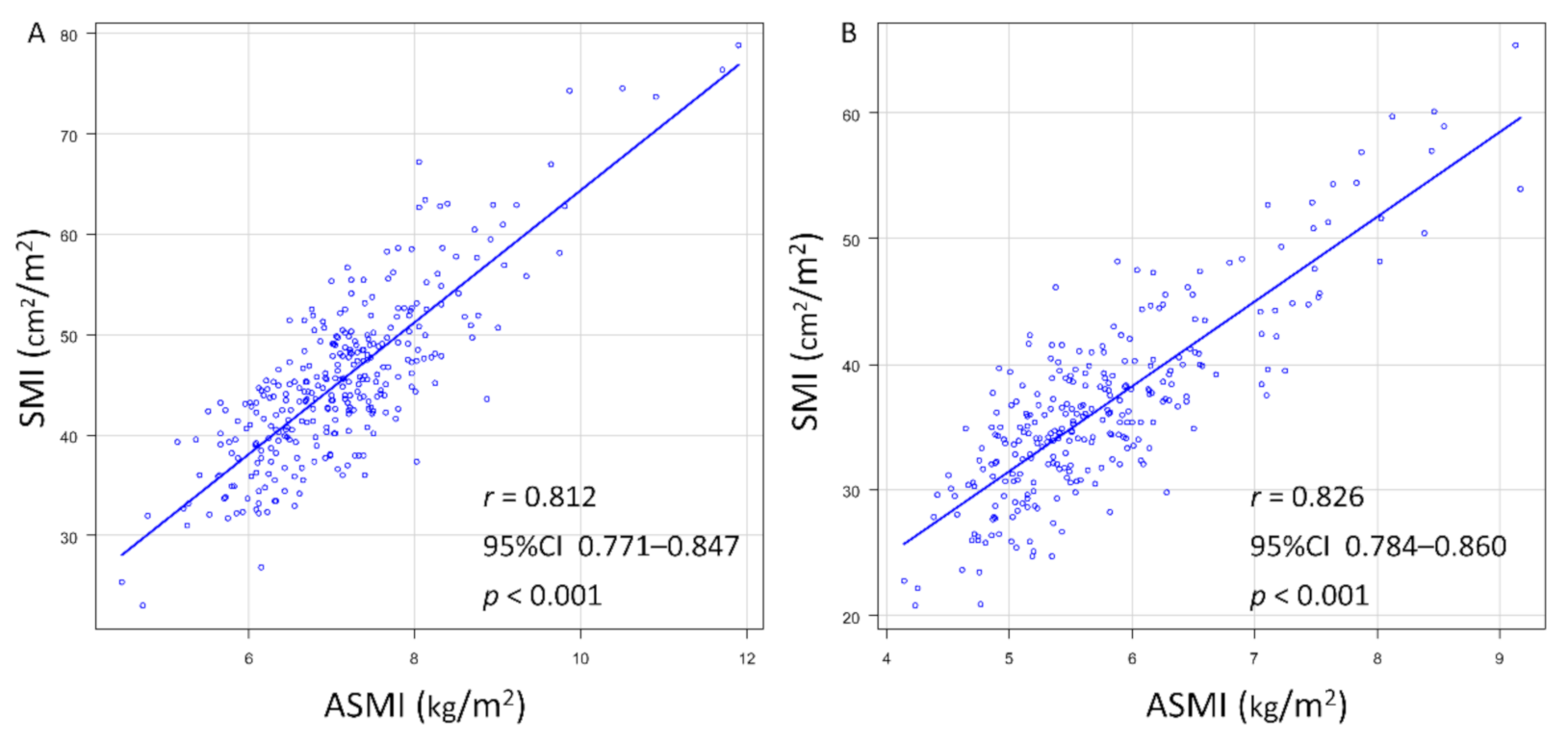
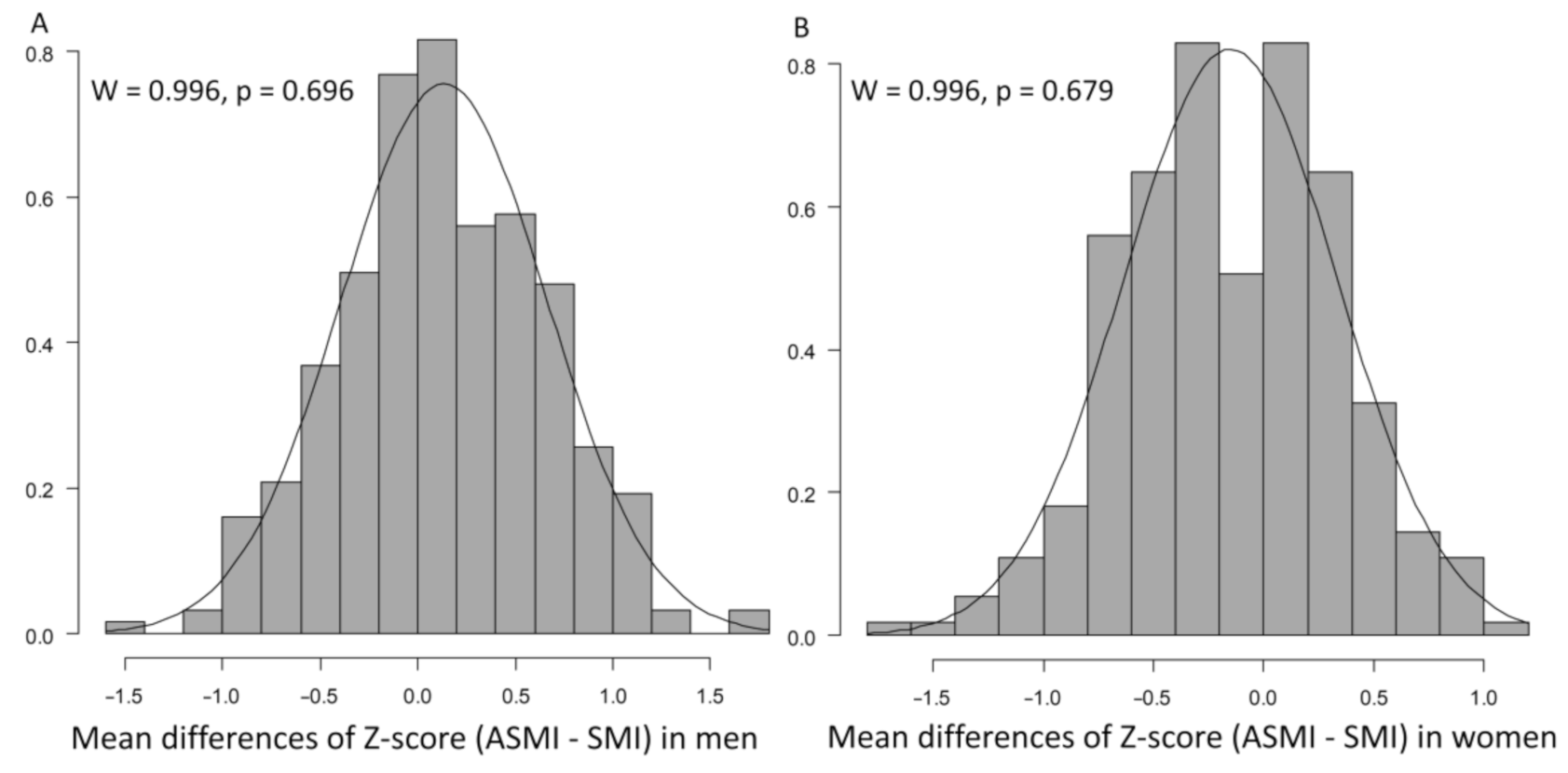
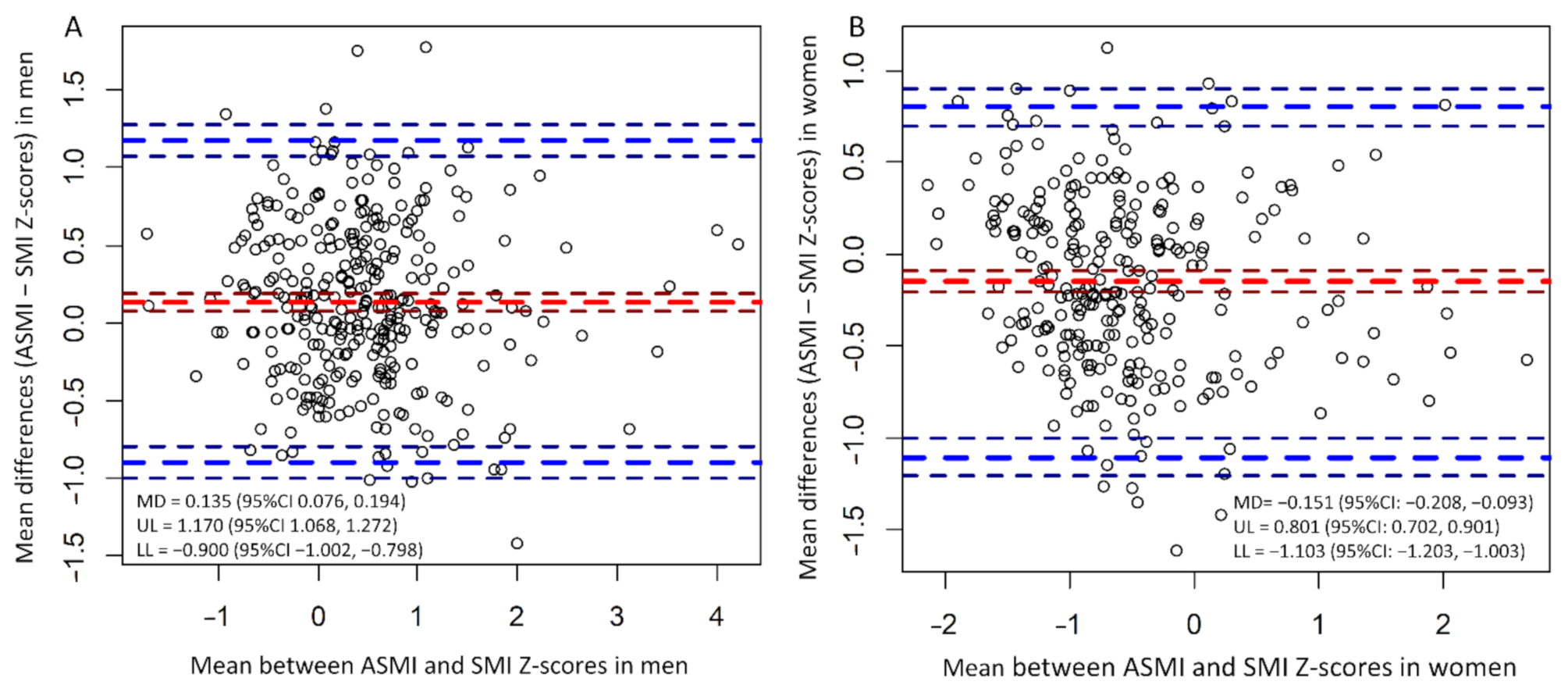
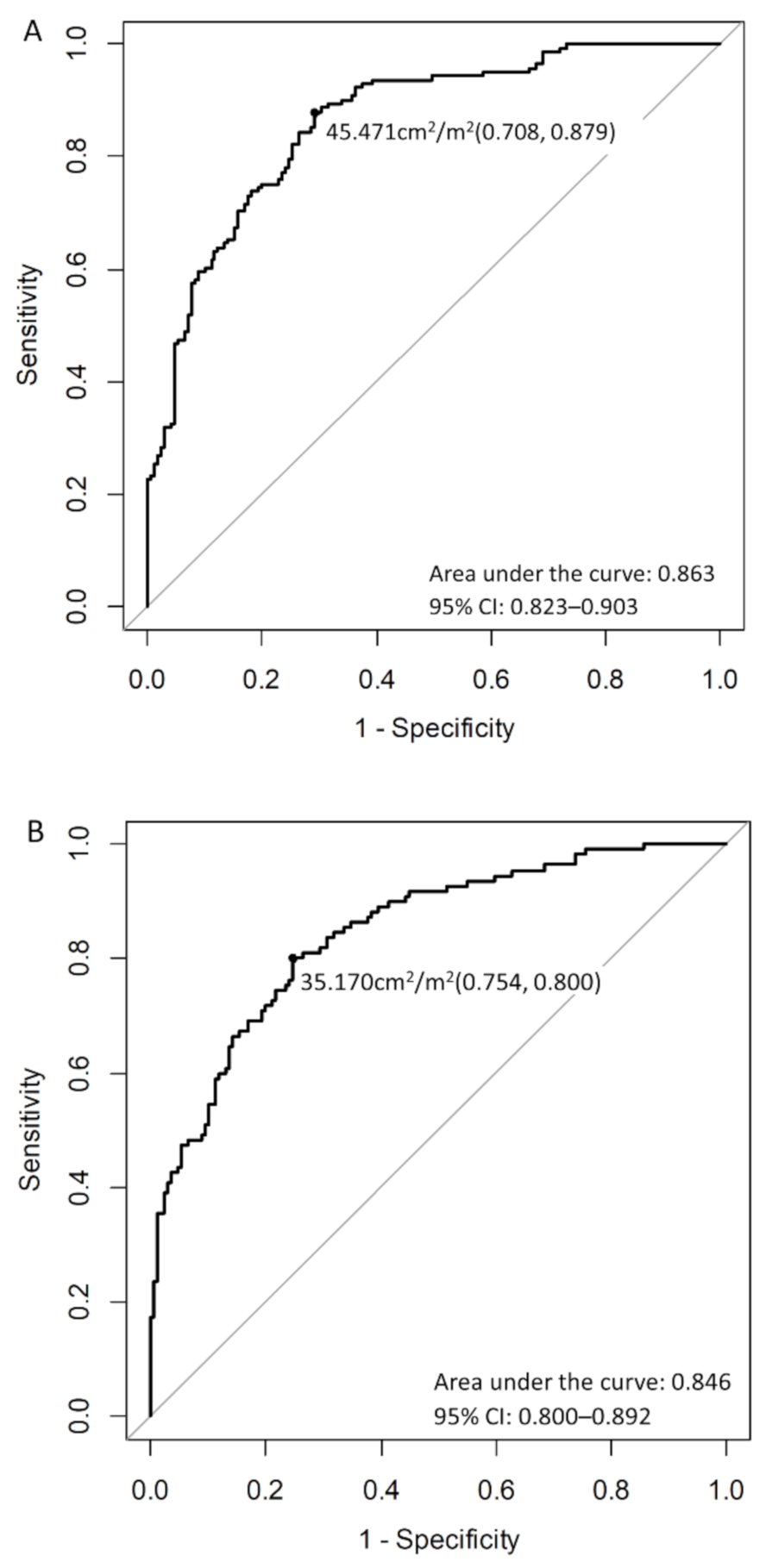
| Factor | All Patients, n = 589 | Men, n = 312 | Women, n = 277 | p-Value |
|---|---|---|---|---|
| Age, years | 64.60 (13.35) | 63.15 (13.69) | 66.22 (12.79) | 0.005 |
| Body mass index, kg/m2 | 23.33 (3.90) | 23.62 (3.71) | 23.00 (4.10) | 0.057 |
| Hepatocellular carcinoma, yes | 123 (20.9%) | 90 (28.8%) | 33 (11.9%) | <0.001 |
| Liver cirrhosis, yes | 164 (27.8%) | 97 (45.1%) | 67 (24.2%) | 0.066 |
| Presarcopenia (by AWGS), yes | 251 (42.6%) | 141 (45.2%) | 110 (39.7%) | 0.183 |
| Presarcopenia (by JSH), yes | 278 (47.2%) | 98 (31.4%) | 180 (65.0%) | <0.001 |
| Skeletal muscle mass index by CT, cm2/m2 | 41.21 (8.94) | 45.41(8.22) | 36.47(7.18) | <0.001 |
| Skeletal muscle mass index by DXA, cm2/m2 | 6.47 (1.17) | 7.11(1.02) | 5.74(0.88) | <0.001 |
| Whole-body bone mineral density, g/cm2 | 1.00 (0.14) | 1.07 (0.12) | 0.92 (0.12) | <0.001 |
| Etiology (Virus/Alcohol/NASH/others) | 313/55/116/54/51 | 176/48/57/31 | 137/7/59/74 | <0.001 |
| ALBI score | −2.77 (0.43) | −2.77 (0.45) | −2.77 (0.42) | 0.908 |
| ALBI grade (1/2/3) | 437/146/6 | 226/82/4 | 211/64/2 | 0.518 |
| FIB-4 index | 2.80 (2.62) | 2.55 (1.94) | 3.07 (3.20) | 0.015 |
| Platelet counts, ×104/μL | 19.56 (7.99) | 19.49 (8.55) | 19.64 (7.33) | 0.814 |
| Serum albumin, g/dL | 4.06 (0.47) | 4.07 (0.50) | 4.05 (0.41) | 0.593 |
| Total bilirubin, mg/dL | 1.09(8.59) | 0.75(0.52) | 1.48(12.51) | 0.298 |
| Aspartate aminotransferase, U/L | 46.18 (106.20) | 38.22 (44.51) | 55.04 (147.13) | 0.055 |
| Alanine aminotransferase, U/L | 46.02 (115.30) | 39.19 (47.38) | 53.71 (160.25) | 0.127 |
| Men (Our Result) | Men (JSH) | Women (Our Result) | Women (JSH) | |
|---|---|---|---|---|
| Prevalence (95% CI) | 0.558 (0.501–0.614) | 0.314 (0.263–0.369) | 0.466 (0.406–0.526) | 0.650 (0.590–0.706) |
| kappa (95% CI) | 0.575 (0.485–0.665) | 0.515 (0.417–0.612) | 0.539 (0.438–0.639) | 0.402 (0.298–0.505) |
| Sensitivity (95% CI) | 0.879 (0.814–0.928) | 0.589 (0.503–0.671) | 0.800 (0.713–0.870) | 0.918 (0.850–0.962) |
| Specificity (95% CI) | 0.708 (0.633–0.775) | 0.912 (0.859–0.950) | 0.754 (0.682–0.818) | 0.527 (0.448–0.605) |
| PPV (95% CI) | 0.713 (0.639–0.779) | 0.847 (0.760–0.912) | 0.682 (0.594–0.761) | 0.561 (0.485–0.635) |
| NPV (95% CI) | 0.877 (0.810–0.927) | 0.729 (0.664–0.787) | 0.851 (0.784–0.904) | 0.907 (0.831–0.957) |
| PLR (95% CI) | 3.008 (2.363–3.827) | 6.711 (4.059–11.093) | 3.259 (2.458–4.319) | 1.941 (1.638–2.300) |
| NLR (95% CI) | 0.170 (0.108–0.269) | 0.451 (0.368–0.552) | 0.265 (0.181–0.389) | 0.155 (0.082–0.295) |
| Accuracy (95% CI) | 0.785 (0.735–0.830) | 0.766 (0.715–0.812) | 0.773 (0.719–0.821) | 0.682 (0.624–0.737) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohashi, K.; Ishikawa, T.; Hoshii, A.; Hokari, T.; Noguchi, H.; Suzuki, M.; Hirosawa, H.; Imai, M.; Mitobe, Y.; Yoshida, T. Optimal Skeletal Muscle Mass Index Cut-Off Values for Presarcopenia Evaluated by Computed Tomography against Dual-Energy X-ray Absorptiometry in Patients with Chronic Liver Disease. J. Clin. Med. 2021, 10, 1419. https://doi.org/10.3390/jcm10071419
Ohashi K, Ishikawa T, Hoshii A, Hokari T, Noguchi H, Suzuki M, Hirosawa H, Imai M, Mitobe Y, Yoshida T. Optimal Skeletal Muscle Mass Index Cut-Off Values for Presarcopenia Evaluated by Computed Tomography against Dual-Energy X-ray Absorptiometry in Patients with Chronic Liver Disease. Journal of Clinical Medicine. 2021; 10(7):1419. https://doi.org/10.3390/jcm10071419
Chicago/Turabian StyleOhashi, Kazuki, Toru Ishikawa, Asami Hoshii, Tamaki Hokari, Hirohito Noguchi, Mitsuyuki Suzuki, Hiroshi Hirosawa, Michitaka Imai, Yuta Mitobe, and Toshiaki Yoshida. 2021. "Optimal Skeletal Muscle Mass Index Cut-Off Values for Presarcopenia Evaluated by Computed Tomography against Dual-Energy X-ray Absorptiometry in Patients with Chronic Liver Disease" Journal of Clinical Medicine 10, no. 7: 1419. https://doi.org/10.3390/jcm10071419
APA StyleOhashi, K., Ishikawa, T., Hoshii, A., Hokari, T., Noguchi, H., Suzuki, M., Hirosawa, H., Imai, M., Mitobe, Y., & Yoshida, T. (2021). Optimal Skeletal Muscle Mass Index Cut-Off Values for Presarcopenia Evaluated by Computed Tomography against Dual-Energy X-ray Absorptiometry in Patients with Chronic Liver Disease. Journal of Clinical Medicine, 10(7), 1419. https://doi.org/10.3390/jcm10071419






