Effects of Different Dialysis Strategies on Inflammatory Cytokine Profile in Maintenance Hemodialysis Patients with COVID-19: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Cytokine Determinations
2.3. Statistical Analysis
3. Results
3.1. Clinical Presentation, Radiological Findings and Outcome of COVID-19 in HD Patients
3.2. Basal Laboratory Characteristics
3.3. Effects of Dialysis Treatment on Cytokine Removal
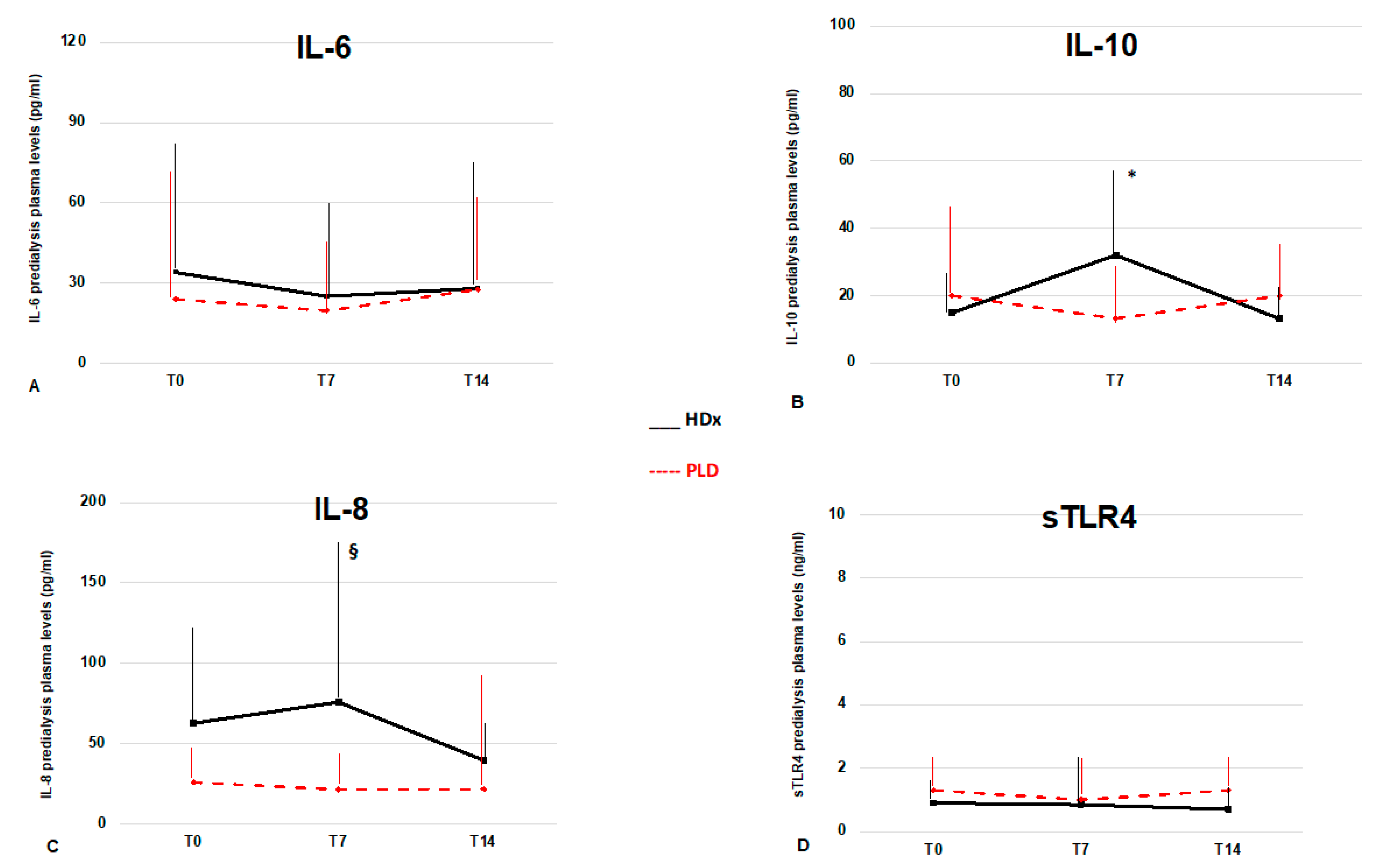
3.4. Longitudinal Cytokine Profile According to Dialysis Modalities
3.5. Cytokine Levels and Clinical Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Vena, A.; Giacobbe, D.R.; Di Biagio, A.; Mikulska, M.; Taramasso, L.; De Maria, A.; Ball, L.; Brunetti, I.; Loconte, M.; Patroniti, N.A.; et al. GECOVID study group. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin. Microbiol. Infect. 2020, 26, 1537–1544. [Google Scholar]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Rampino, T.; Gregorini, M.; Perotti, L.; Ferrari, F.; Pattonieri, E.F.; Grignano, M.A.; Valente, M.; Garrone, A.; Islam, T.; Libetta, C.; et al. Hemoperfusion with CytoSorb as Adjuvant Therapy in Critically Ill Patients with SARS-CoV2 Pneumonia. Blood Purif. 2020, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Maintenance hemodialysis and Coronavirus Disease 2019 (COVID-19): Saving lives with caution, care, and courage. Kidney Med. 2020, 2, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Esposito, P.; Taramasso, L.; Magnasco, L.; Saio, M.; Briano, F.; Russo, C.; Dettori, S.; Vena, A.; Di Biagio, A.; et al. GECOVID study group. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J. Nephrol. 2020, 6, 1–11. [Google Scholar]
- Gansevoort, R.T.; Hilbrands, L.B. CKD is a key risk factor for COVID-19 mortality. Nat. Rev. Nephrol. 2020, 16, 705–706. [Google Scholar]
- Ma, Y.; Diao, B.; Lv, X.; Zhu, J.; Chen, C.; Liu, L.; Zhang, S.; Shen, B.; Wang, H. Epidemiological, Clinical, and Immunological Features of a Cluster of COVID-19-Contracted Hemodialysis Patients. Kidney Int. Rep. 2020, 5, 1333–1341. [Google Scholar]
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef]
- Derosa, G.; Libetta, C.; Esposito, P.; Borettaz, I.; Tinelli, C.; D’angelo, A.; Maffioli, P. Effects of two different dialytic treatments on inflammatory markers in people with end-stage renal disease with and without type 2 diabetes mellitus. Cytokine 2017, 92, 75–79. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Pindel, M.; Pietruczuk, K.; Kuźmiuk-Glembin, I.; Storoniak, H.; Dębska-Ślizień, A.; Witkowski, J.M. The influence of a single hemodialysis procedure on human T lymphocytes. Sci. Rep. 2019, 9, 5041. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C. The rise of expanded hemodialysis. Blood Purif. 2017, 44, I–VIII. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Magagnoli, L.; Ciceri, P.; Conte, F.; Galassi, A. Effects of a medium cut-off (Theranova) dialyser on haemodialysis patients: A prospective, cross-over study. Clin. Kidney J. 2019, 14, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Benedetti, S.; Floridi, A.; Canestrari, F.; Piroddi, M.; Buoncristiani, E.; Buoncristiani, U. Glycoxidation and inflammatory markers in patients on treatment with PMMA-based protein-leaking dialyzers. Kidney Int. 2005, 67, 750–759. [Google Scholar] [CrossRef]
- Korevaar, J.C.; van Manen, J.G.; Dekker, F.W.; de Waart, D.R.; Boeschoten, E.W.; Krediet, R.T.; NECOSAD Study Group. Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. J. Am. Soc. Nephrol. 2004, 15, 2916–2922. [Google Scholar] [CrossRef]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020, 189, 428–437. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, H.; Chen, H.; Qi, W.; Chen, L.; Chen, G.; Yan, W.; Chen, T.; Ning, Q.; Han, M.; et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit. Care. 2020, 24, 525. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Ng, T.H.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef]
- Brandão, S.C.S.; Ramos, J.O.X.; Dompieri, L.T.; Godoi, E.T.A.M.; Figueiredo, J.L.; Sarinho, E.S.C.; Chelvanambi, S.; Aikawa, M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2020, S1359-6101, 30205–30207. [Google Scholar]
- Esposito, P.; La Porta, E.; Grignano, M.A.; Verzola, D.; Milanesi, S.; Ansaldo, F.; Gregorini, M.; Libetta, C.; Garibotto, G.; Rampino, T. Soluble Toll-like Receptor 4: A New Player in Subclinical Inflammation and Malnutrition in Hemodialysis Patients. J. Ren. Nutr. 2018, 28, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, J.K.; Tan, J.T.; Whitton, J.L. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005, 201, 1053–1059. [Google Scholar] [CrossRef]
- Sepe, V.; Gregorini, M.; Rampino, T.; Esposito, P.; Coppo, R.; Galli, F.; Libetta, C. Vitamin e-loaded membrane dialyzers reduce hemodialysis inflammaging. BMC Nephrol. 2019, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transpl. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef]
- Betjes, M.G.; Langerak, A.W.; van der Spek, A.; de Wit, E.A.; Litjens, N.H. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011, 80, 208–217. [Google Scholar] [CrossRef]
- Libetta, C.; Esposito, P.; Sepe, V.; Portalupi, V.; Margiotta, E.; Canevari, M.; Dal Canton, A. Dialysis treatment and regulatory T cells. Nephrol. Dial. Transplant. 2010, 25, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, C.; Waldman, M.; Zaza, G.; Riella, L.V.; Cravedi, P. COVID-19 and the Kidneys: An Update. Front. Med. 2020, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Yalın, S.F.; Altıparmak, M.R.; Dincer, M.T.; Yadigar, S.; Murt, A.; Parmaksiz, E.; Ronco, C. Medium Cut-Off Dialysis Membranes: Can They Have Impact on Outcome of COVID-19 Hemodialysis Patients? Blood Purif. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Contin-Bordes, C.; Lacraz, A.; de Précigout, V. Potential role of the soluble form of CD40 in deficient immunological function of dialysis patients: New findings of its amelioration using polymethylmethacrylate (PMMA) membrane. NDT Plus. 2010, 3, i20–i27. [Google Scholar] [CrossRef] [PubMed]
- Tarakçioğlu, M.; Erbağci, A.B.; Usalan, C.; Deveci, R.; Kocabaş, R. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediat. Inflamm. 2003, 12, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.M.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; de Vries, H.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transpl. 2020, 35, 1973–1983. [Google Scholar] [CrossRef]
| COVID-19 HD | NO COVID-19 HD | p | |
|---|---|---|---|
| N | 31 | 14 | |
| Age, years | 68.9 ± 15.6 | 66.3 ± 15.2 | 0.5 |
| Sex, M/F | 17/14 | 9/5 | 0.5 |
| Dialysis vintage, months | 46 (56) | 28 (50) | 0.86 |
| Qb, mL/min | 292 ± 18 | 300 | 0.12 |
| Diabetes, N (%) | 7 (27) | 5 (35) | 0.71 |
| Hypertension, N (%) | 21 (77) | 10 (70) | 0.7 |
| CVD disease, N (%) | 20 (72) | 11 (78) | 0.9 |
| WBC, ×109/L | 5.8 ± 3.6 | 7.9 ± 2.3 | 0.003 |
| Neutrophils, ×109/L | 4.5 ± 3.6 | 5.5 ± 2.3 | 0.04 |
| Lymphocytes, ×109/L | 0.7 ± 0.36 | 1.4 ± 0.6 | <0.0001 |
| N/L ratio | 5.5 (5) | 3.5 (3.6) | 0.2 |
| CRP, mg/L | 27.5 (45) | 7.25 (19.8) | 0.29 |
| Ferritin, µg/L | 500 (904) | 158 (165) | 0.001 |
| LDH, U/L | 238 ± 69 | 182.2 ± 40 | 0.01 |
| Fibrinogen, g/L | 4.8 (4.9) | 4.2 (3.1) | 0.2 |
| PCT, µg/L | 1.6 (4.3) | 0.6 (0.48) | 0.02 |
| CD3+CD4+, cells/µL | 283 ± 158 | 781 ± 378 | 0.001 |
| CD3+CD8+, cells/µL | 150 (133) | 356 (390) | 0.002 |
| CD19+, cells/µL | 59 (68) | 189 (189) | 0.15 |
| NK, cells/µL | 83(132) | 163 (131) | 0.02 |
| IL-6, pg/mL | 24 (43) | 12 (53) | 0.1 |
| IL-8, pg/mL | 35.1 (100) | 24.1 (234) | 0.9 |
| IL-10, pg/mL | 15.2 (12.5) | 1.2 (1.4) | <0.001 |
| sTLR4, ng/mL | 1 (1.1) | 1.1 (1.6) | 0.16 |
| HDx (n,15) | PLD (n,14) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-HD | Post-HD | p | RR | Pre-HD | Post-HD | p | RR | |
| IL-6, pg/mL | 34 (67) | 39.5 (39) | 0.5 | 0.1 (0.83) | 24 (51.5) | 27.4 (51.6) | 0.58 | −0.02 (0.49) |
| IL-8, pg/mL | 62.8 (59.5) | 18.5 (35.8) | <0.001 | 0.5 (0.46) | 26 (20) | 18.7 (10.3) | 0.001 | 0.28 (0.49) |
| IL-10, pg/mL | 14.9 (12) | 10.6 (8.6) | 0.03 | 0.29 (0.54) | 20 (17.6) | 9 (5.1) | 0.1 | 0.27 (0.4) |
| sTLR4, ng/mL, | 0.9 (0.7) | 0.92 (1.4) | 0.6 | 0.06 (0.5) | 1.3 (1.1) | 0.88 (0.8) | 0.2 | 0.15 (0.9) |
| Albumin, g/dL | 3.3 ± 0.5 | 3.6 ± 0.6 | <0.01 | −0.06 (0.036) | 3.4 ± 0.3 | 3.8 ± 0.4 | <0.01 | −0.1 (0.05) |
| HDx | PLD | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T7 | T14 | p | T0 | T7 | T14 | p | |
| N | 15 | 11 | 10 | 14 | 11 | 11 | ||
| WBC, × 109/L | 5.9 ± 3.3 | 5.8 ± 2.7 | 6.4 ± 2.6 | 0.9 | 6 ± 4.3 | 5.7 ± 1.5 | 5.5 ± 2.3 | 0.34 |
| Neutrophils, × 109/L | 4.7 ± 3.1 | 4.5 ± 2.6 | 4.1 ± 3 | 0.13 | 4.4 ± 4.3 | 4.2 ± 1.3 | 2.5 ± 1.5 | 0.09 |
| Lymphocytes, × 109/L | 0.6 ± 0.24 | 0.73 ± 0.26 | 0.63 ± 0.41 | 0.6 | 0.86 ± 0.4 | 0.85 ± 0.4 | 0.8 ± 0.4 | 0.7 |
| N/L ratio | 7.1 (7.1) | 5.2 (7.8) | 6.4 (7.9) | 0.7 | 4.6 (4.5) | 5.3 (3.2) | 3 (2.2) * | * 0.03 vs. T0 |
| CRP, mg/L | 15.3 (41.7) | 13.1 (19) | 11.4 (15.9) * | * 0.02 vs. T0,T7 | 24 (64) | 12.2 (15) | 24.5 (45) | 0.33 |
| Ferritin, µg/L | 638 (903) | 717 (629) | 433 (586) | 0.6 | 308 (1008) | 358 (1036) | 554 (2485) | 0.48 |
| LDH, U/L | 230 ± 75 | 193 ± 34 | 206 ± 41.8 | 0.2 | 253 ± 65 | 247 ± 70 | 283 ± 135 | 0.54 |
| PCT, µg/L | 1.8 (8.3) | 0.7 (2.7) | 0.72 (0.7) * | * 0.009 vs. T0 | 2 (3.7) | 1 (1.4) | 1.1 (1.45) | 0.5 |
| CD3+CD4+cells/µL | 282 ± 173 | 430 ± 133 | 410 ± 182 | 0.08 | 283 ± 147 | 478 ± 291 | 432 ± 222 | 0.04 |
| CD3+CD8+cells/µL | 160 ± 128 | 202 ± 81,8 | 202 ± 81,8 | 0.7 | 227 ± 113 | 326 ± 189 | 280 ± 117 | 0.24 |
| CD19+, cells/µL | 63.7 ± 37 | 47.5 ± 48.4 | 60.6 ± 41 | 0.38 | 51.4 ± 40 | 54.3 ± 39.7 | 56 ± 50 | 0.8 |
| NK, cells/µL | 87.5 (103.5) | 78 (218) | 117 (73) | 0.7 | 82 (645) | 127 192) | 148 (235) | 0.26 |
| IL-6, pg/mL | 34 (67) | 25 (47.5) | 28.1 (68.9) | 0.6 | 24 (51.5) | 19.6 (29.8) | 27.6 (32) | 0.4 |
| IL-8, pg/mL | 62.8 (59.5) | 76 (99) § | 39.6 (22.7) | 0.27 | 26 (20) | 21.5 (22) | 21.8 (69) | 0.6 |
| IL-10, pg/mL | 14.9 (12.4) | 32 (26.9) * | 13.1 (12.6) | * 0.04 vs. T0 | 20 (24) | 13.2 (17.8) | 19.9 (18.2) | 0.65 |
| sTLR4, ng/mL | 0.9 (0.7) | 0.86 (1.7) | 0.7 (0.75) | 0.48 | 1.3 (1.1) | 1 (1.3) | 1.3 (1.1) | 0.26 |
| IFN-γ, pg/mL | 0.1 (0.1) | 0.1 (0) | 0.1 (0) | - | 0.1 (0.05) | 0.1 (0) | 0.1 (0) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, P.; Cipriani, L.; Verzola, D.; Grignano, M.A.; De Amici, M.; Testa, G.; Grosjean, F.; Russo, E.; Garibotto, G.; Rampino, T.; et al. Effects of Different Dialysis Strategies on Inflammatory Cytokine Profile in Maintenance Hemodialysis Patients with COVID-19: A Randomized Trial. J. Clin. Med. 2021, 10, 1383. https://doi.org/10.3390/jcm10071383
Esposito P, Cipriani L, Verzola D, Grignano MA, De Amici M, Testa G, Grosjean F, Russo E, Garibotto G, Rampino T, et al. Effects of Different Dialysis Strategies on Inflammatory Cytokine Profile in Maintenance Hemodialysis Patients with COVID-19: A Randomized Trial. Journal of Clinical Medicine. 2021; 10(7):1383. https://doi.org/10.3390/jcm10071383
Chicago/Turabian StyleEsposito, Pasquale, Leda Cipriani, Daniela Verzola, Maria Antonietta Grignano, Mara De Amici, Giorgia Testa, Fabrizio Grosjean, Elisa Russo, Giacomo Garibotto, Teresa Rampino, and et al. 2021. "Effects of Different Dialysis Strategies on Inflammatory Cytokine Profile in Maintenance Hemodialysis Patients with COVID-19: A Randomized Trial" Journal of Clinical Medicine 10, no. 7: 1383. https://doi.org/10.3390/jcm10071383
APA StyleEsposito, P., Cipriani, L., Verzola, D., Grignano, M. A., De Amici, M., Testa, G., Grosjean, F., Russo, E., Garibotto, G., Rampino, T., & Viazzi, F. (2021). Effects of Different Dialysis Strategies on Inflammatory Cytokine Profile in Maintenance Hemodialysis Patients with COVID-19: A Randomized Trial. Journal of Clinical Medicine, 10(7), 1383. https://doi.org/10.3390/jcm10071383







