A Novel Combination of Accelerometry and Ecological Momentary Assessment for Post-Stroke Paretic Arm/Hand Use: Feasibility and Validity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Instruments
2.4. Procedure
2.5. Data Analysis—Accelerometry
2.6. Outcome Measures
2.6.1. Aim 1: Feasibility
- Accelerometer wearing time: We calculated each participants’ overall and daily accelerometer wearing time to rule out novelty or fatigue effects during the 5 days. If the participant showed “no movement” for any 3-h segment (i.e., the activity counts in every epoch within the 3-h period were <2), we considered that as non-wearing time [8]. Accelerometry data were included in further analyses only when there was a clear indication that both accelerometers were worn.
- EMA response rates: Participants received a total of 30 scheduled prompts (6×/day for 5 days) and could self-trigger a prompt anytime during participation. Duplicate prompts were defined when (1) the time interval between 2 adjacent prompts was less than 1 min and (2) the responses of the 2 prompts were the same on the close-ended EMA questions. After removing duplicate prompts, the overall response rate and the response rate by day were calculated for each participant and then averaged across participants for both scheduled and self-triggered prompts.
- Participants’ feedback: During the exit interview, participants were asked whether they experienced any problems or technical issues with either device (open-ended questions). Their responses were noted and summarized by the experimenter (Y.C.). Specific to EMA, we asked three 7-point Likert-type questions: (a) “To what extent, did you find responding to the survey questions easy to do?” (1 = not easy at all; 4 = neutral; 7 = very easy), (b) “Generally, what do you think of the total number of prompts you got?” (1 = too many prompts; 4 = about right; 7 = need more prompts to reflect the activities I did), and (c) “To what extent, did you find the prompts on the smartphone disruptive in your day?” (1 = not disruptive at all; 4 = neutral; 7 = very disruptive). Participants were also encouraged to share feedback or comments, such as the reasons for missing prompts and their use of the self-trigger function.
2.6.2. Aim 2: Validity
- EMA response bias: We evaluated whether the likelihood of responding to EMA prompts was related to participants’ arm/hand use behavior. We compared the time metrics (i.e., TimeR, TimeL, and TimeB) within the before-prompt 10-min window between answered and unanswered scheduled prompts. If, for example, no response to prompts was associated with lower levels of arm/hand movement, it would suggest that participants might have dismissed the EMA prompt due to social desirability to avoid answering “no use” when they did not use their arm/hand. Similarly, the time metrics within the before-prompt 10-min window were also compared between the answered scheduled prompts and the self-triggered prompts to evaluate whether participants tended to have higher arm/hand use levels prior to a self-triggered EMA prompt.
- EMA measurement reactivity: We examined the immediate and accumulative effects of EMA measurement reactivity. To determine the immediate effect, we compared the arm/hand movements before and after each EMA prompt. A significant difference of the time metrics between before- and after-prompts would suggest that the act of monitoring behavior itself may have influenced arm/hand use behavior. The accumulative effect was examined by comparing the time metrics of each day across the 5 days. To facilitate comparison across participants, the daily time metrics were normalized by each participant’s daily wearing time and converted to a percentage.
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Aim 1: Feasibility
3.2.1. Accelerometer Wearing Time
3.2.2. EMA Response Rates
3.2.3. Participants’ Feedback
- “The [self-triggered] function is a great idea. I know I can make up some … so I didn’t feel too worried when I missed a survey.”
- “I think it is great. I can make sure that I did at least 30 prompts during the week.”
- “It is really hard to follow the [prompt] schedule for an active person like me. … I usually enter one [prompt] when I have time.”
3.3. Aim 2: Validity
3.3.1. EMA Response Bias
3.3.2. EMA Measurement Reactivity
4. Discussion
4.1. High Feasibility of the Combined Methodology
4.2. The Advantages of EMA Self-Triggered Function
4.3. No EMA Response Bias Associated with Arm/Hand Movement
4.4. Unexpected Accumulative EMA Measurement Reactivity: Increased Right (Paretic) Arm/Hand Movement over Days
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
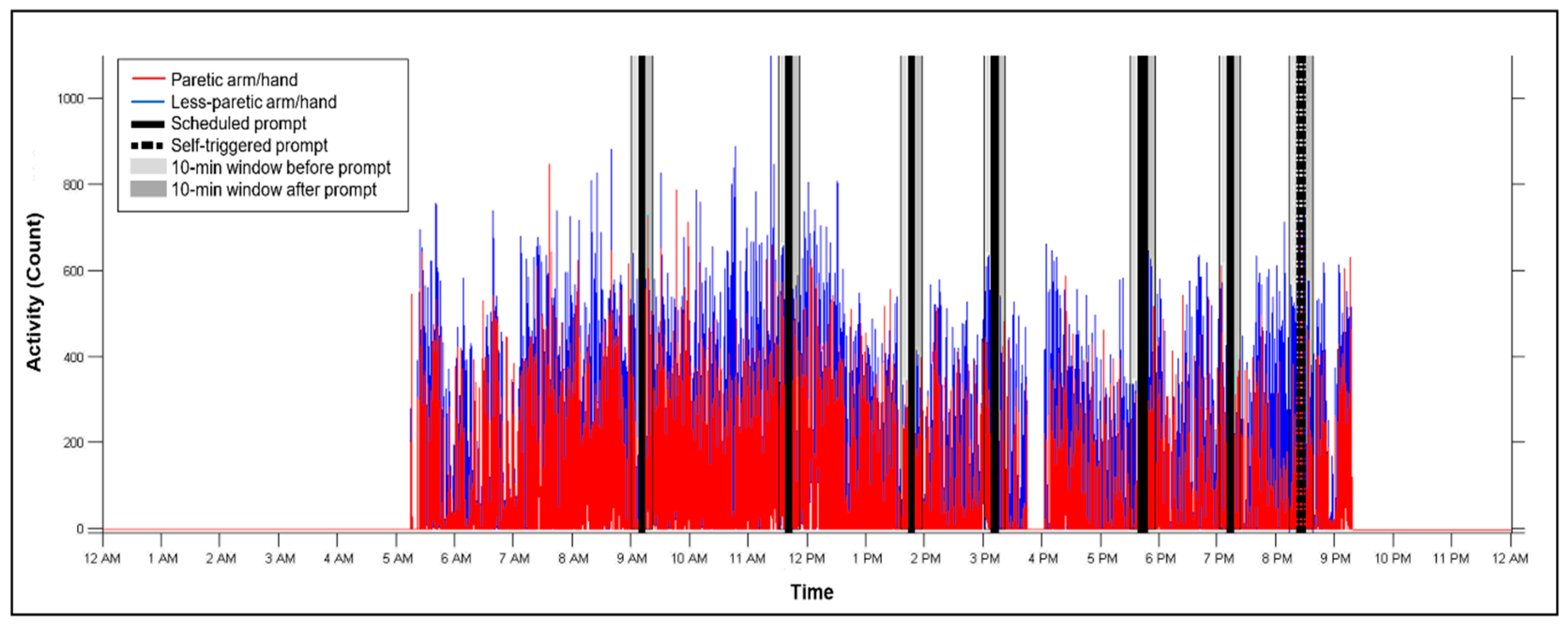
Appendix B
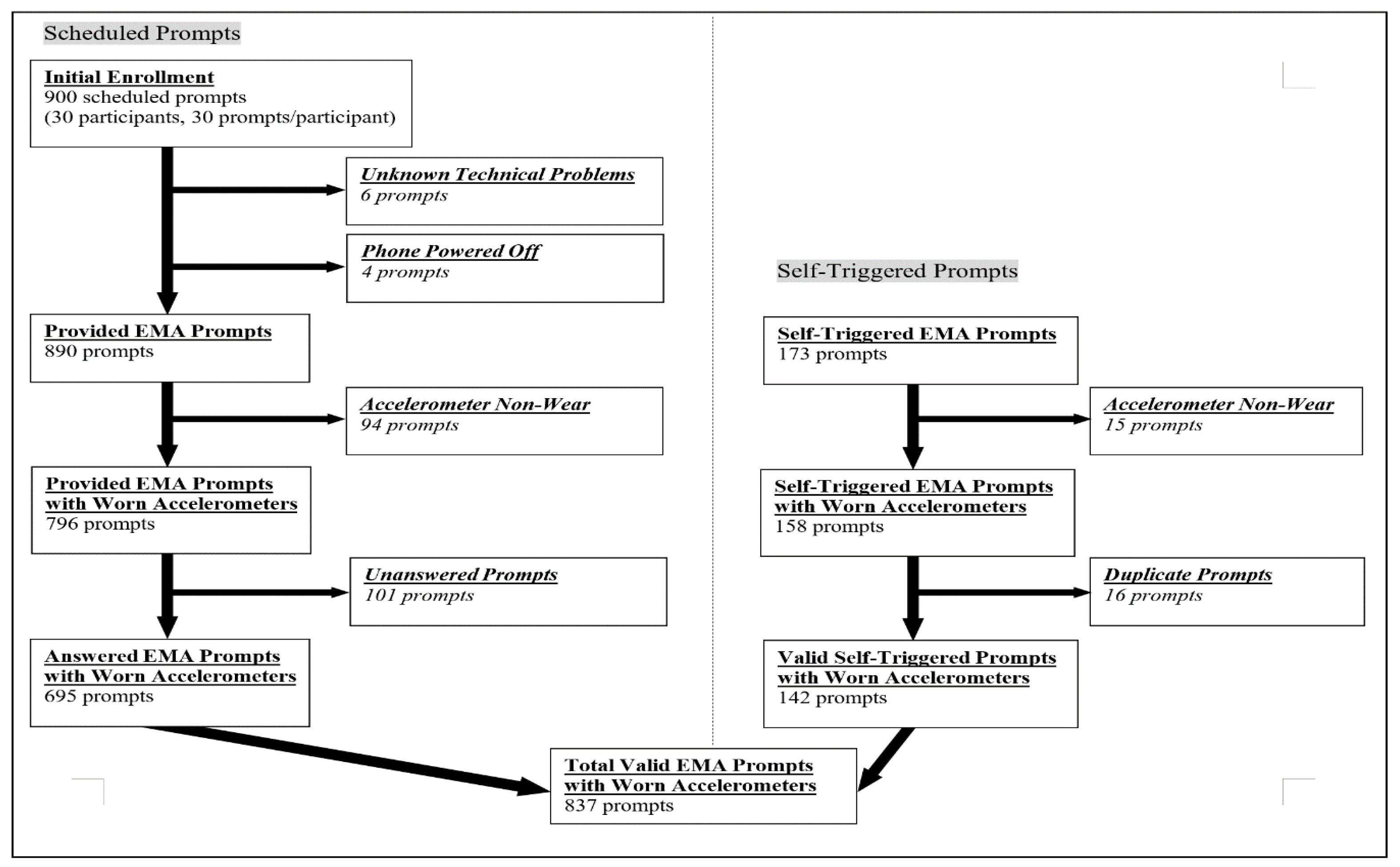
References
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Taub, E.; Uswatte, G.; Morris, D.; Giuliani, C.; Light, K.E.; Nichols-Larsen, D. Effect of constraint-induced movement on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA 2006, 296, 2095–2104. [Google Scholar] [CrossRef]
- Hidaka, Y.; Han, C.E.; Wolf, S.L.; Winstein, C.J.; Schweighofer, N. Use it and improve it or lose it: Interactions between arm function and use in humans post-stroke. PLoS Comput. Biol. 2012, 8, e1002343. [Google Scholar] [CrossRef]
- Waddell, K.J.; Strube, M.J.; Bailey, R.R.; Klaesner, J.W.; Birkenmeier, R.L.; Dromerick, A.W.; Lang, C.E. Does task-specific training improve upper limb performance in daily life poststroke? NNR 2017, 31, 290–300. [Google Scholar] [CrossRef]
- Andrews, K.; Stewart, J. Stroke recovery: He can but does he? Rheumatol. Rehabil. 1979, 18, 43–48. [Google Scholar] [CrossRef]
- Stewart, J.C.; Cramer, S.C. Patient-reported measures provide unique insights into motor function after stroke. Stroke 2013, 44, 1111–1116. [Google Scholar] [CrossRef]
- Chen, H.L.; Lin, K.C.; Hsieh, Y.W.; Wu, C.Y.; Liing, R.J.; Chen, C.L. A study of predictive validity, responsiveness, and minimal clinically important difference of arm accelerometer in real-world activity of patients with chronic stroke. Clin. Rehabil. 2018, 32, 75–83. [Google Scholar] [CrossRef]
- Uswatte, G.; Foo, W.L.; Olmstead, H.; Lopez, K.; Holand, A.; Simms, L.B.; Uswatte, G.; Taub, E.; Uswatte, G.; Miltner, W.H.; et al. Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Giuliani, C.; Winstein, C.; Zeringue, A.; Hobbs, L.; Wolf, S.L.; Article, O. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the Extremity Constraint-Induced Therapy Evaluation trial. Arch. Phys. Med. Rehabil. 2006, 87, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. An accelerometry-based methodology for assessment of real-world bilateral upper extremity activity. PLoS ONE 2014, 9, e103135. [Google Scholar] [CrossRef]
- Bailey, R.R.; Klaesner, J.W.; Lang, C.E. Quantifying real-world upper-limb activity in nondisabled adults and adults with chronic stroke. NNR 2015, 29, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, R.J.M.; Janssen-Potten, Y.J.M.; Timmermans, A.A.A.; Smeets, R.J.E.M.; Seelen, H.A.M. Recognizing complex upper extremity activities using body worn sensors. PLoS ONE 2015, 10, e0118642. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.S.; Eng, J.J.; Boyd, L.A.; Lakhani, B.; Bernhardt, J.; Lang, C.E. Exploring the Role of Accelerometers in the Measurement of Real World Upper-Limb Use After Stroke. Brain Impair. 2016, 17, 16–33. [Google Scholar] [CrossRef]
- Wade, E.; Chen, C.; Winstein, C.J. Spectral analyses of wrist motion in individuals poststroke: The development of a performance measure with promise for unsupervised settings. NNR 2014, 28, 169–178. [Google Scholar] [CrossRef]
- Bochniewicz, E.M.; Emmer, G.; McLeod, A.; Barth, J.; Dromerick, A.W.; Lum, P. Measuring Functional Arm Movement after Stroke Using a Single Wrist-Worn Sensor and Machine Learning. J. Stroke Cerebrovasc. Dis. 2017, 26, 2880–2887. [Google Scholar] [CrossRef]
- van Kuppevelt, D.; Heywood, J.; Hamer, M.; Sabia, S.; Fitzsimons, E.; van Hees, V. Segmenting accelerometer data from daily life with unsupervised machine learning. PLoS ONE 2019, 14, e0208692. [Google Scholar]
- Lee, S.I.; Adans-Dester, C.P.; Grimaldi, M.; Dowling, A.V.; Horak, P.C.; Black-Schaffer, R.M.; Bonato, P.; Gwin, J.T. Enabling Stroke Rehabilitation in Home and Community Settings: A Wearable Sensor-Based Approach for Upper-Limb Motor Training. IEEE J. Transl. Eng. Health Med. 2018, 6, 2100411. [Google Scholar] [CrossRef] [PubMed]
- Kayes, N.M.; McPherson, K.M. Measuring what matters: Does ‘objectivity’ mean good science? Disabil. Rehabil. 2010, 32, 1011–1019. [Google Scholar] [CrossRef]
- Berges, I.M.; Seale, G.S.; Ostir, G.V. The role of positive affect on social participation following stroke. Disabil. Rehabil. 2012, 34, 2119–2123. [Google Scholar] [CrossRef]
- Torkia, C.; Best, K.L.; Miller, W.C.; Eng, J.J. Balance Confidence: A Predictor of Perceived Physical Function, Perceived Mobility, and Perceived Recovery 1 Year after Inpatient Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2016, 97, 1064–1071. [Google Scholar] [CrossRef]
- Caetano, L.C.G.; Pacheco, B.D.; Samora, G.A.R.; Teixeira-Salmela, L.F.; Scianni, A.A. Self-Efficacy to Engage in Physical Exercise and Walking Ability Best Predicted Exercise Adherence after Stroke. Stroke Res. Treat. 2020, 2020, 2957623. [Google Scholar] [CrossRef]
- Stone, A.A. The science of real-time data capture: Self-reports in health research. J. Epidemiol. Community Health 2008, 62, 471. [Google Scholar]
- Dunton, G.F.; Liao, Y.; Kawabata, K.; Intille, S. Momentary assessment of adults’ physical activity and sedentary behavior: Feasibility and validity. Front. Psychol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Liao, Y.; Intille, S.S.; Dunton, G.F. Using ecological momentary assessment to understand where and with whom adults’ physical and sedentary activity occur. Int. J. Behav. Med. 2015, 22, 51–61. [Google Scholar] [CrossRef]
- Johnson, E.I.; Sibon, I.; Renou, P.; Rouanet, F.; Allard, M.; Swendsen, J. Feasibility and validity of computerized ambulatory monitoring in stroke patients. Neurology 2009, 73, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Jean, F.A.; Swendsen, J.D.; Sibon, I.; Feher, K.; Husky, M. Daily life behaviors and depression risk following stroke: A preliminary study using ecological momentary assessment. J. Geriatr. Psychiatry Neurol. 2013, 26, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Villain, M.; Sibon, I.; Renou, P.; Poli, M.; Swendsen, J. Very early social support following mild stroke is associated with emotional and behavioral outcomes three months later. Clin. Rehabil. 2016, 31, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Scollon, C.N.; Chu, K.-P.; Diener, E. Experience sampling: Promises and pitfalls, strengths and weaknesses. J. Happiness Stud. 2003, 4, 5–34. [Google Scholar] [CrossRef]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Albert, M.L. A simple test of visual neglect. Neurology 1973, 23, 658–664. [Google Scholar] [CrossRef]
- Wampold, B.E.; Freund, R.D. Use of multiple regression in counseling psychology research: A flexible data-analytic strategy. J. Couns. Psychol. 1987, 34, 372–382. [Google Scholar] [CrossRef]
- Rinehart, J.K.; Singleton, R.D.; Adair, J.C.; Sadek, J.R.; Haaland, K.Y. Arm use after left or right hemiparesis is influenced by hand preference. Stroke 2009, 40, 545–550. [Google Scholar] [CrossRef]
- Harris, J.; Eng, J. Individuals with the dominant hand affected following stroke demonstrate less impairement than those with the nondominant hand affected. NNR 2006, 20, 380–389. [Google Scholar]
- McCombe Waller, S.; Whitall, J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clin. Rehabil. 2005, 19, 544–551. [Google Scholar] [CrossRef]
- ActiGraph Support Center. What Are Counts? 2016. Available online: https://actigraph.desk.com/customer/en/portal/articles/2515580-what-are-counts (accessed on 12 December 2020).
- Kanning, M.; Ebner-Priemer, U.; Schlicht, W. Using activity triggered e-diaries to reveal the associations between physical activity and affective states in older adult’s daily living. Int. J. Behav. Nutr. Phys. Act 2015, 12, 111. [Google Scholar] [CrossRef]
- Reichert, M.; Tost, H.; Reinhard, I.; Zipf, A.; Salize, H.-J.; Meyer-Lindenberg, A.; Ebner-Priemer, U.W. Within-subject associations between mood dimensions and non-exercise activity: An ambulatory assessment approach using repeated real-time and objective data. Front. Psychol. 2016, 7, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; McCombe Waller, S. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Penguin Books Ltd.: London, UK, 2009. [Google Scholar]



| ID | Gender | Age (Years) | Onset (Years) | MoCA | FMA | |
|---|---|---|---|---|---|---|
| 1 | Male | 65.3 | 8.1 | 26 | 61 | Mild |
| 2 | Male | 59.1 | 12.0 | 25 | 38 | Moderate |
| 3 | Male | 59.8 | 2.8 | 25 | 59 | Mild |
| 4 | Female | 63.6 | 9.0 | 19 | 30 | Moderate |
| 5 | Male | 31.8 | 1.5 | 21 | 66 | Mild |
| 6 | Male | 59.9 | 0.7 | 30 | 65 | Mild |
| 7 | Male | 61.2 | 4.6 | 25 | 58 | Mild |
| 8 | Male | 66.8 | 14.8 | 19 | 22 | Severe |
| 9 | Male | 60.0 | 1.2 | 25 | 63 | Mild |
| 10 | Male | 70.5 | 2.3 | 25 | 21 | Severe |
| 11 | Female | 24.4 | 1.7 | 29 | 48 | Mild |
| 12 | Male | 77.6 | 4.8 | 24 | 39 | Moderate |
| 13 | Male | 44.0 | 2.8 | 23 | 61 | Mild |
| 14 | Male | 72.3 | 1.9 | 23 | 66 | Mild |
| 15 | Male | 58.0 | 8.6 | 27 | 53 | Mild |
| 16 | Male | 75.6 | 5.9 | 20 | 53 | Mild |
| 17 | Male | 54.5 | 6.0 | 26 | 54 | Mild |
| 18 | Male | 61.7 | 10.6 | 26 | 52 | Mild |
| 19 | Female | 70.5 | 11.7 | 27 | 47 | Mild |
| 20 | Male | 79.4 | 2.1 | 23 | 57 | Mild |
| 21 | Male | 47.6 | 3.1 | 28 | 45 | Mild |
| 22 | Female | 57.6 | 2.8 | 27 | 55 | Mild |
| 23 | Female | 71.1 | 1.1 | 23 | 30 | Moderate |
| 24 | Female | 58.6 | 0.8 | 29 | 41 | Moderate |
| 25 | Female | 60.2 | 0.9 | 30 | 20 | Severe |
| 26 | Female | 73.0 | 3.8 | 19 | 23 | Severe |
| 27 | Male | 53.1 | 2.8 | 27 | 28 | Severe |
| 28 | Female | 68.7 | 2.8 | 21 | 40 | Moderate |
| 29 | Male | 82.9 | 1.7 | 30 | 62 | Mild |
| 30 | Male | 47.1 | 7.9 | 20 | 61 | Mild |
| Mean ± SD | 21M:9F | 61.2 ± 13.1 | 4.7 ± 3.9 | 24.7 ± 3.4 | 47.3 ± 14.9 | |
| (Range) | - | (24.4–82.9) | (0.7–14.8) | (19–30) | (20–66) | |
| Variables (Mean ± SD) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|---|---|---|---|---|
| Wearing time (hours) | 14.6 ± 3.2 | 14.0 ± 3.6 | 13.5 ± 3.1 | 13.2 ± 3.4 | 12.9 ± 4.3 |
| Time (%) # | - | - | - | - | - |
| TimeR | 4.7 ± 3.9 * | 6.0 ± 4.6 | 5.7 ± 4.3 ** | 6.4 ± 4.5 | 6.2 ± 4.5 |
| (38.5 min) & | (48.7 min) | (46.8 min) | (52.7 min) | (50.9 min) | |
| TimeL | 22.7 ± 9.9 | 21.7 ± 8.8 | 23.3 ± 9.6 | 21.8 ± 7.4 | 23.1 ± 9.8 |
| (3.1 h) | (3.0 h) | (3.2 h) | (3.0 h) | (3.2 h) | |
| TimeB | 30.3 ± 13.8 | 29.5 ± 13.0 | 30.1 ± 13.3 | 27.8 ± 13.3 | 28.2 ± 15.2 |
| (4.1 h) | (4.0 h) | (4.1 h) | (3.8 h) | (3.9 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Demers, M.; Lewthwaite, R.; Schweighofer, N.; Monterosso, J.R.; Fisher, B.E.; Winstein, C. A Novel Combination of Accelerometry and Ecological Momentary Assessment for Post-Stroke Paretic Arm/Hand Use: Feasibility and Validity. J. Clin. Med. 2021, 10, 1328. https://doi.org/10.3390/jcm10061328
Chen Y-A, Demers M, Lewthwaite R, Schweighofer N, Monterosso JR, Fisher BE, Winstein C. A Novel Combination of Accelerometry and Ecological Momentary Assessment for Post-Stroke Paretic Arm/Hand Use: Feasibility and Validity. Journal of Clinical Medicine. 2021; 10(6):1328. https://doi.org/10.3390/jcm10061328
Chicago/Turabian StyleChen, Yi-An, Marika Demers, Rebecca Lewthwaite, Nicolas Schweighofer, John R. Monterosso, Beth E. Fisher, and Carolee Winstein. 2021. "A Novel Combination of Accelerometry and Ecological Momentary Assessment for Post-Stroke Paretic Arm/Hand Use: Feasibility and Validity" Journal of Clinical Medicine 10, no. 6: 1328. https://doi.org/10.3390/jcm10061328
APA StyleChen, Y.-A., Demers, M., Lewthwaite, R., Schweighofer, N., Monterosso, J. R., Fisher, B. E., & Winstein, C. (2021). A Novel Combination of Accelerometry and Ecological Momentary Assessment for Post-Stroke Paretic Arm/Hand Use: Feasibility and Validity. Journal of Clinical Medicine, 10(6), 1328. https://doi.org/10.3390/jcm10061328








