Factors Associated with Increased Risk of Early Severe Neonatal Morbidity in Late Preterm and Early Term Infants
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
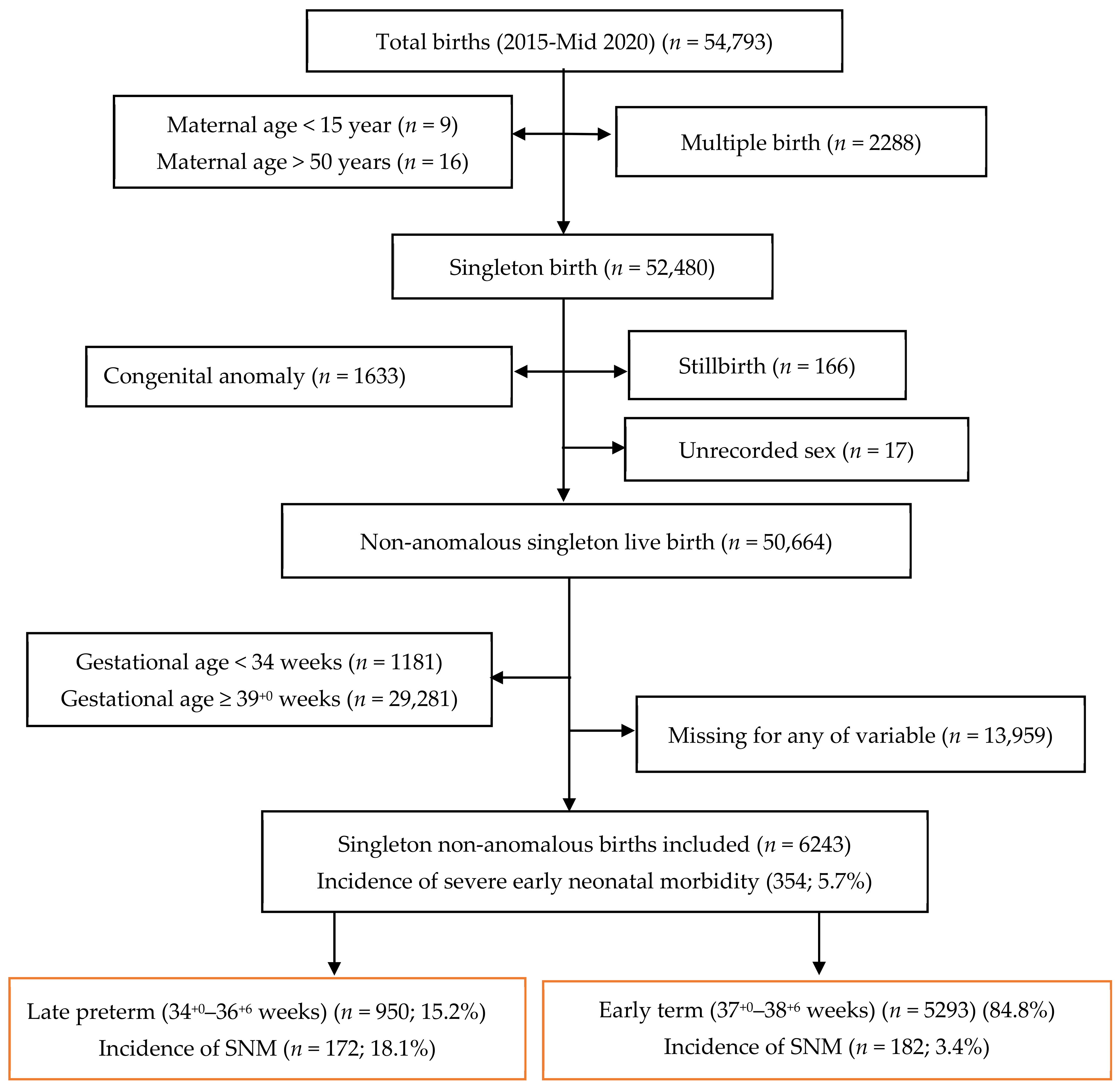
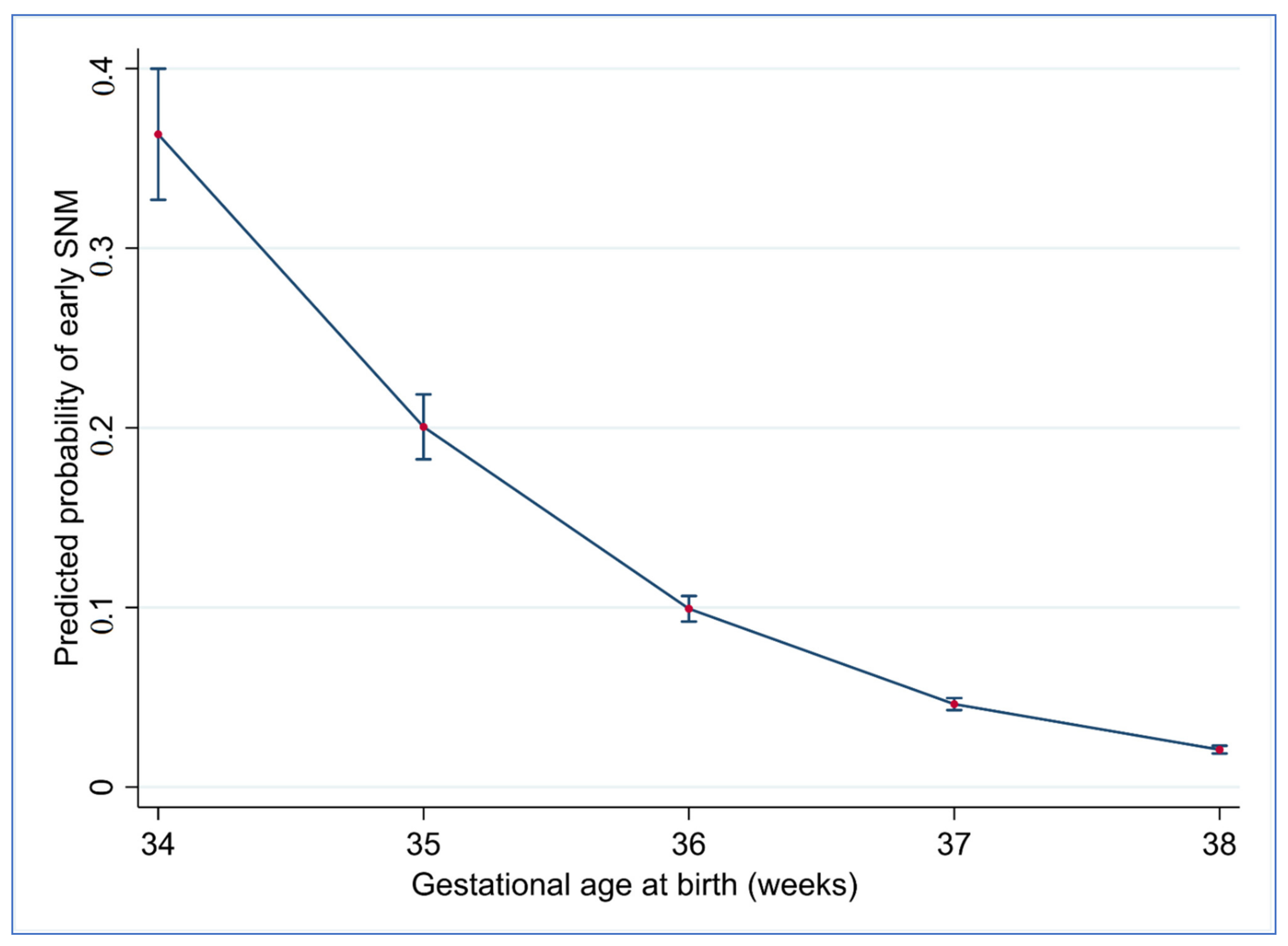
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016, 215, 103.e1–103.e14. [Google Scholar] [CrossRef]
- Beck, S.; Wojdyla, D.; Say, L.; Bertran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; van Look, P.F.A. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef]
- Souza, J.P.; Widmer, M.; Gülmezoglu, A.M.; Lawrie, T.A.; Adejuyigbe, E.A.; Carroli, G.; Crowther, C.; Currie, S.M.; Dowswell, T.; Hofmeyr, J.; et al. Maternal and perinatal health research priorities beyond 2015: An international survey and prioritization exercise. Reprod. Health 2014, 11, 61. [Google Scholar] [CrossRef]
- Brown, H.K.; Speechley, K.N.; Macnab, J.; Natale, R.; Campbell, M.K. Neonatal morbidity associated with late preterm and early term birth: The roles of gestational age and biological determinants of preterm birth. Int. J. Epidemiol. 2014, 43, 802–814. (In English) [Google Scholar] [CrossRef]
- Delnord, M.; Zeitlin, J. Epidemiology of late preterm and early term births—An international perspective. Semin. Fetal Neonatal Med. 2019, 24, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N. The “late preterm” birth—ten years later. Pediatrics 2017, 139, e20163331. [Google Scholar] [CrossRef]
- Engle, W.A. Morbidity and Mortality in Late Preterm and Early Term Newborns: A Continuum. Clin. Perinatol. 2011, 38, 493–516. (In English) [Google Scholar] [CrossRef]
- Bonamy, A.K.E.; Zeitlin, J.; Piedvache, A.; Maier, R.F.; van Heijst, A.; Varendi, H.; Manktelow, B.N.; Fenton, A.; Mazela, J.; Cuttini, M.; et al. Wide variation in severe neonatal morbidity among very preterm infants in European regions. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F36–F45. [Google Scholar] [CrossRef] [PubMed]
- Flatley, C.; Gibbons, K.; Hurst, C.; Flenady, V.; Kumar, S. Cross-validated prediction model for severe adverse neonatal outcomes in a term, non-anomalous, singleton cohort. BMJ Paediatr. Open 2019, 3, e000424. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. (In English) [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Loftin, R.W.; Habli, M.; Snyder, C.C.; Cormier, C.M.; Lewis, D.F.; DeFranco, E.A. Late Preterm Birth. Rev. Obstet. Gynecol. 2010, 3, 10–19. [Google Scholar] [PubMed]
- Verburg, P.E.; Dekker, G.A.; Venugopal, K.; Scheil, W.; Erwich, J.J.H.M.; Mol, B.W.; Roberts, C.T. Long-term Trends in Singleton Preterm Birth in South Australia from 1986 to 2014. Obstet. Gynecol. 2018, 131, 79–89. [Google Scholar] [CrossRef]
- Cheong, J.L.; Doyle, L.W. Increasing rates of prematurity and epidemiology of late preterm birth. J. Paediatr. Child Health 2012, 48, 784–788. (In English) [Google Scholar] [CrossRef]
- Pink, B. Information Paper: An Introduction to Socio-Economic Indexes for Areas (SEIFA), 2006; Australian Bureau of Statistics (ABS): Canberra, Australia, 2008.
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Courvoisier, D.S.; Combescure, C.; Agoritsas, T.; Gayet-Ageron, A.; Perneger, T.V. Performance of logistic regression modeling: Beyond the number of events per variable, the role of data structure. J. Clin. Epidemiol. 2011, 64, 993–1000. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wynants, L.; Bouwmeester, W.; Moons, K.; Moerbeek, M.; Timmerman, D.; van Huffel, S.; van Calster, B.; Vergouwe, Y. A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. J. Clin. Epidemiol. 2015, 68, 1406–1414. [Google Scholar] [CrossRef]
- Cui, J. QIC Program and Model Selection in GEE Analyses. Stata J. Promot. Commun. Stat. Stata 2007, 7, 209–220. [Google Scholar] [CrossRef]
- Madden, J.V.; Flatley, C.J.; Kumar, S. Term small-for-gestational-age infants from low-risk women are at significantly greater risk of adverse neonatal outcomes. Am. J. Obstet. Gynecol. 2018, 218, 525.e1–525.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lodge, J.; Flatley, C.; Kumar, S. The burden of adverse obstetric and perinatal outcomes from maternal smoking in an Australian cohort. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 356–361. [Google Scholar] [CrossRef]
- Hayashi, K.; Matsuda, Y.; Kawamichi, Y.; Shiozaki, A.; Saito, S. Smoking During Pregnancy Increases Risks of Various Obstetric Complications: A Case-Cohort Study of the Japan Perinatal Registry Network Database. J. Epidemiol. 2011, 21, 61–66. [Google Scholar] [CrossRef]
- Azuine, R.E.; Ji, Y.; Chang, H.-Y.; Kim, Y.; Ji, H.; DiBari, J.; Hong, X.; Wang, G.; Singh, G.K.; Pearson, C.; et al. Prenatal Risk Factors and Perinatal and Postnatal Outcomes Associated with Maternal Opioid Exposure in an Urban, Low-Income, Multiethnic US Population. JAMA Netw. Open 2019, 2, e196405. [Google Scholar] [CrossRef] [PubMed]
- Sarah, S.; Emma, W.; Hemant, A.; Theodore, D.; Anne, G. Maternal smoking and cannabis use during pregnancy and infant outcomes. J. Perinat. Med. 2020, 48, 168–172. (In English) [Google Scholar]
- Gibberd, A.J.; Simpson, J.M.; Jones, J.; Williams, R.; Stanley, F.; Eades, S.J. A large proportion of poor birth outcomes among Aboriginal Western Australians are attributable to smoking, alcohol and substance misuse, and assault. BMC Pregnancy Childbirth 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Broekhuijsen, K.; Ravelli, A.C.J.; Langenveld, J.; van Pampus, M.G.; Berg, P.P.V.D.; Mol, B.W.J.; Franssen, M.T.M. Maternal and neonatal outcomes of pregnancy in women with chronic hypertension: A retrospective analysis of a national register. Acta Obstet. Gynecol. Scand. 2015, 94, 1337–1345. (In English) [Google Scholar] [CrossRef]
- Clapp, M.A.; James, K.E.; Bates, S.V.; Kaimal, A.J. Patient and Hospital Factors Associated With Unexpected Newborn Complications Among Term Neonates in US Hospitals. JAMA Netw. Open 2020, 3, e1919498. [Google Scholar] [CrossRef]
- Hibbard, J.U.; Wilkins, I.; Sun, L.; Gregory, K.; Haberman, S.; Hoffman, M.; Kominiarek, M.A.; Reddy, U.; Bailit, J.; Consortium on Safe Labor; et al. Respiratory Morbidity in Late Preterm Births. JAMA 2010, 304, 419–425. [Google Scholar] [CrossRef][Green Version]
- Melamed, N.; Klinger, G.; Tenenbaum-Gavish, K.; Herscovici, T.; Linder, N.; Hod, M.; Yogev, Y. Short-term Neonatal Outcome in Low-Risk, Spontaneous, Singleton, Late Preterm Deliveries. Obstet. Gynecol. 2009, 114, 253–260. [Google Scholar] [CrossRef]
- Bastek, J.A.; Sammel, M.D.; Pare, E.; Srinivas, S.K.; Posencheg, M.A.; Elovitz, M.A. Adverse neonatal outcomes: Examining the risks between preterm, late preterm, and term infants. Am. J. Obstet. Gynecol. 2008, 199, 367.e1–367.e8. [Google Scholar] [CrossRef] [PubMed]
- Ventolini, G.; Neiger, R.; Mathews, L.; Adragna, N.; Belcastro, M. Incidence of Respiratory Disorders in Neonates Born between 34 and 36 Weeks of Gestation Following Exposure to Antenatal Corticosteroids between 24 and 34 Weeks of Gestation. Am. J. Perinatol. 2008, 25, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Reddy, U.M.; Ko, C.-W.; Raju, T.N.; Willinger, M. Delivery Indications at Late-Preterm Gestations and Infant Mortality Rates in the United States. Pediatrics 2009, 124, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Besser, L.; Sabag-Shaviv, L.; Yitshak-Sade, M.; Mastrolia, S.A.; Landau, D.; Beer-Weisel, R.; Klaitman, V.; Benshalom-Tirosh, N.; Mazor, M.; Erez, O. Medically indicated late preterm delivery and its impact on perinatal morbidity and mortality: A retrospective population-based cohort study. J. Matern. Neonatal Med. 2019, 32, 3278–3287. [Google Scholar] [CrossRef] [PubMed]
- Grace, R.; Greer, M.; Kumar, S. Perinatal consequences of a category 1 caesarean section at term. BMJ Open 2015, 5. [Google Scholar] [CrossRef]
- Thavarajah, H.; Flatley, C.; Kumar, S. The relationship between the five minute Apgar score, mode of birth and neonatal outcomes. J. Matern. Neonatal Med. 2018, 31, 1335–1341. [Google Scholar] [CrossRef]
- Prior, T.; Kumar, S. Mode of delivery has an independent impact on neonatal condition at birth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Carrion, V.; Shelton, J.; Wynn, R.J.; Ryan, R.M.; Singhal, K.; Lakshminrusimha, S. Adverse Neonatal Outcomes Associated with Early-Term Birth. JAMA Pediatr. 2013, 167, 1053–1059. [Google Scholar] [CrossRef]
- Knight, H.E.; Oddie, S.J.; Harron, K.L.; Aughey, H.K.; van der Meulen, J.H.; Gurol-Urganci, I.; Cromwell, D.A. Establishing a composite neonatal adverse outcome indicator using English hospital administrative data. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F502–F509. [Google Scholar] [CrossRef]
- Mendez-Figueroa, H.; Truong, V.T.T.; Pedroza, C.; Khan, A.M.; Chauhan, S.P. Small-for-gestational-age infants among uncomplicated pregnancies at term: A secondary analysis of 9 Maternal-Fetal Medicine Units Network studies. Am. J. Obstet. Gynecol. 2016, 215, 628.e1–628.e7. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Rice, M.M.; Grobman, W.A.; Bailit, J.; Reddy, U.M.; Wapner, R.J.; Varner, M.W.; Thorp, J.M.; Leveno, K.J.; Caritis, S.N.; et al. Neonatal Morbidity of Small- and Large-for-Gestational-Age Neonates Born at Term in Uncomplicated Pregnancies. Obstet. Gynecol. 2017, 130, 511–519. [Google Scholar] [CrossRef]
- Delnord, M.; Mortensen, L.; Hindori-Mohangoo, A.D.; Blondel, B.; Gissler, M.; Kramer, M.R.; Richards, J.L.; Deb-Rinker, P.; Rouleau, J.; Morisaki, N.; et al. International variations in the gestational age distribution of births: An ecological study in 34 high-income countries. Eur. J. Public Health 2017, 28, 303–309. [Google Scholar] [CrossRef]
- Newnham, J.P.; Kemp, M.W.; White, S.W.; Arrese, C.A.; Hart, R.J.; Keelan, J.A. Applying Precision Public Health to Prevent Preterm Birth. Front. Public Health 2017, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.J.; Temmink, J.D.; Wertaschnigg, D.; Ganzevoort, W.; Reddy, M.; Davey, M.; Wallace, E.M.; Mol, B. Trends in singleton preterm birth in Victoria, 2007 to 2017: A consecutive cross-sectional study. Acta Obstet. Gynecol. Scand. 2020. [Google Scholar] [CrossRef]
- Bouchet, N.; Gayet-Ageron, A.; Areta, M.L.; Pfister, R.E.; de Tejada, B.M. Avoiding late preterm deliveries to reduce neonatal complications: An 11-year cohort study. BMC Pregnancy Childbirth 2018, 18, 17. [Google Scholar] [CrossRef] [PubMed]

| Variables | Late Preterm Cohort | Early Term Cohort |
|---|---|---|
| Early SNM Present (n = 172) | Early SNM Present (n = 182) | |
| Maternal age (mean, sd) year | 32.3 (5.3) | 32.0 (5.4) |
| Ethnicity | ||
| Caucasian | 68.6% (118/172) | 67.0% (122/182) |
| Indigenous | 2.9% (5/172) | 2.7% (5/182) |
| Asian | 15.7% (27/172) | 19.2% (35/182) |
| Other | 12.8% (22/172) | 11.0% (20/182) |
| SEIFA Score (median, IQR) | 1035 (999–1067) | 1035 (996–1067) |
| Smoking | ||
| No smoking | 14.5% (25/172) | 76.9% (140/182) |
| Mother smokes | 7.6% (13/172) | 12.1% (22/182) |
| Partner smokes | 5.8% (10/172) | 11.0% (20/182) |
| Illicit drug use | 5.8% (10/172) | 6.6% (12/182) |
| Nulliparity | 40.1% (69/172) | 51.6% (94/182) |
| Maternal BMI (kg/m2) | 23.9 (20.4–28.2) | 26.0 (22.0–31.6) |
| Maternal diabetes status | ||
| No diabetes | 70.9% (122/172) | 48.4% (88/182) |
| Pre-existing diabetes | 12.8% (22/172) | 11.0% (20/182) |
| Gestational diabetes | 16.3% (28/172) | 40.7% (74/182) |
| Hypertension | ||
| No hypertension | 81.4% (140/172) | 85.7% (156/182) |
| Essential/gestational hypertension | 11.0% (19/172) | 8.8% (16/182) |
| Pre-eclampsia/Eclampsia/HELLP syndrome | 7.6% (13/172) | 5.5% (10/182) |
| Assisted reproduction | 13.4% (23/172) | 10.4% (19/182) |
| Chorioamnionitis | 4.7% (8/172) | 0.0% (0/182) |
| Antepartum haemorrhage | 15.7% (27/172) | 8.2% (15/182) |
| Induction of labour (IOL) | 15.1% (26/172) | 46.7% (85/182) |
| Method of birth | ||
| Spontaneous vaginal birth | 22.7% (39/172) | 22.5% (41/182) |
| Instrumental birth | 6.4% (11/172) | 19.2% (35/182) |
| Emergency CS for FTP | 1.7% (3/172) | 6.6% (12/182) |
| Emergency CS for NRFS | 7.6% (13/172) | 6.0% (11/182) |
| Emergency CS Other | 42.4% (73/172) | 17.6% (32/182) |
| Elective CS | 19.2% (33/172) | 28.0% (51/182) |
| Birth weight (g) (mean, sd) | 2614 (577) | 3343 (562) |
| Infant’s sex | ||
| Male | 62.2% (107/172) | 65.4% (119/182) |
| Female | 37.8% (65/172) | 34.6% (63/182) |
| Variables | Late Preterm Cohort (n = 950) | Early Term Cohort (n = 5293) | ||
|---|---|---|---|---|
| Unadjusted OR (95%CI) | Adjusted OR (95%CI) † | Unadjusted OR (95%CI) | Adjusted OR (95%CI) † | |
| Maternal age (mean, sd) year | 0.99 (0.96–1.03) | 1.00 (0.97–1.03) | 0.98 (0.95–1.00) | 0.98 (0.95–1.01) |
| Ethnicity | ||||
| Caucasian | Reference | Reference | Reference | Reference |
| indigenous | 0.75 (0.28–2.00) | 0.72 (0.27–1.93) | 0.57 (0.23–1.41) | 0.53 (0.21–1.33) |
| Asian | 0.66 (0.42–1.04) | 0.59 (0.37–0.96) * | 0.65 (0.44–0.95) * | 0.65 (0.45–0.96) * |
| Other | 0.87 (0.53–1.45) | 0.84 (0.50–1.43) | 0.76 (0.47–1.23) | 0.76 (0.47–1.23) |
| SEIFA Score (median, IQR) | 1.00 (0.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) * | 1.00 (1.00–1.00) |
| Smoking | ||||
| No smoking | Reference | Reference | Reference | Reference |
| Mother smokes | 0.90 (0.54–1.51) | 0.77 (0.46-1.29) | 1.36 (0.86–2.14) | 1.26 (0.79–2.00) |
| Partner smokes | 0.86 (0.45–1.68) | 0.69 (0.36–1.33) | 1.61 (0.99–2.60) | 1.60 (0.99–2.60) |
| Illicit drug use | 0.75 (0.38–1.48) | 0.57 (0.29–1.14) | 1.18 (0.65–2.15) | 1.14 (0.62–2.09) |
| Nullipara | 0.64 (0.46–0.89) ** | 0.59 (0.42–0.83) ** | 1.40 (1.04–1.88) * | 1.38 (1.03–1.86) * |
| Maternal BMI (kg/m2) | 0.99 (0.96–1.02) | 0.98 (0.96–1.01) | 1.03 (1.01–1.05) *** | 1.03 (1.01–1.05) *** |
| Maternal diabetes Status | ||||
| No diabetes | Reference | Reference | Reference | Reference |
| Pre-existing diabetes | 2.91 (1.51–5.60) *** | 3.27 (1.62–6.63) *** | 4.67 (2.79–7.82) *** | 4.20 (2.48–7.09) *** |
| Gestational diabetes | 0.72 (0.45–1.14) | 0.75 (0.46–1.22) | 1.39 (1.01–2.00) * | 145 (1.06–1.99) * |
| Hypertension | ||||
| No Hypertension | Reference | Reference | Reference | Reference |
| Essential/gestational hypertension | 1.01 (0.63–1.62) | 0.97 (0.57–1.66) | 1.33 (0.79–2.24) | 1.19 (0.70–2.02) |
| Pre-eclampsia/Eclampsia/HELLP syndrome | 0.70 (0.38–1.31) | 0.73 (0.38–1.40) | 1.59 (0.82–3.06) | 1.22 (0.63–2.37) |
| Assisted reproduction | 0.77 (0.45–1.34) | 0.86 (0.50–1.47) | 0.79 (0.49–1.28) | 0.77 (0.47–1.24) |
| Chorioamnionitis | 1.14 (0.52–2.53) | 0.81 (0.36–1.85) | -- | -- |
| Antepartum hemorrhage | 1.51 (0.95–2.42) | 1.23 (0.75–2.01) | 1.80 (1.05–3.11) * | 1.64 (0.95–2.84) |
| Induction of labour (IOL) | 0.64 (0.41–1.00) * | 0.76 (0.48–1.20) | 1.19 (0.88–1.60) | 1.21 (0.90–1.62) |
| Method of birth | ||||
| Spontaneous vaginal birth | Reference | Reference | Reference | Reference |
| Instrumental birth | 1.50 (0.74–3.04) | 1.78 (0.84–3.80) | 3.45 (2.17–5.46) *** | 3.46 (2.18–5.49) *** |
| Emergency CS for FTP | 1.16 (0.33–4.07) | 1.53 (0.44–5.25) | 3.09 (1.60–5.58) *** | 3.17 (1.63–6.17) *** |
| Emergency CS for NRFS | 2.99 (1.48–6.04) ** | 3.20 (1.53–6.69) ** | 3.23 (1.63–6.40) *** | 3.17 (1.59–6.33) *** |
| Emergency CS Other | 2.62 (1.71–3.99) *** | 2.57 (1.67–3.98) *** | 3.53 (2.20 -5.66) *** | 3.21 (1.99–5.18) *** |
| Elective CS | 1.65 (1.00–2.71) * | 1.63 (0.99–2.70) | 1.66 (1.10–2.52) * | 1.72 (1.13–2.60) * |
| Birth weight (g) (mean, sd) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) ** | 1.00 (1.00–1.00) *** |
| Infant’s sex (Female vs. male) | 0.60 (0.43–0.84) ** | 0.59 (0.42–0.84) ** | 0.58 (0.43–0.79) *** | 0.58 (0.42–0.79) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mengistu, T.S.; Schreiber, V.; Flatley, C.; Fox, J.; Kumar, S. Factors Associated with Increased Risk of Early Severe Neonatal Morbidity in Late Preterm and Early Term Infants. J. Clin. Med. 2021, 10, 1319. https://doi.org/10.3390/jcm10061319
Mengistu TS, Schreiber V, Flatley C, Fox J, Kumar S. Factors Associated with Increased Risk of Early Severe Neonatal Morbidity in Late Preterm and Early Term Infants. Journal of Clinical Medicine. 2021; 10(6):1319. https://doi.org/10.3390/jcm10061319
Chicago/Turabian StyleMengistu, Tesfaye S., Veronika Schreiber, Christopher Flatley, Jane Fox, and Sailesh Kumar. 2021. "Factors Associated with Increased Risk of Early Severe Neonatal Morbidity in Late Preterm and Early Term Infants" Journal of Clinical Medicine 10, no. 6: 1319. https://doi.org/10.3390/jcm10061319
APA StyleMengistu, T. S., Schreiber, V., Flatley, C., Fox, J., & Kumar, S. (2021). Factors Associated with Increased Risk of Early Severe Neonatal Morbidity in Late Preterm and Early Term Infants. Journal of Clinical Medicine, 10(6), 1319. https://doi.org/10.3390/jcm10061319






