Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Schistosoma Species DNA Samples
2.2. Urine Samples Spiked with gDNA from Schistosoma Species
2.3. Schisto-LAMP Assays
2.4. Specificity and Sensibility of Schisto-LAMP Assays in Detecting Schistosome Hybrids
2.5. Detection of LAMP Products
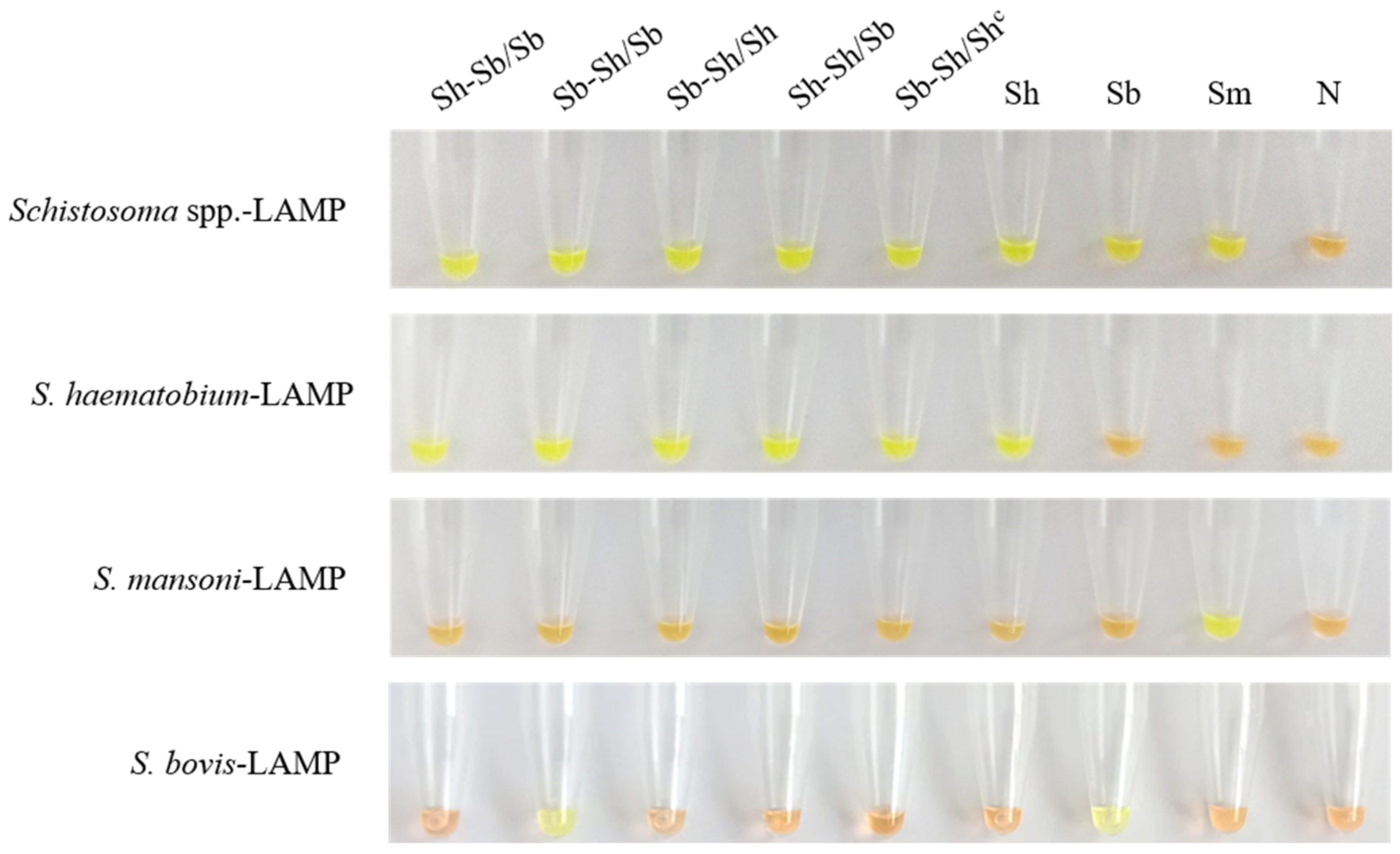
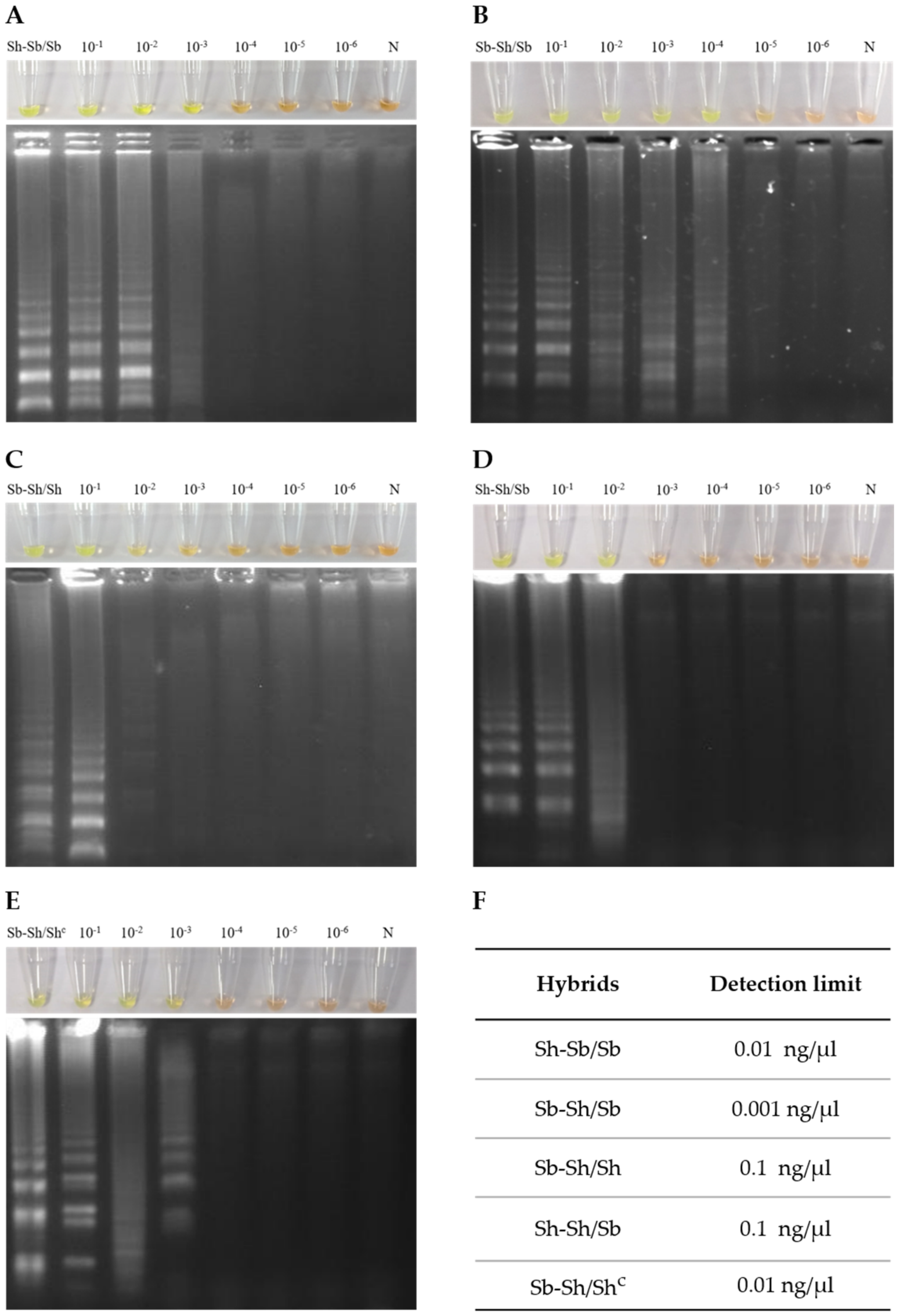
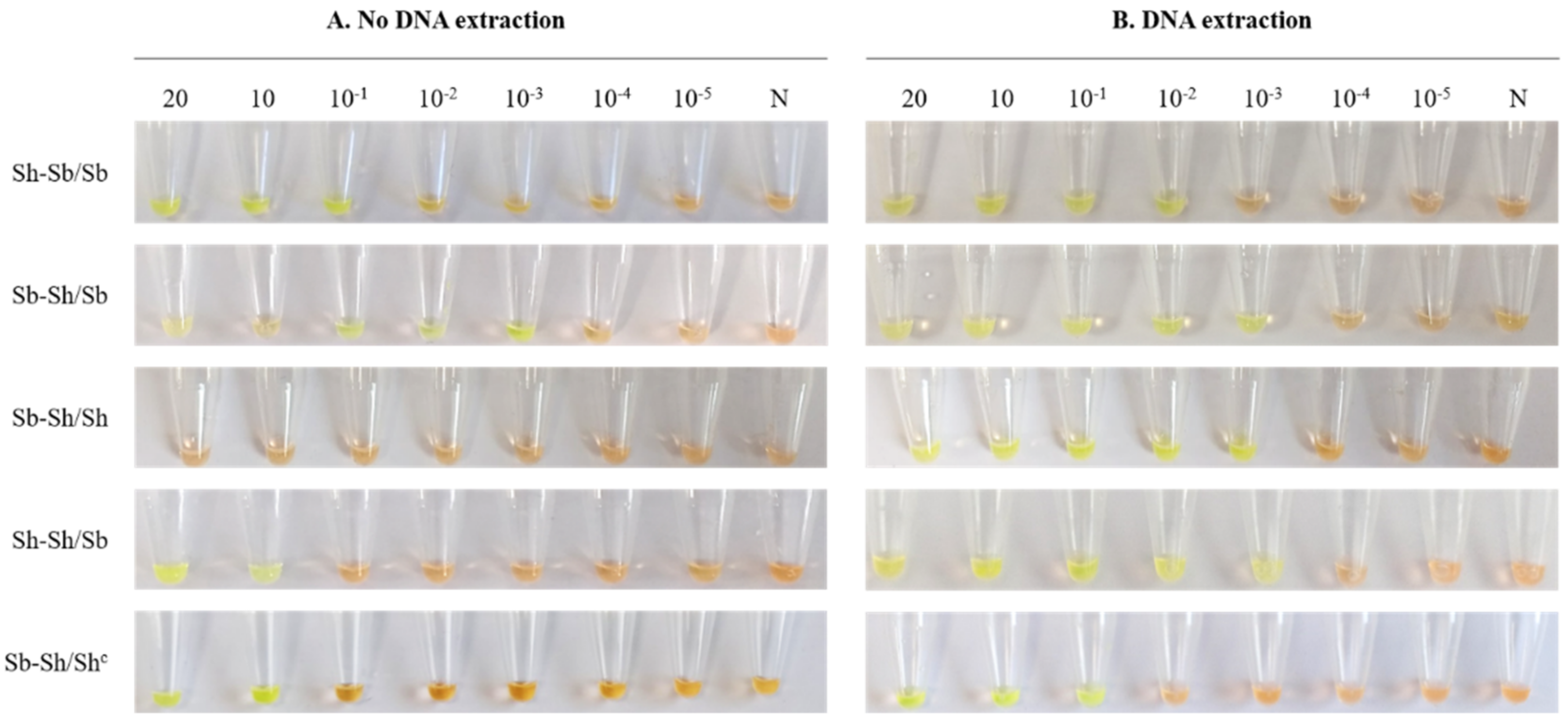
3. Results
3.1. Schisto-LAMP Assays Performance
3.2. Sensitivity of Genus-Specific-LAMP Assay in Detection of Hybrid Schistosomes
3.3. Detection Limit of Genus-Specific LAMP Assay in Simulated Human Urine Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- King, K.C.; Stelkens, R.B.; Webster, J.P.; Smith, D.F.; Brockhurst, M.A. Hybridization in parasites: Consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Webster, J.P.; Gower, C.M.; Knowles, S.C.L.; Molyneux, D.H.; Fenton, A. One health—An ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol. Appl. 2016, 9, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Leger, E.; Webster, J.P. Hybridizations within the genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology 2017, 144, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- World Health Organization. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 18 November 2020).
- Ohmae, H.; Sinuon, M.; Kirinoki, M.; Matsumoto, J.; Chigusa, Y.; Socheat, D.; Matsuda, H. Schistosomiasis mekongi: From discovery to control. Parasitol. Int. 2004, 53, 135–142. [Google Scholar] [CrossRef]
- Webster, B.L.; Southgate, V.R.; Timothy, D.; Littlewood, J. A revision of the interrelationships of Schistosoma including the recently described Schistosoma guineensis. Int. J. Parasitol. 2006, 36, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Latif, B.; Heo, C.C.; Razuin, R.; Shamalaa, D.V.; Tappe, D. Autochthonous human schistosomiasis, Malaysia. Emerg. Infect. Dis. 2013, 19, 1340–1341. [Google Scholar] [CrossRef]
- Weyher, A.H.; Phillips-Conroy, J.E.; Fischer, K.; Weil, G.J.; Chansa, W.; Fischer, P.U. Molecular identification of Schistosoma mattheei from feces of kinda (Papio cynocephalus kindae) and grayfoot baboons (Papio ursinus griseipes) in Zambia. J. Parasitol. 2010, 96, 184–190. [Google Scholar] [CrossRef]
- De Bont, J.; Vercruysse, J. The epidemiology and control of cattle schistosomiasis. Parasitol. Today 1997, 13, 255–262. [Google Scholar] [CrossRef]
- Webster, B.L.; Tchuem Tchuenté, L.A.; Jourdane, J.; Southgate, V.R. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005, 79, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Southgate, V.R.; van Wijk, H.B.; Wright, C.A. Schistosomiasis at Loum, Cameroun; Schistosoma haematobium, Schistosoma haematobium, S. intercalatum and their natural hybrid. Z. Parasitenkd. 1976, 49, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Southgate, V.R.; Tchuem Tchuenté, L.-A. Isoenzyme analysis of Schistosoma haematobium, S. intercalatum and their hybrids and occurrences of natural hybridization in Cameroon. J. Helminthol. 2003, 77, 269–274. [Google Scholar] [CrossRef]
- Webster, B.L.; Alharbi, M.H.; Kayuni, S.; Makaula, P.; Halstead, F.; Christiansen, R.; Juziwelo, L.; Stanton, M.C.; LaCourse, E.J.; Rollinson, D.; et al. Schistosome interactions within the Schistosoma haematobium group, Malawi. Emerg. Infect. Dis. 2019, 25, 1245–1247. [Google Scholar] [CrossRef]
- Boissier, J.; Grech-Angelini, S.; Webster, B.L.; Allienne, J.F.; Huyse, T.; Mas-Coma, S.; Toulza, E.; Barré-Cardi, H.; Rollinson, D.; Kincaid-Smith, J.; et al. Outbreak of urogenital schistosomiasis in Corsica (France): An epidemiological case study. Lancet Infect. Dis. 2016, 16, 971–979. [Google Scholar] [CrossRef]
- De la Torre-Escudero, E.; Pérez-Sánchez, R.; Manzano-Román, R.; Oleaga, A. Schistosoma bovis-host interplay: Proteomics for knowing and acting. Mol. Biochem. Parasitol. 2017, 215, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kincaid-Smith, J.; Rey, O.; Toulza, E.; Berry, A.; Boissier, J. Emerging Schistosomiasis in Europe: A need to quantify the risks. Trends Parasitol. 2017, 33, 600–609. [Google Scholar] [CrossRef]
- Oey, H.; Zakrzewski, M.; Gravermann, K.; Young, N.D.; Korhonen, P.K.; Gobert, G.N.; Nawaratna, S.; Hasan, S.; Martínez, D.M.; You, H.; et al. Whole-genome sequence of the bovine blood fluke Schistosoma bovis supports interspecific hybridization with S. haematobium. PLoS Pathog. 2019, 15, 1–16. [Google Scholar] [CrossRef]
- Savassi, B.A.E.S.; Mouahid, G.; Lasica, C.; Mahaman, S.D.K.; Garcia, A.; Courtin, D.; Allienne, J.F.; Ibikounlé, M.; Moné, H. Cattle as natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 interactions, with new cercarial emergence and genetic patterns. Parasitol. Res. 2020, 119, 2189–2205. [Google Scholar] [CrossRef]
- De Bont, J.; Vercruysse, J.; Southgate, V.R.; Rollinson, D.; Kaukas, A. Cattle Schistosomiasis in Zambia. J. Helminthol. 1994, 68, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Angora, E.K.; Allienne, J.F.; Rey, O.; Menan, H.; Touré, A.O.; Coulibaly, J.T.; Raso, G.; Yavo, W.; N’Goran, E.K.; Utzinger, J.; et al. High prevalence of Schistosoma haematobium x Schistosoma bovis hybrids in schoolchildren in Côte d’Ivoire. Parasitology 2020, 147, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Pennance, T.; Allan, F.; Emery, A.; Rabone, M.; Cable, J.; Garba, A.D.; Hamidou, A.A.; Webster, J.P.; Rollinson, D.; Webster, B.L. Interactions between Schistosoma haematobium group species and their Bulinus spp. intermediate hosts along the Niger River Valley. Parasites Vectors 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soentjens, P.; Cnops, L.; Huyse, T.; Yansouni, C.; De Vos, D.; Bottieau, E.; Clerinx, J.; Van Esbroeck, M. Diagnosis and clinical management of Schistosoma haematobium-Schistosoma bovis hybrid infection in a cluster of travelers returning from Mali. Clin. Infect. Dis. 2016, 63, 1626–1629. [Google Scholar] [CrossRef]
- Holtfreter, M.C.; Moné, H.; Müller-Stöver, I.; Mouahid, G.; Richter, J. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Eurosurveillance 2014, 19, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Moné, H.; Holtfreter, M.C.; Mouahid, G.; Richter, J. Difficulties in Schistosomiasis assessment, Corsica, France. Emerg. Infect. Dis. 2016, 22, 762–763. [Google Scholar] [CrossRef]
- Bärenbold, O.; Raso, G.; Coulibaly, J.T.; N’Goran, E.K.; Utzinger, J.; Vounatsou, P. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl. Trop. Dis. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Chuah, C.; Gobert, G.N.; Latif, B.; Heo, C.C.; Leow, C.Y. Schistosomiasis in Malaysia: A review. Acta Trop. 2019, 190, 137–143. [Google Scholar] [CrossRef]
- Weerakoon, K.G.A.D.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 2015, 28, 939–967. [Google Scholar] [CrossRef]
- Hinz, R.; Schwarz, N.G.; Hahn, A.; Frickmann, H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell. Probes 2017, 31, 2–21. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Weerakoon, K.G.; Gordon, C.A.; McManus, D.P. DNA diagnostics for schistosomiasis control. Trop. Med. Infect. Dis. 2018, 3, 81. [Google Scholar] [CrossRef]
- Schols, R.; Carolus, H.; Hammoud, C.; Mulero, S.; Mudavanhu, A.; Huyse, T. A rapid diagnostic multiplex PCR approach for xenomonitoring of human and animal schistosomiasis in a “One Health” context. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): Expansion of its practical application as a tool to achieve universal health coverage. J. Infect. Chemother. 2020, 26, 13–17. [Google Scholar] [CrossRef]
- Avendaño, C.; Patarroyo, M.A. Loop-mediated isothermal amplification as point-of-care diagnosis for neglected parasitic infections. Int. J. Mol. Sci. 2020, 21, 7981. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Avendaño, C.; Sala-Vizcaíno, A.; Crego-Vicente, B.; Febrer-Sendra, B.; García-Bernalt Diego, J.; Oleaga, A.; López-Abán, J.; Vicente, B.; Patarroyo, M.A.; et al. Molecular markers for detecting Schistosoma species by Loop-Mediated Isothermal Amplification. Dis. Markers 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.A.; Liang, Y.S.; Raghavan, N.; Knight, M. The NIH-NIAID schistosomiasis resource center. PLoS Negl. Trop. Dis. 2008, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gandasegui, J.; Fernández-Soto, P.; Carranza-Rodríguez, C.; Pérez-Arellano, J.L.; Vicente, B.; López-Abán, J.; Muro, A. The rapid-heat LAMPellet method: A potential diagnostic method for human urogenital schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, 1–23. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui Arahuetes, J.; Sánchez Hernández, A.; López Abán, J.; Vicente Santiago, B.; Muro, A. A Loop-Mediated Isothermal Amplification (LAMP) assay for early detection of Schistosoma mansoni in stool samples: A diagnostic approach in a murine model. PLoS Negl. Trop. Dis. 2014, 8, e3126. [Google Scholar] [CrossRef] [PubMed]
- Pennance, T.; Ame, S.M.; Amour, A.K.; Suleiman, K.R.; Allan, F.; Rollinson, D.; Webster, B.L. Occurrence of Schistosoma bovis on Pemba Island, Zanzibar: Implications for urogenital schistosomiasis transmission monitoring. Parasitology 2018, 145, 1732. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Webster, J.P.; Rollinson, D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: Species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013, 7, e2110. [Google Scholar] [CrossRef]
- Léger, E.; Garba, A.; Hamidou, A.A.; Webster, B.L.; Pennance, T.; Rollinson, D.; Webster, J.P. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg. Infect. Dis. 2016, 22, 2212–2214. [Google Scholar] [CrossRef]
- Huyse, T.; Van Den Broeck, F.; Hellemans, B.; Volckaert, F.A.M.; Polman, K. Hybridisation between the two major African schistosome species of humans. Int. J. Parasitol. 2013, 43, 687–689. [Google Scholar] [CrossRef]
- Catalano, S.; Sène, M.; Diouf, N.D.; Fall, C.B.; Borlase, A.; Léger, E.; Bâ, K.; Webster, J.P. Rodents as natural hosts of zoonotic Schistosoma species and hybrids: An epidemiological and evolutionary perspective from West Africa. J. Infect. Dis. 2018, 218, 429–433. [Google Scholar] [CrossRef]
- Catalano, S.; Léger, E.; Fall, C.B.; Borlase, A.; Diop, S.D.; Berger, D.; Webster, B.L.; Faye, B.; Diouf, N.D.; Rollinson, D.; et al. Multihost transmission of Schistosoma mansoni. Emerg. Infect. Dis. 2020, 26, 1234–1242. [Google Scholar]
- Pena, H.B.; De Souza, C.P.; Simpson, A.J.G.; Pena, S.D.J. Intracellular promiscuity in Schistosoma mansoni: Nuclear transcribed DNA sequences are part of a mitochondrial minisatellite region. Proc. Natl. Acad. Sci. USA 1995, 92, 915–919. [Google Scholar] [CrossRef]
- Gandasegui, J.; Fernández-Soto, P.; Muro, A.; Simões Barbosa, C.; Lopes de Melo, F.; Loyo, R.; de Souza Gomes, E.C. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui, J.; Rodríguez, C.C.; Pérez-Arellano, J.L.; Crego-Vicente, B.; García-Bernalt Diego, J.; López-Abán, J.; Vicente, B.; Muro, A. Detection of Schistosoma mansoni-derived DNA in human urine samples by loop-mediated isothermal amplification (LAMP). PLoS ONE 2019, 14, e0214125. [Google Scholar] [CrossRef] [PubMed]
- Le Govic, Y.; Kincaid-Smith, J.; Allienne, J.F.; Rey, O.; de Gentile, L.; Boissier, J. Schistosoma haematobium- Schistosoma mansoni hybrid parasite in migrant boy, France, 2017. Emerg. Infect. Dis. 2019, 25, 365–367. [Google Scholar] [CrossRef]
- Zhao, G.H.; Mo, X.H.; Zou, F.C.; Li, J.; Weng, Y.B.; Lin, R.Q.; Xia, C.M.; Zhu, X.Q. Genetic variability among Schistosoma japonicum isolates from different endemic regions in China revealed by sequences of three mitochondrial DNA genes. Vet. Parasitol. 2009, 162, 67–74. [Google Scholar] [CrossRef]
- Vilas, R.; Criscione, C.D.; Blouin, M.S. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 2005, 131, 839–846. [Google Scholar] [CrossRef]
- Webster, B.L.; Culverwell, C.L.; Khamis, I.S.; Mohammed, K.A.; Rollinson, D.; Stothard, J.R. DNA barcoding of Schistosoma haematobium on Zanzibar reveals substantial genetic diversity and two major phylogenetic groups. Acta Trop. 2013, 128, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.J.; Cribb, T.H. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv. Parasitol. 2005, 60, 101–163. [Google Scholar] [CrossRef]
- Cutmore, S.C.; Bennett, M.B.; Cribb, T.H. Staphylorchis cymatodes (Gorgoderidae: Anaporrhutinae) from carcharhiniform, orectolobiform and myliobatiform elasmobranchs of Australasia: Low host specificity, wide distribution and morphological plasticity. Parasitol. Int. 2010, 59, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.A.; Rollinson, D. Repetitive sequences in the ribosomal DNA internal transcribed spacer of Schistosoma haematobium, Schistosoma intercalatum and Schistosoma mattheei. Mol. Biochem. Parasitol. 1994, 63, 153–156. [Google Scholar] [CrossRef]
- Dvořák, J.; Vaňáčová, Š.; Hampl, V.; Flegr, J.; Horák, P. Comparison of European Trichobilharzia species based on ITS1 and ITS2 sequences. Parasitology 2002, 124, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Van Herwerden, L.; Blair, D.; Agatsuma, T. Intra- and inter-specific variation in nuclear ribosomal internal transcribed spacer 1 of the Schistosoma japonicum species complex. Parasitology 1998, 116, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.A.; Rollinson, D. Comparison of the intergenic spacers and 3’ end regions of the large subunit (28S) ribosomal RNA gene from three species of Schistosoma. Parasitology 1998, 117, 235–242. [Google Scholar] [CrossRef]
- El Bali, L.; Diman, A.; Bernard, A.; Roosens, N.H.C.; Dekeersmaecker, S.C.J. Comparative study of seven commercial kits for human DNA extraction from urine samples suitable for DNA biomarker-based public health studies. J. Biomol. Tech. 2014, 25, 96–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Study Location | RD-PCR Analysis | Sequence Analysis | Abbreviation | |
|---|---|---|---|---|
| cox1 | cox 1 haplotypes | ITS2 alleles | ||
| Agboville | S. haematobium | S. haematobium | S. bovis + S. bovis | Sh-Sb/Sb |
| S. bovis | S. bovis | S. haematobium + S. bovis | Sb-Sh/Sb | |
| S. bovis | S. bovis | S. haematobium+ S. haematobium | Sb-Sh/Sh | |
| S. haematobium | S. haematobium | S. haematobium + S. bovis | Sh-Sh/Sb | |
| Corsica | S. bovis | S. bovis | S. haematobium + S. haematobium | Sb-Sh/Shc |
| Schisto-LAMP | Primer Sets | Sequence 5′→3′ | Length (bp) | Ref. |
|---|---|---|---|---|
| S. mansoni | F3 | TTATCGTCTATAGTACGGTAGG | 22 | [45] |
| B3 | ATACTTTAACCCCCACCAA | 19 | ||
| FIP | GCCAAGTAGAGACACAAACATCTT-TGGGTAAGGTAGAAAATGTTGT | 47 | ||
| BIP | AGAAGTGTTTAACTTGATGAAGGGG-AAACAAAACCGAAACCACTA | 45 | ||
| S. haematobium | F3 | CTTTCTAAGCCCGCGATA | 18 | [44] |
| B3 | GCGCATTACACTTGGTCT | 18 | ||
| FIP | TACCCCTAACTTCGTGGTCTCC-CCCCCTTATTTTAGGGTGC | 41 | ||
| BIP | CTCCCTATATAACATGGCGAGTAAG-ACTATGAAATCAGTGTTTTTCGG | 48 | ||
| S. bovis | F3 | TTCATTGTTAGGTTGCGT | 18 | [42] |
| B3 | TCTATATTCTACTCTAATCCCTCT | 24 | ||
| FIP | TCAGTATCATCTCAAACATCACACT-AGTAGTATGTTCTGTCTTAAGTT | 48 | ||
| BIP | TTTGTAGTACCTCTGGTTTACATCA-TTCACTCTCAGACTCTACAT | 45 | ||
| LF | ACTTAGACCATGAACATCAACCTAT | 25 | ||
| LB | TACTAAGTGAGAGTAATCGAACACC | 25 | ||
| Schistosoma spp. | F3 | TTGACCGGGGTACCTAGC | 18 | [42] |
| B3 | CGTGAATGGCAAGCCAAAC | 19 | ||
| FIP | ATCGCCCTTGGCAGATCAGG-CTGTCGTATGCCCTGATGG | 39 | ||
| BIP | ATATGCATGCAAATCCGCCCCG-CGGATCGCTTCAACAGTGTA | 43 | ||
| LF | CAGATCAGGCAACCCGAAAG | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crego-Vicente, B.; Fernández-Soto, P.; Febrer-Sendra, B.; García-Bernalt Diego, J.; Boissier, J.; Angora, E.K.; Oleaga, A.; Muro, A. Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids. J. Clin. Med. 2021, 10, 1308. https://doi.org/10.3390/jcm10061308
Crego-Vicente B, Fernández-Soto P, Febrer-Sendra B, García-Bernalt Diego J, Boissier J, Angora EK, Oleaga A, Muro A. Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids. Journal of Clinical Medicine. 2021; 10(6):1308. https://doi.org/10.3390/jcm10061308
Chicago/Turabian StyleCrego-Vicente, Beatriz, Pedro Fernández-Soto, Begoña Febrer-Sendra, Juan García-Bernalt Diego, Jérôme Boissier, Etienne K. Angora, Ana Oleaga, and Antonio Muro. 2021. "Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids" Journal of Clinical Medicine 10, no. 6: 1308. https://doi.org/10.3390/jcm10061308
APA StyleCrego-Vicente, B., Fernández-Soto, P., Febrer-Sendra, B., García-Bernalt Diego, J., Boissier, J., Angora, E. K., Oleaga, A., & Muro, A. (2021). Application of a Genus-Specific LAMP Assay for Schistosome Species to Detect Schistosoma haematobium x Schistosoma bovis Hybrids. Journal of Clinical Medicine, 10(6), 1308. https://doi.org/10.3390/jcm10061308








