TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review
Abstract
1. Introduction
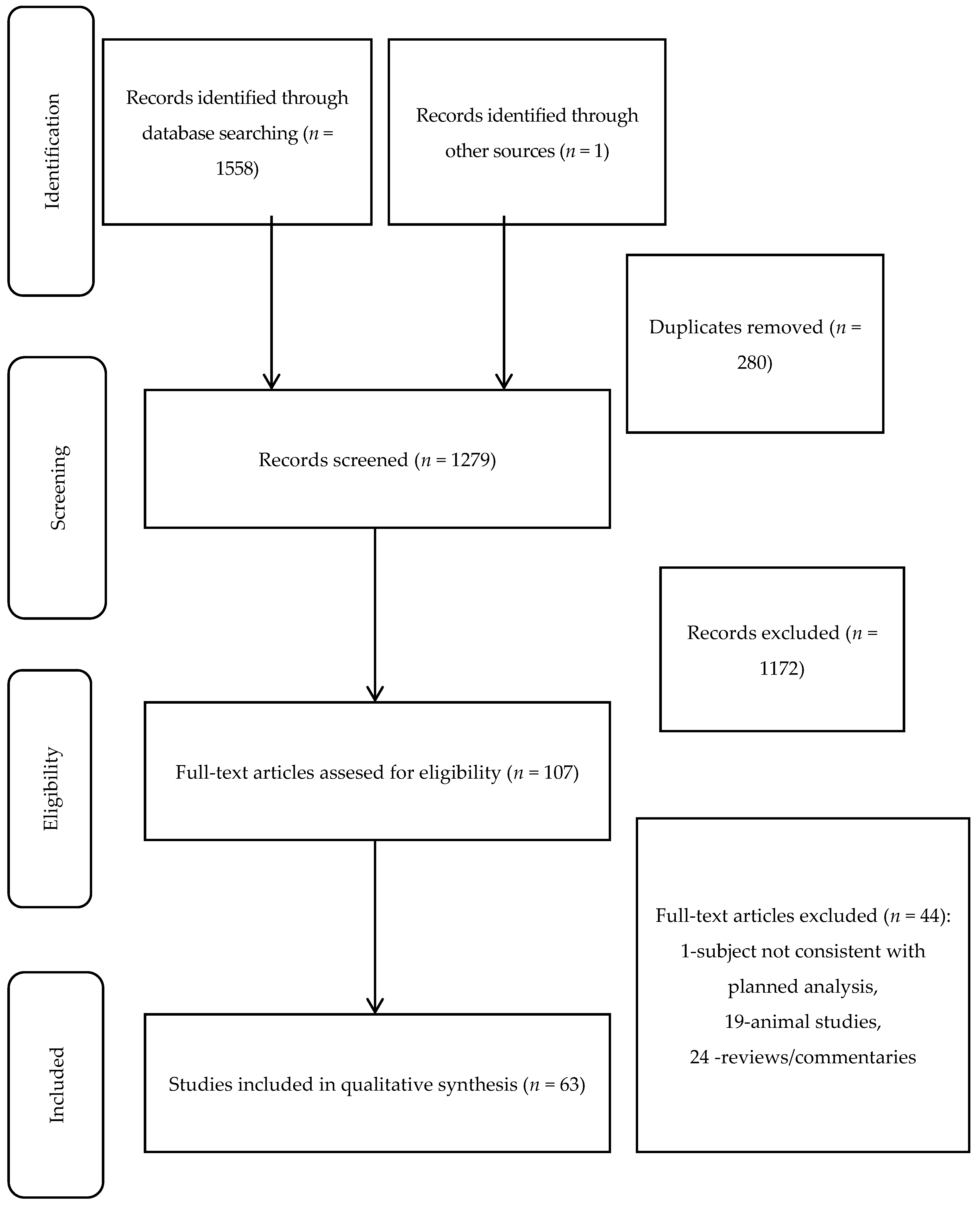
2. Materials and Methods
3. Results
4. Discussion
4.1. Heart Failure
4.2. Coronary Artery Disease
4.3. Atrial Fibrillation
4.4. Cerebral Ischemia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, A.; Magiorkinis, E.; Sakorafas, G.H.; Androutsos, G. A Brief History of Apoptosis: From Ancient to Modern Times. Oncol. Res. Treat. 2008, 31, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Sawa, A.; Wiegand, G.W.; Cooper, J.K.; Margolis, R.L.; Sharp, A.H.; Lawler, J.F.; Greenamyre, J.T.; Snyder, S.H.; Ross, C.A. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 1999, 5, 1194–1198. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.-M. Apoptosis in Human Acute Myocardial Infarction. Circulation 1997, 95, 320–323. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.-P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Tecchio, C.; Huber, V.; Scapini, P.; Calzetti, F.; Margotto, D.; Todeschini, G.; Pilla, L.; Martinelli, G.; Pizzolo, G.; Rivoltini, L.; et al. IFNα-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood 2004, 103, 3837–3844. [Google Scholar] [CrossRef]
- Kimberley, F.C.; Screaton, G.R. Following a TRAIL: Update on a ligand and its five receptors. Cell Res. 2004, 14, 359–372. [Google Scholar] [CrossRef]
- Stuckey, D.W.; Shah, K. TRAIL on trial: Preclinical advances in cancer therapy. Trends Mol. Med. 2013, 19, 685–694. [Google Scholar] [CrossRef]
- Secchiero, P.; Zerbinati, C.; Rimondi, E.; Corallini, F.; Milani, D.; Grill, V.; Forti, G.; Capitani, S.; Zauli, G. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell. Mol. Life Sci. 2004, 61, 1965–1974. [Google Scholar] [CrossRef]
- Spierings, D.C.; De Vries, E.G.; Vellenga, E.; Heuvel, F.A.V.D.; Koornstra, J.J.; Wesseling, J.; Hollema, H.; De Jong, S. Tissue distribution of the death ligand TRAIL and its receptors. J. Histochem. Cytochem. 2004, 52, 821–831. [Google Scholar] [CrossRef]
- Tanner, M.A.; Thomas, T.P.; Grisanti, L.A. Death receptor 5 contributes to cardiomyocyte hypertrophy through epidermal growth factor receptor transactivation. J. Mol. Cell. Cardiol. 2019, 136, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, B.; Bernardi, S.; Candido, R.; Zacchigna, S.; Fabris, B.; Secchiero, P. TRAIL shows potential cardioprotective activity. Investig. New Drugs 2011, 30, 1257–1260. [Google Scholar] [CrossRef]
- Di Bartolo, B.A.; Cartland, S.P.; Harith, H.H.; Bobryshev, Y.V.; Schoppet, M.; Kavurma, M.M. TRAIL-Deficiency Accelerates Vascular Calcification in Atherosclerosis via Modulation of RANKL. PLoS ONE 2013, 8, e74211. [Google Scholar] [CrossRef]
- Watt, V.; Chamberlain, J.; Steiner, T.; Francis, S.; Crossman, D. TRAIL attenuates the development of atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 2011, 215, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Martin-Villalba, A.; Herr, I.; Jeremias, I.; Hahne, M.; Brandt, R.; Vogel, J.; Schenkel, J.; Herdegen, T.; Debatin, K.-M. CD95 Ligand (Fas-L/APO-1L) and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Mediate Ischemia-Induced Apoptosis in Neurons. J. Neurosci. 1999, 19, 3809–3817. [Google Scholar] [CrossRef]
- Hameed, A.G.; Arnold, N.D.; Chamberlain, J.; Pickworth, J.A.; Paiva, C.; Dawson, S.; Cross, S.; Long, L.; Zhao, L.; Morrell, N.W.; et al. Inhibition of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J. Exp. Med. 2012, 209, 1919–1935. [Google Scholar] [CrossRef] [PubMed]
- Bumdelger, B.; Kokubo, H.; Kamata, R.; Fujii, M.; Yoshimura, K.; Aoki, H.; Orita, Y.; Ishida, T.; Ohtaki, M.; Nagao, M.; et al. Osteoprotegerin Prevents Development of Abdominal Aortic Aneurysms. PLoS ONE 2016, 11, e0147088. [Google Scholar] [CrossRef] [PubMed]
- Venuraju, S.M.; Yerramasu, A.; Corder, R.; Lahiri, A. Osteoprotegerin as a Predictor of Coronary Artery Disease and Cardiovascular Mortality and Morbidity. J. Am. Coll. Cardiol. 2010, 55, 2049–2061. [Google Scholar] [CrossRef]
- Osmančík, P.; Heřman, D.; Stros, P.; Línková, H.; Vondrak, K.; Paskova, E. Changes and Prognostic Impact of Apoptotic and Inflammatory Cytokines in Patients Treated with Cardiac Resynchronization Therapy. Cardiology 2013, 124, 190–198. [Google Scholar] [CrossRef]
- Stenemo, M.; Nowak, C.; Byberg, L.; Sundström, J.; Giedraitis, V.; Lind, L.; Ingelsson, E.; Fall, T.; Ärnlöv, J. Circulating proteins as predictors of incident heart failure in the elderly. Eur. J. Heart Fail. 2017, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Schoppet, M.; Ruppert, V.; Hofbauer, L.C.; Henser, S.; Al-Fakhri, N.; Christ, M.; Pankuweit, S.; Maisch, B. TNF-related apoptosis-inducing ligand and its decoy receptor osteoprotegerin in nonischemic dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2005, 338, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Lula, J.F.; Rocha, M.O.D.C.; Nunes, M.D.C.P.; Ribeiro, A.L.P.; Teixeira, M.M.; Bahia, M.T.; Talvani, A. Plasma concentrations of tumour necrosis factor-alpha, tumour necrosis factor-related apoptosis-inducing ligand, and FasLigand/CD95L in patients with Chagas cardiomyopathy correlate with left ventricular dysfunction. Eur. J. Heart Fail. 2009, 11, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Osmančík, P.; Peroutka, Z.; Budera, P.; Heřman, D.; Stros, P.; Straka, Z.; Vondrak, K. Decreased Apoptosis following Successful Ablation of Atrial Fibrillation. Cardiology 2010, 116, 302–307. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Kossyvakis, C.; Raisakis, K.; Angelidis, C.; Efremidis, M.; Panagopoulou, V.; Kaoukis, A.; Theodorakis, A.; Toli, K.; et al. Association of post-cardioversion transcardiac concentration gradient of soluble tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL) and inflammatory biomarkers to atrial fibrillation recurrence. Clin. Biochem. 2013, 46, 1020–1025. [Google Scholar] [CrossRef]
- Müller, P.; Deneke, T.; Schiedat, F.; Bösche, L.; Strauch, J.; Dietrich, J.W.; Vogt, M.; Tannapfel, A.; Stiegler, H.; Mügge, A.; et al. Increased Preoperative Serum Apoptosis Marker Fas Ligand Correlates with Histopathology and New-Onset of Atrial Fibrillation in Patients After Cardiac Surgery. J. Cardiovasc. Electrophysiol. 2013, 24, 1110–1115. [Google Scholar] [CrossRef]
- Rewiuk, K.; Grodzicki, T. Osteoprotegerin and TRAIL in Acute Onset of Atrial Fibrillation. BioMed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.; Purmah, Y.; Cardoso, V.R.; Gkoutos, G.V.; Tull, S.P.; Neculau, G.; Thomas, M.R.; Kotecha, D.; Lip, G.Y.H.; Kirchhof, P.; et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur. Heart J. 2019, 40, 1268–1276. [Google Scholar] [CrossRef]
- Kawano, N.; Mori, K.; Emoto, M.; Lee, E.; Kobayashi, I.; Yamazaki, Y.; Urata, H.; Morioka, T.; Koyama, H.; Shoji, T.; et al. Association of serum TRAIL levels with atherosclerosis in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2011, 91, 316–320. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Kossyvakis, C.; Kaoukis, A.; Raisakis, K.; Panagopoulou, V.; Miliou, A.; Theodorakis, A.; Driva, M.; Pyrgakis, V.; et al. Association of soluble tumour necrosis factor-related apoptosis-inducing ligand levels with coronary plaque burden and composition. Heart 2011, 98, 214–218. [Google Scholar] [CrossRef]
- Arcidiacono, M.V.; Rimondi, E.; Maietti, E.; Melloni, E.; Tisato, V.; Gallo, S.; Valdivielso, J.M.; Fernández, E.; Betriu, À.; Voltan, R.; et al. Relationship between low levels of circulating TRAIL and atheromatosis progression in patients with chronic kidney disease. PLoS ONE 2018, 13, e0203716. [Google Scholar] [CrossRef]
- Schoppet, M.; Sattler, A.M.; Schaefer, J.R.; Hofbauer, L.C. Osteoprotegerin (OPG) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) levels in atherosclerosis. Atherosclerosis 2006, 184, 446–447. [Google Scholar] [CrossRef]
- Satoh, D.; Inami, N.; Shimazu, T.; Kajiura, T.; Yamada, K.; Iwasaka, T.; Nomura, S. Soluble TRAIL prevents RANTES-dependent restenosis after percutaneous coronary intervention in patients with coronary artery disease. J. Thromb. Thrombolysis 2009, 29, 471–476. [Google Scholar] [CrossRef]
- Mori, K.; Ikari, Y.; Jono, S.; Shioi, A.; Ishimura, E.; Emoto, M.; Inaba, M.; Hara, K.; Nishizawa, Y. Association of serum TRAIL level with coronary artery disease. Thromb. Res. 2010, 125, 322–325. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Beltrami, A.P.; Ceconi, C.; Bonasia, V.; Di Chiara, A.; Ferrari, R.; Zauli, G. An imbalanced OPG/TRAIL ratio is associated to severe acute myocardial infarction. Atherosclerosis 2010, 210, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.G.; El-Shehaby, A.; Nabih, M. Possible Role of Osteoprotegerin and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand as Markers of Plaque Instability in Coronary Artery Disease. Angiology 2010, 61, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.; Giannopoulos, G.; Panagopoulou, V.; Raisakis, K.; Kossyvakis, C.; Kaoukis, A.; Tzalamouras, V.; Mavri, M.; Pyrgakis, V.; Cleman, M.W.; et al. Inverse Association of Coronary Soluble Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (sTRAIL) Levels to In-Stent Neointimal Hyperplasia. Cardiology 2012, 123, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Choi, K.; Kwon, K.; Choi, C. Soluble tumor necrosis factor-related apoptosis-inducing ligand after percutaneous coronary intervention: A potential biomarker for vascular remodeling. J. Cardiovasc. Med. 2012, 13, 292–293. [Google Scholar] [CrossRef]
- Luz, A.; Santos, M.; Magalhães, R.; Oliveira, J.C.; Pacheco, A.; Silveira, J.; Cabral, S.; Torres, S.; Leite-Moreira, A.F.; Carvalho, H. Soluble TNF-related apoptosis induced ligand (sTRAIL) is augmented by Post-Conditioning and correlates to infarct size and left ventricle dysfunction in STEMI patients: A substudy from a randomized clinical trial. Heart Vessel. 2016, 32, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cholan, P.M.; Cartland, S.P.; Dang, L.; Rayner, B.S.; Patel, S.; Thomas, S.R.; Kavurma, M.M. TRAIL protects against endothelial dysfunction in vivo and inhibits angiotensin-II-induced oxidative stress in vascular endothelial cells in vitro. Free. Radic. Biol. Med. 2018, 126, 341–349. [Google Scholar] [CrossRef]
- Teringova, E.; Kozel, M.; Knot, J.; Kocka, V.; Benesova, K.; Tousek, P. Relationship between TRAIL and Left Ventricular Ejection Fraction in Patients with ST-Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Chasseraud, M.; Liabeuf, S.; Mozar, A.; Mentaveri, R.; Brazier, M.; Massy, Z.A.; Kamel, S. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand and Vascular Calcification. Ther. Apher. Dial. 2011, 15, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.R.; Park, Y.; Chang, J.H.; Lee, S.S. Inverse regulation of serum osteoprotegerin and tumor necrosis factor-related apoptosis-inducing ligand levels in patients with leg lesional vascular calcification. Medicine 2019, 98, e14489. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Nardi, K.; Chiasserini, D.; Eusebi, P.; Tantucci, M.; Di Piero, V.; Altieri, M.; Marini, C.; Russo, T.; Silvestrini, M.; et al. Immunological Profile of Silent Brain Infarction and Lacunar Stroke. PLoS ONE 2013, 8, e68428. [Google Scholar] [CrossRef]
- Kang, Y.H.; Park, M.-G.; Noh, K.-H.; Park, H.R.; Lee, H.W.; Son, S.M.; Park, K.-P. Low serum TNF-related apoptosis-inducing ligand (TRAIL) levels are associated with acute ischemic stroke severity. Atherosclerosis 2015, 240, 228–233. [Google Scholar] [CrossRef]
- Pan, X.; Pang, M.; Ma, A.; Wang, K.; Zhang, Z.; Zhong, Q.; Yang, S. Association of TRAIL and Its Receptors with Large-Artery Atherosclerotic Stroke. PLoS ONE 2015, 10, e0136414. [Google Scholar] [CrossRef]
- O’Sullivan, E.P.; Ashley, D.T.; Davenport, C.; Kelly, J.; Devlin, N.; Crowley, R.; Leahy, A.L.; Kelly, C.J.; Agha, A.; Thompson, C.J.; et al. Osteoprotegerin is higher in peripheral arterial disease regardless of glycaemic status. Thromb. Res. 2010, 126, e423–e427. [Google Scholar] [CrossRef]
- Karatolios, K.; Pankuweit, S.; Goettsch, C.; Hofbauer, L.C.; Timmesfeld, N.; Al-Fakhri, N.; Maisch, B.; Schoppet, M. Osteoprotegerin (OPG) and TNF-related apoptosis-inducing ligand (TRAIL) levels in malignant and benign pericardial effusions. Clin. Biochem. 2012, 45, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Long, Y.; Yang, H.; Zhu, C.; Ma, Q.; Zhang, Y. TRAIL Is Decreased Before 20 Weeks Gestation in Women with Hypertensive Disorders of Pregnancy. PLoS ONE 2015, 10, e0128425. [Google Scholar] [CrossRef]
- Liu, H.; Yang, E.; Lu, X.; Zuo, C.; He, Y.; Jia, D.; Zhu, Q.; Yu, Y.; Lv, A. Serum levels of tumor necrosis factor-related apoptosis-inducing ligand correlate with the severity of pulmonary hypertension. Pulm. Pharmacol. Ther. 2015, 33, 39–46. [Google Scholar] [CrossRef]
- Wigren, M.; Svenungsson, E.; Mattisson, I.Y.; Gustafsson, J.T.; Gunnarsson, I.; Zickert, A.; Elvin, K.; Jensen-Urstad, K.; Bengtsson, A.; Gullstrand, B.; et al. Cardiovascular disease in systemic lupus erythematosus is associated with increased levels of biomarkers reflecting receptor-activated apoptosis. Atherosclerosis 2018, 270, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Singh, P.; Tengryd, C.; Cavalera, M.; Mattisson, I.Y.; Nitulescu, M.; Persson, A.F.; Volkov, P.; Engström, G.; Orho-Melander, M.; et al. sTRAIL-R2 (Soluble TNF [Tumor Necrosis Factor]-Related Apoptosis-Inducing Ligand Receptor 2) a Marker of Plaque Cell Apoptosis and Cardiovascular Events. Stroke 2019, 50, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Damås, J.K.; Eiken, H.G.; Holm, T.; Haug, T.; Simonsen, S.; Frøland, S.S.; Gullestad, L.; Aukrust, P. Increased gene expression of tumor necrosis factor superfamily ligands in peripheral blood mononuclear cells during chronic heart failure. Cardiovasc. Res. 2002, 54, 175–182. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Xi, L.; Røe, O.D.; Chen, Y.; Wang, N. Dysregulated atrial gene expression of osteoprotegerin/receptor activator of nuclear factor-?B (RANK)/RANK ligand axis in the development and progression of atrial fibrillation. Circ. J. 2011, 75, 2781–2788. [Google Scholar] [CrossRef]
- Schoppet, M.; Al-Fakhri, N.; Franke, F.E.; Katz, N.; Barth, P.J.; Maisch, B.; Preissner, K.T.; Hofbauer, L.C. Localization of Osteoprotegerin, Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand, and Receptor Activator of Nuclear Factor-κB Ligand in Mönckeberg’s Sclerosis and Atherosclerosis. J. Clin. Endocrinol. Metab. 2004, 89, 4104–4112. [Google Scholar] [CrossRef]
- Michowitz, Y.; Goldstein, E.; Roth, A.; Afek, A.; Abashidze, A.; Ben Gal, Y.; Keren, G.; George, J. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J. Am. Coll. Cardiol. 2005, 45, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Niessner, A.; Sato, K.; Chaikof, E.L.; Colmegna, I.; Goronzy, J.J.; Weyand, C.M. Pathogen-Sensing Plasmacytoid Dendritic Cells Stimulate Cytotoxic T-Cell Function in the Atherosclerotic Plaque Through Interferon-α. Circulation 2006, 114, 2482–2489. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Yanase, N.; Oshima, K.; Sasame, A.; Hara, T.; Fukazawa, S.; Takata, R.; Hata, K.; Mukai, K.; Yamashina, A.; et al. Enhanced Expression of the Apoptosis Inducing Ligand TRAIL in Mononuclear Cells After Myocardial Infarction. Jpn. Heart J. 2003, 44, 833–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, K.; Niessner, A.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 2006, 203, 239–250. [Google Scholar] [CrossRef]
- Corallini, F.; Secchiero, P.; Beltrami, A.P.; Cesselli, D.; Puppato, E.; Ferrari, R.; Beltrami, C.A.; Zauli, G. TNF-α modulates the migratory response of mesenchymal stem cells to TRAIL. Cell. Mol. Life Sci. 2010, 67, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nuki, T.; Gomita, K.; Weyand, C.M.; Hagiwara, N. Statins reduce endothelial cell apoptosis via inhibition of TRAIL expression on activated CD4 T cells in acute coronary syndrome. Atherosclerosis 2010, 213, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Gonelli, A.; Corallini, F.; Ceconi, C.; Ferrari, R.; Zauli, G. Metalloproteinase 2 cleaves in vitro recombinant TRAIL: Potential implications for the decreased serum levels of TRAIL after acute myocardial infarction. Atherosclerosis 2010, 211, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Winrow, V.R.; Horrocks, M.; Stevens, C.R. Differential expression of TRAIL and its receptors relative to calcification in AAA. Biochem. Biophys. Res. Commun. 2007, 358, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Brunetti, G.; Oranger, A.; Greco, G.; Di Benedetto, A.; Mori, G.; Colucci, S.; Zallone, A.; Paparella, D.; Grano, M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int. J. Cardiol. 2013, 169, 296–304. [Google Scholar] [CrossRef]
- Tisato, V.; Secchiero, P.; Rimondi, E.; Gianesini, S.; Menegatti, E.; Casciano, F.; Zamboni, P.; Zauli, G. GM-CSF Exhibits Anti-Inflammatory Activity on Endothelial Cells Derived from Chronic Venous Disease Patients. Mediat. Inflamm. 2013, 2013, 1–9. [Google Scholar] [CrossRef][Green Version]
- Niessner, A.; Hohensinner, P.J.; Rychli, K.; Neuhold, S.; Zorn, G.; Richter, B.; Hülsmann, M.; Berger, R.; Mörtl, D.; Huber, K.; et al. Prognostic value of apoptosis markers in advanced heart failure patients. Eur. Heart J. 2008, 30, 789–796. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Ceconi, C.; Parrinello, G.; Volpato, S.; Ferrari, R.; Zauli, G. Potential Prognostic Significance of Decreased Serum Levels of TRAIL after Acute Myocardial Infarction. PLoS ONE 2009, 4, e4442. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Chasseraud, M.; Brazier, M.; Choukroun, G.; Kamel, S.; Massy, Z.A. The circulating soluble TRAIL is a negative marker for inflammation inversely associated with the mortality risk in chronic kidney disease patients. Nephrol. Dial. Transpl. 2010, 25, 2596–2602. [Google Scholar] [CrossRef]
- Volpato, S.; Ferrucci, L.; Secchiero, P.; Corallini, F.; Zuliani, G.; Fellin, R.; Guralnik, J.M.; Bandinelli, S.; Zauli, G. Association of tumor necrosis factor-related apoptosis-inducing ligand with total and cardiovascular mortality in older adults. Atherosclerosis 2011, 215, 452–458. [Google Scholar] [CrossRef]
- Osmancik, P.; Teringova, E.; Toušek, P.; Paulu, P.; Widimsky, P. Prognostic Value of TNF-Related Apoptosis Inducing Ligand (TRAIL) in Acute Coronary Syndrome Patients. PLoS ONE 2013, 8, e53860. [Google Scholar] [CrossRef]
- Mori, K.; Okuno, S.; Shoji, T.; Emoto, M.; Kakutani, Y.; Yamakawa, K.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; Shoji, S.; et al. Tumor necrosis factor-related apoptosis-inducing ligand as an independent predictor of mortality in hemodialysis patents. Cytokine 2013, 61, 912–916. [Google Scholar] [CrossRef]
- Richter, B.; Koller, L.; Hohensinner, P.J.; Zorn, G.; Brekalo, M.; Berger, R.; Mörtl, D.; Maurer, G.; Pacher, R.; Huber, K.; et al. A multi-biomarker risk score improves prediction of long-term mortality in patients with advanced heart failure. Int. J. Cardiol. 2013, 168, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Kuzniewski, M.; Fedak, D.; Dumnicka, P.; Stępień, E.; Kusnierz-Cabala, B.; Cwynar, M.; Sułowicz, W. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv. Med. Sci. 2016, 61, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Michaëlsson, E.; Linde, C.; Donal, E.; Daubert, J.-C.; Gan, L.-M.; Lund, L.H. Inflammatory Biomarkers Predict Heart Failure Severity and Prognosis in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Genet. 2017, 10, e001633. [Google Scholar] [CrossRef]
- Mattisson, I.Y.; Björkbacka, H.; Wigren, M.; Edsfeldt, A.; Melander, O.; Fredrikson, G.N.; Bengtsson, E.; Gonçalves, I.; Orho-Melander, M.; Engström, G.; et al. Elevated Markers of Death Receptor-Activated Apoptosis are Associated with Increased Risk for Development of Diabetes and Cardiovascular Disease. EBioMedicine 2017, 26, 187–197. [Google Scholar] [CrossRef]
- Skau, E.; Henriksen, E.; Wagner, P.; Hedberg, P.; Siegbahn, A.; Leppert, J. GDF-15 and TRAIL-R2 are powerful predictors of long-term mortality in patients with acute myocardial infarction. Eur. J. Prev. Cardiol. 2017, 24, 1576–1583. [Google Scholar] [CrossRef]
- Ajala, O.; Zhang, Y.; Gupta, A.; Bon, J.; Sciurba, F.; Chandra, D. Decreased serum TRAIL is associated with increased mortality in smokers with comorbid emphysema and coronary artery disease. Respir. Med. 2018, 145, 21–27. [Google Scholar] [CrossRef]
- Nowak, C.; Carlsson, A.C.; Östgren, C.J.; Nyström, F.H.; Alam, M.; Feldreich, T.; Sundström, J.; Carrero, J.-J.; Leppert, J.; Hedberg, P.; et al. Multiplex proteomics for prediction of major cardiovascular events in type 2 diabetes. Diabetologia 2018, 61, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Feldreich, T.; Nowak, C.; Fall, T.; Carlsson, A.C.; Carrero, J.-J.; Ripsweden, J.; Qureshi, A.R.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; et al. Circulating proteins as predictors of cardiovascular mortality in end-stage renal disease. J. Nephrol. 2019, 32, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Sharma, A.; Mehta, C.; Bakris, G.; Rossignol, P.; White, W.B.; Zannad, F. Multi-proteomic approach to predict specific cardiovascular events in patients with diabetes and myocardial infarction: Findings from the EXAMINE trial. Clin. Res. Cardiol. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Candido, R.; Corallini, F.; Zacchigna, S.; Toffoli, B.; Rimondi, E.; Fabris, B.; Giacca, M.; Zauli, G. Systemic Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Delivery Shows Antiatherosclerotic Activity in Apolipoprotein E–Null Diabetic Mice. Circulation 2006, 114, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Bossi, F.; Toffoli, B.; Fabris, B. Roles and Clinical Applications of OPG and TRAIL as Biomarkers in Cardiovascular Disease. BioMed. Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, R.; Zauli, G. Emerging non-apoptotic functions of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/Apo2L. J. Cell. Physiol. 2004, 201, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s path in the immune system. Immunology 2009, 127, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Cziupka, K.; Busemann, A.; Partecke, L.I.; Pötschke, C.; Rath, M.; Traeger, T.; Koerner, P.; Von Bernstorff, W.; Kessler, W.; Diedrich, S.; et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) improves the innate immune response and enhances survival in murine polymicrobial sepsis. Crit. Care Med. 2010, 38, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tao, T.; Zhu, J.; Zou, Y.; Wang, J.; Li, J.; Bo, L.; Deng, X. Soluble Tumor Necrosis Factor Related Apoptosis Inducing Ligand Level as a Predictor of Severity of Sepsis and the Risk of Mortality in Septic Patients. PLoS ONE 2013, 8, e82204. [Google Scholar] [CrossRef]
- Liao, X.; Wang, X.; Gu, Y.; Chen, Q.; Chen, L.-Y. Involvement of death receptor signaling in mechanical stretch-induced cardiomyocyte apoptosis. Life Sci. 2005, 77, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Kirkiles-Smith, N.C.; McNiff, J.M.; Pober, J.S. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J. Immunol. 2003, 171, 1526–1533. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Oever, I.A.M.V.D.; Raterman, H.G.; Nurmohamed, M.T.; Simsek, S. Endothelial Dysfunction, Inflammation, and Apoptosis in Diabetes Mellitus. Mediat. Inflamm. 2010, 2010, 1–15. [Google Scholar] [CrossRef]
- Boldt, A.; Wetzel, U.; Lauschke, J.; Weigl, J.; Gummert, J.; Hindricks, G.; Kottkamp, H.; Dhein, S. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004, 90, 400–405. [Google Scholar] [CrossRef]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, G.; Risuglia, N.; Lombardo, G.; Lempereur, L.; Nicoletti, F.; Memo, M.; Bernardini, R. Protective effects of estradiol on TRAIL-induced apoptosis in a human oligodendrocytic cell line: Evidence for multiple sites of interactions. Cell Death Differ. 2004, 11, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, G.; Uberti, D.; Carsana, T.; Lombardo, G.; Bernardini, R.; Memo, M. Neutralization of TRAIL death pathway protects human neuronal cell line from β-amyloid toxicity. Cell Death Differ. 2003, 10, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, L.; Liang, X.; Ma, X.; Liu, Y.; Yang, M.; Liu, K.; Wei, X.; Zhou, Z.; Chen, Y.H.; et al. Blocking TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal damage after transient global cerebral ischemia. Neurobiol. Dis. 2010, 39, 138–147. [Google Scholar] [CrossRef]

| Author | Year | Design of the Study | Population | Number of Patients | Parameters Assessed | Results and Key Observations |
|---|---|---|---|---|---|---|
| Heart Failure | ||||||
| Osmancik et al. [20] | 2013 | Prospective observational study | Patients with heart failure treated with CRT | 81 | Six-month evaluation and two-year follow-up after implantation | TRAIL did not differ in responders and nonresponders, TRAIL did not predict mortality |
| Stenemo et al. [21] | 2017 | Prospective observational study | Elderly patients without heart failure at baseline | 1586 | Eight-year and 10-year follow-up/proteomics profilling | TRAIL-R2 associated with incident heart failure at follow-up and worsened LV systolic function at baseline |
| Cardiomyopathy | ||||||
| Schoppet et al. [22] | 2005 | Case-control study | Man with nonischemic dilated cardiomyopathy | 105 and 86 controls | Comparison to control group | TRAIL elevated comparing to controls, correlating with LV end-diastolic diameter |
| Lula et al. [23] | 2009 | Case-control study | Patients with Chagas cardiomyopathy | 31 and 15 controls | TRAIL concentration compared between groups and hemodynamic parameters | TRAIL concentration enhanced and correlate with LV ejection fraction and LV diastolic diameter |
| Atrial Fibrillation (AF) | ||||||
| Osmancik et al. [24] | 2010 | Prospective observational study | Patients with AF | 25 | Intervention-ablation | TRAIL concentration decreased in patients successfully ablated |
| Deftereos et al. [25] | 2012 | Prospective observational study | Patients with persisted AF, successfully cardioverted to sinus rhythm | 45 | Six-month follow-up, measured transcardiac gradients (coronary sinus concentration minus aortic root concentration) | TRAIL concentration without differences in group with and without AF recurrence, TRAIL transcardiac gradient was negative predictor of AF recurrence |
| Muller et al. [26] | 2013 | Prospective observational study | Patients with sinus rhythm, without history of AF, undergoing cardiac surgery | 33 | TRAIL concentration measured pre- and postoperatively | TRAIL did not predict postoperative AF |
| Rewiuk et al. [27] | 2015 | Prospective observational study | Patients with acute onset of AF | 60 | TRAIL concentration measured initially and 7 to 10 days after pharmacological cardioversion | TRAIL did not predict restoration of sinus rhythm, increase in TRAIL concentration in sinus rhythm maintenance group |
| Chua et al. [28] | 2019 | Prospective observational study | Patients with known AF | 638 | Logistic regression with machine learning algorithms to determine AF risk factors | TRAIL-R2 associated with AF |
| Atherosclerosis | ||||||
| Kawano et al. [29] | 2011 | Cross-sectional study | Patients with type 2 diabetes without symptoms of CAD | 416 | TRAIL concentration measured in correlation with atherosclerotic lesion (IMT) | TRAIL did not correlate with IMT and not differ in group with calcified plaque and without |
| Deftereos et al. [30] | 2011 | Cross-sectional study | Patients with stable angina or positive ischemia noninvasive test | 56 | TRAIL concentration measured during left cardiac catherization; IVUS plaque assessment | TRAIL associated with histologic prototype of plaque |
| Arcidiacono et al. [31] | 2018 | Prospective observational study | Patients with chronic kidney disease without previous CV events | 378 | TRAIL concentration compared with appearance of plaque baseline and after 24-month of follow-up | TRAIL associated negatively with plaque at baseline and with new atheromatous plaques after 24 months |
| Coronary Artery Disease | ||||||
| Schoppet et al. [32] | 2005 | Cross-sectional study | Men undergoing coronary angiography for suspected CAD | 363 | TRAIL concentration compared between group with and without CAD | TRAIL and TRAIL/OPG ratio not correlated with presence or severity of CAD |
| Satoh et al. [33] | 2009 | Prospective observational study | Patients with CAD treated with percutaneous coronary intervention | 85 and 50 controls | 0.5-year follow-up | TRAIL concentration higher in CAD patients and non-restenosis group |
| Mori et al. [34] | 2010 | Cross-sectional study | Patients undergoing coronary angiography | 285 | TRAIL concentration assessed with CAD severity | TRAIL concentration was inversely correlated with severity of CAD |
| Secchiero et al. [35] | 2010 | Case-control study | Patients with AMI | 113 and 21 with unstable angina and 120 controls | TRAIL concentration compared between groups | TRAIL concentration decreased in AMI patients, CAD patients characterized by an increased OPG/TRAIL ratio |
| Shaker et al. [36] | 2010 | Case-control study | Male patients with AMI and CAD | 28 with AMI, 30 with CAD and 20 controls | TRAIL concentration compared between groups | TRAIL concentration lover in AMI patients |
| Deftereos et al. [37] | 2012 | Prospective observational study | Patients undergoing percutaneous coronary intervention with drug-eluting stent | 67 | 12-months follow-up | TRAIL negatively correlated indices of neointimal hyperplasia and positively correlated in-stent minimum lumen area |
| Song et al. [38] | 2012 | Case-control study | Patients with CAD undergoing stent implantation | 42 and 17 controls | 0.5-year follow-up | TRAIL concentration was increased 1 month after angioplasty |
| Luz et al. [39] | 2016 | Prospective observational study | Patients with STEMI | 66 | TRAIL concentration measured between group treated with postconditioning and without, nine-month follow-up | TRAIL was augmented by postconditioning and correlated to infarct size and LVEF |
| Manuneedhi Cholan et al. [40] | 2018 | Case-control study | Patients with stable CAD | 9 and 7 controls | TRAIL and F2-isoprostanes concentration compared between groups | TRAIL concentration was reduced in CAD patients and correlated with marker of oxidative stress |
| Teringova et al. [41] | 2018 | Prospective observational study | Patients with STEMI treated with primary percutaneous coronary intervention | 101 | TRAIL concentration measured at baseline and one month after, two-year follow-up | TRAIL reaches its lowest serum concentration after reperfusion, low TRAIL concentration is associated with worse LVEF |
| Vascular Calcification | ||||||
| Chasseraud et al. [42] | 2011 | Cross-sectional study | Hemodialysis patients | 38 | TRAIL concentration measured in hemodialysis patients and compared with calcification levels | TRAIL decreased in serum of hemodialysis patients but not correlate with calcification |
| Moon et al. [43] | 2019 | Cross-sectional study | Patients with diabetes and without diabetes | 71 | Diagnosis of PAD based on ABI results; calcification determined by computed tomography scan | TRAIL was downregulated in patient with PAD and vascular calcification |
| Cerebrovascular Disease | ||||||
| Sarchielli et al. [44] | 2013 | Case-control study | Patients with silent brain infarction and lacunar infarct | 49 and 31 controls | Assessment of pathophysiology of silent brain infarction | TRAIL concentration was higher in patients with silent brain infarction |
| Kang et al. [45] | 2015 | Cross-sectional study | Patients with ischemic acute stroke | 293 | Assessment of TRAIL correlation with stroke volume | Low concentration of TRAIL correlated with stroke severity |
| Pan et al. [46] | 2015 | Prospective observational study | Patients with large artery atherosclerosis stroke | 132 and 60 controls | Three-month follow-up | TRAIL concentration lower in patients with large artery atherosclerosis stroke; TRAIL negatively correlated with prognosis |
| Other | ||||||
| O’Sullivan et al. [47] | 2010 | Case-control study | Patients with PAD | 83 and 21 controls | PAD assessed using ABI, TRAIL concentration measured between groups with PAD and diabetes | TRAIL concentration was higher in patients with PAD |
| Karatolios et al. [48] | 2011 | Cross-sectional study | Patients with pericardial effusion | 83 | Assessment TRAIL concentration in pericardial effusion associated with malignancy, CAD and non-malignant | TRAIL concentration was higher in pericardial effusion associated with malignancy and CAD |
| Zhou et al. [49] | 2014 | Validation study | Pregnant women | 812 | Serum samples taken from 8 to 20 week gestation; than assessed those who developed hypertension | TRAIL concentration lower in patients who developed pregnancy hypertension than uncomplicated pregnancies |
| Liu et al. [50] | 2015 | Prospective observational study | Patients with pulmonary hypertension | 78 and 80 controls | Comparison of TRAIL concentration between groups, 2-years follow-up | TRAIL concentration higher in patients with pulmonary hypertension; elevated TRAIL associated with eventual complications |
| General Cardiovascular Risk | ||||||
| Wigren et al. [51] | 2018 | Cross-sectional study | Patients with systemic lupus erythematosus | 484 and 253 controls | Comparison of TRAIL-R2 concentration between groups | 14% of patients had CVD (CAD, cerebrovascular disease, PAD); patients with CVD had higher concentration of TRAIL-R2 than those without |
| Goncalves et al. [52] | 2019 | Prospective observational study | CPIP cohort (patients with atherosclerosis) | 558 | 37-month follow-up | Higher TRAIL-R2 associated with higher CV risk in future |
| Author | Year | Investigated Disease | Population | Number of Patients | Parameters Assessed | Results and Key Observations |
|---|---|---|---|---|---|---|
| Yndestad et al. [53] | 2002 | Heart failure | Patients with heart failure | 8 and 8 controls | Analysis of gene expression in peripheral blood mononuclear cells | TRAIL gene downregulated in chronic heart failure patients |
| Cao et al. [54] | 2011 | Atrial fibrillation | Patients with AF | 48 and 48 controls | Tissue obtained during mitral valve surgery | TRAIL gene expression upregulated |
| Schoppet et al. [55] | 2004 | Atherosclerosis | Patients’ samples of vascular tissue | 8 and 4 normal vessels | Analysis of samples of Mönckeberg’s sclerosis and atherosclerotic arteries | TRAIL detected in calcified regions of Mönckeberg’s sclerosis and atherosclerotic arteries |
| Michowitz et al. [56] | 2005 | Atherosclerosis | Patient’s samples of atherosclerotic plaques | 24 | TRAIL expression assessed on peripheral mononuclear cells when stimulated by oxLDL | TRAIL expression enhanced by oxLDL in atherosclerotic lesions; TRAIL serum concentration reduced in patients with unstable CAD |
| Niessner et al. [57] | 2006 | Atherosclerosis | Patients with ACS | 31 | TRAIL expression assessed on CD4 T-cells when stimulated by IFNα produced by activated plasmacytoid dendritic cells | TRAIL expression is enhanced by IFNα in atherosclerotic lesions |
| Goncalvez et al. | 2019 | Atherosclerosis | Patients’ samples of carotid plaques | 202 | TRAIL expression analyzed in atherosclerotic lesion | TRAIL-R2 and TRAIL expression were increased in symptomatic carotid plaques |
| Nakajima et al. [58] | 2003 | Coronary artery disease | Patients with AMI | 26 and 16 controls | Compared expression of TRAIL on peripheral blood mononuclear cells between groups | TRAIL expression was enhanced in AMI patients |
| Sato et al. [59] | 2006 | Coronary artery disease | Patients with ACS | 50 and 33 controls | Examined whether the TRAIL pathway was involved in CD4 T cell-mediated apoptosis | TRAIL expression enhanced on CD4 T-cells in patients with ACS |
| Corallini et al. [60] | 2009 | Coronary artery disease | Patients with AMI | 80 and 40 controls | Analysed the relationship of TRAIL with mesenchymal stem cells (role in regeneration after AMI) | TNFα enhanced the migration of mesenchymal stem cells in response to TRAIL |
| Sato et al. [61] | 2010 | Coronary artery disease | Patients with ACS | 55 and 34 controls | Intervention-administration of statins and TRAIL-specific antibodies | TRAIL-R2 upregulated on endothelial cells, T cell mediated endothelial death was dependent on the TRAIL pathway |
| Secchiero et al. [62] | 2010 | Coronary artery disease | Patients with AMI | 30 | Evaluated the potential role of metalloproteinase 2 in promoting the cleavage of TRAIL after AMI | TRAIL concentration showed inverse correlation with MMP2/TIMP2 ratio |
| Liu et al. [63] | 2007 | Vascular calcification | Patients’ aortic samples taken during abdominal aortic aneurysm | 33 and 8 controls | calcification levels were determined by computed tomography scan | TRAIL and TRAIL-R1 expression were higher in aneurysm samples and in calcified samples |
| Galeone et al. [64] | 2013 | Vascular calcification | Patients’ samples of severe calcific aortic stenosis taken during valve replacement | 10 and 10 controls | Immunohistochemistry and confocal microscopy used for TRAIL immunostaining | higher TRAIL concentration detected in calcific aortic valves and serum |
| Hameed et al. [17] | 2012 | Pulmonary hypertension | Specimens of human pulmonary artery smooth muscle cells | - | Assessment of TRAIL expression in smooth muscle cells | TRAIL upregulated in pulmonary artery smooth muscle cells in patients with pulmonary hypertension |
| Tisato et al. [65] | 2013 | Chronic venous disease | Specimens of venous endothelial cells from patients with chronic venous disease | 20 | Assessment of TRAIL expression in correlation with hemodynamic parameters and after GM-CSF exposure | TRAIL expression correlated positively with resistance index and GM-CSF |
| Author | Year | Population | Measured Protein | Number of Patients | Follow-Up | Death/CV Deaths | Assessed Outcome | Relation TRAIL to All-Cause Mortality | Relation TRAIL to CV Mortality | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI | Stroke | CV Death | All-Cause Death | Re-Hospitalization | ||||||||||
| Niessner et al. [66] | 2009 | Patients with HF * | TRAIL | 351 | 16 months | 93/ND | - | - | - | yes | yes | inversely p < 0.001 | n/a | TRAIL predicted outcome (all-cause mortality and rehospitalization) |
| Secchiero et al. [67] | 2009 | Patients with AMI | TRAIL | 60 and 60 control group | 12 months | 10/9 | - | - | yes | yes | - | ND | inversely p = 0.001 | TRAIL concentration decreased at baseline, low levels of TRAIL at discharge was prognostic factor of cardiac death and heart failure at 12 months |
| Liabeuf et al. [68] | 2010 | Chronic kidney disease patients | TRAIL | 130 | 2 years | 36/19 | - | - | yes | yes | - | inversely p = 0.010 | NSS | lowest TRAIL was associated with infectious but not CV mortality |
| Volpato et al. [69] | 2011 | inCHIANTI study (older people with CVD) | TRAIL | 1282 | 6 years | 259/112 | - | - | yes | yes | - | inversely p = 0.008 | inversely ND | an association was found between prevalent CV disease and TRAIL |
| Osmancik et al. [70] | 2013 | Patients with ACS | TRAIL | 295 | 0.5 year | 12/ND | yes | yes (but not evaluated) | - | yes | yes | inversely p = 0.001 | n/a | TRAIL predicted all-cause mortality, re-MI and combined end point (death and hospitalization for HF) |
| Mori et al. [71] | 2013 | Male hemodialysis patients | TRAIL | 149 | 36 months | 33/11 | - | - | yes | yes | - | inversely p = 0.011 | NSS | TRAIL associated with infectious and all-cause mortality but not CV mortality |
| Richter et al. [72] | 2013 | Patients with HF * | TRAIL | 349 | 5 years | 195/145 | - | - | - | yes | - | inversely p < 0.001 | n/a | TRAIL predicted all-cause mortality |
| Kuzniewski et al. [73] | 2016 | Hemodialysis patients | TRAIL | 69 and 35 controls | 7 years | 39/31 | - | - | yes | yes | - | NSS | NSS | TRAIL did not predict CV mortality; OPG/TRAIL ratio positively predicted all-cause and CV mortality |
| Hage et al. [74] | 2017 | Patients with HF with preserved ejection fraction/proteomic study | TRAIL and TRAIL-R2 | 86 | 1.5 years | 11/ND | - | - | - | yes | yes | ND (composite outcome) | n/a | TRAIL and TRAIL-R2 predicting outcome (all-cause mortality or re-hospitalization |
| Mattisson et al. [75] | 2017 | MDC study (general population) | TRAIL-R2 | 4742 | 19 years | ND/278 | yes | yes | yes | - | - | n/a | n/a | higher TRAIL-R2 was associated with increased risk of CV events (myocardial infarction and stroke) |
| Skau et al. [76] | 2017 | Patients with AMI | TRAIL-R2 | 847 | 7 years | 207/ND | - | - | - | yes | - | n/a | n/a | TRAIL-R2 predicted all-cause mortality |
| Ajala et al. [77] | 2018 | Smokers | TRAIL | 474 | 8 years | 83/ND | - | - | - | yes | - | inversely p = 0.004 | n/a | TRAIL concentration reduced in smokers with comorbid emphysema and CAD; related to reduced survival/ CAD assessed by quantifying coronary artery calcium |
| Nowak et al. [78] | 2018 | Patients with diabetes | TRAIL-R2 | 1211 | 6 years | ND | yes | yes | - | yes | - | n/a | n/a | TRAIL-R2 increased concentration associated with incident major adverse CV events |
| Feldreich et al. [79] | 2019 | MIMICK study (hemodialysis patients)/proteomic study | TRAIL-R2 | 183 | 43 months | ND/45 | - | - | yes | - | - | n/a | n/a | TRAIL-R2 associated with CV mortality |
| Ferreira et al. [80] | 2020 | Patients with diabetes after MI/proteomic study | TRAIL-R2 | 5131 | 1.5 year | 302/226 | yes | yes | yes | yes | yes | n/a | n/a | TRAIL-R2 prognose all-cause mortality and CV death or HF hospitalization |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakareko, K.; Rydzewska-Rosołowska, A.; Zbroch, E.; Hryszko, T. TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review. J. Clin. Med. 2021, 10, 1252. https://doi.org/10.3390/jcm10061252
Kakareko K, Rydzewska-Rosołowska A, Zbroch E, Hryszko T. TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review. Journal of Clinical Medicine. 2021; 10(6):1252. https://doi.org/10.3390/jcm10061252
Chicago/Turabian StyleKakareko, Katarzyna, Alicja Rydzewska-Rosołowska, Edyta Zbroch, and Tomasz Hryszko. 2021. "TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review" Journal of Clinical Medicine 10, no. 6: 1252. https://doi.org/10.3390/jcm10061252
APA StyleKakareko, K., Rydzewska-Rosołowska, A., Zbroch, E., & Hryszko, T. (2021). TRAIL and Cardiovascular Disease—A Risk Factor or Risk Marker: A Systematic Review. Journal of Clinical Medicine, 10(6), 1252. https://doi.org/10.3390/jcm10061252







