T1a Glottic Cancer: Advances in Vocal Outcome Assessment after Transoral CO2-Laser Microsurgery Using the VEM
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Surgical Procedure and Postoperative Regimen
2.3. Examination Instruments and Criteria
3. Data Analysis
4. Results
4.1. Sample Description and Preoperative Assessment
4.2. Postoperative Assessment
5. Discussion
Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on Larynx Cancer Epidemiology. Chin. J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An Update on Larynx Cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer. 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; WHO: Lyon, France, 2017. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley: Chichester, UK, 2016. [Google Scholar]
- Agaimy, A.; Weichert, W. Grading of Head and Neck Neoplasms. Pathologe 2016, 37, 285–292. [Google Scholar] [CrossRef]
- Williamson, A.J.; Bondje, S. Glottic Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Markou, K.; Christoforidou, A.; Karasmanis, I.; Tsiropoulos, G.; Triaridis, S.; Constantinidis, I.; Vital, V.; Nikolaou, A. Laryngeal Cancer: Epidemiological Data from Nuorthern Greece and Review of the Literature. Hippokratia 2013, 17, 313–318. [Google Scholar] [PubMed]
- Pantel, M.; Guntinas-Lichius, O. Laryngeal Carcinoma: Epidemiology, Risk Factors and Survival. HNO 2012, 60, 32–40. [Google Scholar] [CrossRef]
- Nahavandipour, A.; Jakobsen, K.K.; Gronhoj, C.; Hebbelstrup Jensen, D.; Kim Schmidt Karnov, K.; Klitmoller Agander, T.; Specht, L.; von Buchwald, C. Incidence and Survival of Laryngeal Cancer in Denmark: A Nation-wide Study from 1980 to 2014. Acta Oncol. 2019, 58, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Brandstorp-Boesen, J.; Sorum Falk, R.; Boysen, M.; Brondbo, K. Impact of Stage, Management and Recurrence on Survival Rates in Laryngeal Cancer. PLoS ONE 2017, 12, e0179371. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S. Evidence-Based Review of Laryngeal Cancer Surgery. Laryngorhinootologie 2016, 95 (Suppl. 1), S192–S216. [Google Scholar] [CrossRef]
- Forner, D.; Rigby, M.H.; Corsten, M.; Trites, J.R.; Pyne, J.; Taylor, S.M. Oncological and Functional Outcomes after Repeat Transoral Laser Microsurgery for the Treatment of Recurrent Early Glottic Cancer. J. Laryngol. Otol. 2020, 1–5. [Google Scholar] [CrossRef]
- Luscher, M.S.; Pedersen, U.; Johansen, L.V. Treatment Outcome after Laser Excision of Early Glottic Squamous Cell Carcinoma--A Literature Survey. Acta Oncol. 2001, 40, 796–800. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Q.; Wang, H.; Li, B.; Liu, J.; Hu, J.; Liu, S.; Zou, J. Prognostic Comparison of Transoral Laser Microsurgery for Early Glottic Cancer with or without Anterior Commissure Involvement: A Meta-analysis. Am. J. Otolaryngol. 2021, 42, 102787. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, M.; Sjogren, E.V. Involvement of the Anterior Commissure in Early Glottic Cancer (Tis-T2): A Review of the Literature. Cancers 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Steiner, W. Results of Curative Laser Microsurgery of Laryngeal Carcinomas. Am. J. Otolaryngol. 1993, 14, 116–121. [Google Scholar] [CrossRef]
- Ledda, G.P.; Puxeddu, R. Carbon Dioxide Laser Microsurgery for Early Glottic Carcinoma. Otolaryngol. Head Neck Surg. 2006, 134, 911–915. [Google Scholar] [CrossRef]
- Canis, M.; Ihler, F.; Martin, A.; Matthias, C.; Steiner, W. Transoral Laser Microsurgery for T1a Glottic Cancer: Review of 404 Cases. Head Neck 2015, 37, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Goyal, A.; Goyal, M.; Goel, S. Oncological Outcomes Following Transoral CO2 Laser Microsurgery for T1 Glottic Cancer. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. 1), 542–547. [Google Scholar] [CrossRef] [PubMed]
- Arens, C. Transoral Treatment Strategies for Head and Neck Tumors. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2012, 11. [Google Scholar] [CrossRef]
- Wiegand, S. Evidence and Evidence Gaps of Laryngeal Cancer Surgery. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2016, 15. [Google Scholar] [CrossRef]
- Ambrosch, P.; Fazel, A. Functional Organ Preservation in Laryngeal and Hypopharyngeal Cancer. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2011, 10. [Google Scholar] [CrossRef]
- Chatenoud, L.; Garavello, W.; Pagan, E.; Bertuccio, P.; Gallus, S.; La Vecchia, C.; Negri, E.; Bosetti, C. Laryngeal Cancer Mortality Trends in European Countries. Int. J. Cancer 2016, 138, 833–842. [Google Scholar] [CrossRef]
- Stachler, R.J.; Francis, D.O.; Schwartz, S.R.; Damask, C.C.; Digoy, G.P.; Krouse, H.J.; McCoy, S.J.; Ouellette, D.R.; Patel, R.R.; Reavis, C.C.W.; et al. Clinical Practice Guideline: Hoarseness (Dysphonia) (Update). Otolaryngol. Head Neck Surg. 2018, 158 (Suppl. 1), S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Tikka, T.; Pracy, P.; Paleri, V. Refining the Head and Neck Cancer Referral Guidelines: A Two Centre Analysis of 4715 Referrals. Br. J. Oral Maxillofac. Surg. 2016, 54, 141–150. [Google Scholar] [CrossRef][Green Version]
- Caffier, P.P.; Schmidt, B.; Gross, M.; Karnetzky, K.; Nawka, T.; Rotter, A.; Seipelt, M.; Sedlmaier, B. A Comparison of White Light Laryngostroboscopy versus Autofluorescence Endoscopy in the Evaluation of Vocal Fold Pathology. Laryngoscope 2013, 123, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Whited, C.W.; Dailey, S.H. Evaluation of the Dysphonic Patient (in: Function Preservation in Laryngeal Cancer). Otolaryngol. Clin. N. Am. 2015, 48, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Cocco, D.; De Benedetto, L.; Del Bon, F.; Nicolai, P.; Peretti, G. Narrow Band Imaging and High Definition Television in the Assessment of Laryngeal Cancer: A Prospective Study on 279 Patients. Eur. Arch. Otorhinolaryngol. 2010, 267, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ptok, M.; Schwemmle, C.; Iven, C.; Jessen, M.; Nawka, T. On the Auditory Evaluation of Voice Quality. HNO 2006, 54, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Smith, J.D.; Hogikyan, N.D. The White Lesion, Hyperkeratosis, and Dysplasia. Otolaryngol. Clin N. Am. 2019, 52, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Nawka, T.; Martin, A.; Caffier, P.P. Microlaryngoscopy and Phonomicrosurgery. HNO 2013, 61, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Laoufi, S.; Brasnu, D.F. Voice Outcomes of Transoral Laser Microsurgery of the Larynx. Otolaryngol. Clin. N. Am. 2015, 48, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mau, T.; Palaparthi, A.; Riede, T.; Titze, I.R. Effect of Resection Depth of Early Glottic Cancer on Vocal Outcome: An Optimized Finite Element Simulation. Laryngoscope 2015, 125, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Peeters, A.J.; van Gogh, C.D.; Goor, K.M.; Verdonck-de Leeuw, I.M.; Langendijk, J.A.; Mahieu, H.F. Health Status and Voice Outcome after Treatment for T1a Glottic Carcinoma. Eur. Arch. Otorhinolaryngol. 2004, 261, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Culie, D.; Poissonnet, G.; Dassonville, O. Current Role of Primary Surgical Treatment in Patients with Head and Neck Squamous Cell Carcinoma. Curr. Opin. Oncol. 2019, 31, 138–145. [Google Scholar] [CrossRef]
- Hartl, D.M.; Brasnu, D.F. Contemporary Surgical Management of Early Glottic Cancer. Otolaryngol. Clin. N. Am. 2015, 48, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Baird, B.J.; Sung, C.K.; Beadle, B.M.; Divi, V. Treatment of Early-stage Laryngeal Cancer: A Comparison of Treatment Options. Oral Oncol. 2018, 87, 8–16. [Google Scholar] [CrossRef]
- Huang, G.; Luo, M.; Zhang, J.; Liu, H. Laser Surgery versus Radiotherapy for T1a Glottic Carcinoma: A Meta-analysis of Oncologic Outcomes. Acta Otolaryngol. 2017, 137, 1204–1209. [Google Scholar] [CrossRef]
- Strong, M.S.; Jako, G.J. Laser Surgery in the Larynx. Early Clinical Experience with Continuous CO2 Laser. Ann. Otol. Rhinol. Laryngol. 1972, 81, 791–798. [Google Scholar] [CrossRef]
- Harris, A.T.; Tanyi, A.; Hart, R.D.; Trites, J.; Rigby, M.H.; Lancaster, J.; Nicolaides, A.; Taylor, S.M. Transoral Laser Surgery for Laryngeal Carcinoma: Has Steiner Achieved a Genuine Paradigm Shift in Oncological Surgery? Ann. R. Coll. Surg. Engl. 2018, 100, 2–5. [Google Scholar] [CrossRef]
- Sjogren, E.V. Transoral Laser Microsurgery in Early Glottic Lesions. Curr. Otorhinolaryngol. Rep. 2017, 5, 56–68. [Google Scholar] [CrossRef]
- Peretti, G.; Piazza, C.; Cocco, D.; De Benedetto, L.; Del Bon, F.; Redaelli De Zinis, L.O.; Nicolai, P. Transoral CO(2) Laser Treatment for T(is)-T(3) Glottic Cancer: The University of Brescia Experience on 595 Patients. Head Neck 2010, 32, 977–983. [Google Scholar] [CrossRef]
- Remacle, M.; Van Haverbeke, C.; Eckel, H.; Bradley, P.; Chevalier, D.; Djukic, V.; de Vicentiis, M.; Friedrich, G.; Olofsson, J.; Peretti, G.; et al. Proposal for Revision of the European Laryngological Society Classification of Endoscopic Cordectomies. Eur. Arch. Otorhinolaryngol. 2007, 264, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Caffier, P.P.; Moller, A.; Forbes, E.; Muller, C.; Freymann, M.L.; Nawka, T. The Vocal Extent Measure: Development of a Novel Parameter in Voice Diagnostics and Initial Clinical Experience. BioMed Res. Int. 2018, 2018, 3836714. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Caffier, P.P.; Nawka, T.; Ibrahim-Nasr, A.; Thomas, B.; Muller, H.; Ko, S.R.; Song, W.; Gross, M.; Weikert, S. Development of Three-dimensional Laryngostroboscopy for Office-based Laryngeal Diagnostics and Phonosurgical Therapy. Laryngoscope 2018, 128, 2823–2831. [Google Scholar] [CrossRef]
- Patel, R.R.; Awan, S.N.; Barkmeier-Kraemer, J.; Courey, M.; Deliyski, D.; Eadie, T.; Paul, D.; Švec, J.G.; Hillman, R. Recommended Protocols for Instrumental Assessment of Voice: American Speech-Language-Hearing Association Expert Panel to Develop a Protocol for Instrumental Assessment of Vocal Function. Am. J. Speech Lang. Pathol. 2018, 27, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Dejonckere, P.H.; Bradley, P.; Clemente, P.; Cornut, G.; Crevier-Buchman, L.; Friedrich, G.; Van De Heyning, P.; Remacle, M.; Woisard, V.; Committee on Phoniatrics of the European Laryngological Society (ELS). A Basic Protocol for Functional Assessment of Voice Pathology, Especially for Investigating the Efficacy of (Phonosurgical) Treatments and Evaluating New Assessment Techniques. Guideline Elaborated by the Committee on Phoniatrics of the European Laryngological Society (ELS). Eur. Arch. Otorhinolaryngol. 2001, 258, 77–82. [Google Scholar] [CrossRef]
- Ternström, S.; Pabon, P.; Södersten, M. The Voice Range Profile: Its Function, Applications, Pitfalls and Potential. Acta Acust. United Acust. 2016, 102, 268–283. [Google Scholar] [CrossRef]
- Nawka, T.; Verdonck-de Leeuw, I.M.; De Bodt, M.; Guimaraes, I.; Holmberg, E.B.; Rosen, C.A.; Schindler, A.; Woisard, V.; Whurr, R.; Konerding, U. Item reduction of the voice handicap index based on the original version and on European translations. Folia Phoniatr. Logop. 2009, 61, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, F.L.; De Bodt, M.S.; Molenberghs, G.; Remacle, M.; Heylen, L.; Millet, B.; Van Lierde, K.; Raes, J.; Van de Heyning, P.H. The Dysphonia Severity Index: An Objective Measure of Vocal Quality Based on a Multiparameter Approach. J. Speech Lang. Hear Res. 2000, 43, 796–809. [Google Scholar] [CrossRef]
- Greulich, M.T.; Parker, N.P.; Lee, P.; Merati, A.L.; Misono, S. Voice Outcomes Following Radiation versus Laser Microsurgery for T1 Glottic Carcinoma: Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2015, 152, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, P.M.; Majumdar, S.; Morton, R.P. Quality of Life and Functional Outcomes in the Management of Early Glottic Carcinoma: A Systematic Review of Studies Comparing Radiotherapy and Transoral Laser Microsurgery. Clin. Otolaryngol. 2010, 35, 373–382. [Google Scholar] [CrossRef]
- Cohen, S.M.; Garrett, C.G.; Dupont, W.D.; Ossoff, R.H.; Courey, M.S. Voice-related Quality of Life in T1 Glottic Cancer: Irradiation versus Endoscopic Excision. Ann. Otol. Rhinol. Laryngol. 2006, 115, 581–586. [Google Scholar] [CrossRef]
- Laoufi, S.; Mirghani, H.; Janot, F.; Hartl, D.M. Voice Quality after Treatment of T1a Glottic Cancer. Laryngoscope 2014, 124, 1398–1401. [Google Scholar] [CrossRef]
- Krengli, M.; Policarpo, M.; Manfredda, I.; Aluffi, P.; Gambaro, G.; Panella, M.; Pia, F. Voice Quality after Treatment for T1a Glottic Carcinoma—Radiotherapy versus Laser Cordectomy. Acta Oncol. 2004, 43, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Gupta, S.; Rajopadhye, G. A Comparison of Phonatory outcome Between Trans-oral CO2 Laser Cordectomy and Radiotherapy in T1 Glottic Cancer. Eur. Arch. Otorhinolaryngol. 2018, 275, 2783–2786. [Google Scholar] [CrossRef]
- van Gogh, C.D.; Verdonck-de Leeuw, I.M.; Wedler-Peeters, J.; Langendijk, J.A.; Mahieu, H.F. Prospective Evaluation of Voice Outcome during the First Two Years in Male Patients Treated by Radiotherapy or Laser Surgery for T1a Glottic Carcinoma. Eur. Arch. Otorhinolaryngol. 2012, 269, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.T.; Park, M.J.; Hong, K.H. Characteristics of Speech Production in Patients with T1 Glottic Cancer who Underwent Laser Cordectomy or Radiotherapy. Logoped Phoniatr. Vocol. 2018, 43, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Saito, K.; Yabe, H.; Uno, K.; Ogawa, K. Comparative Multidimensional Assessment of Laryngeal Function and Quality of Life after Radiotherapy and Laser Surgery for Early Glottic Cancer. Head Neck 2016, 38, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Nunez Batalla, F.; Caminero Cueva, M.J.; Senaris Gonzalez, B.; Llorente Pendas, J.L.; Gorriz Gil, C.; Lopez Llames, A.; Alonso Pantiga, R.; Suárez Nieto, C. Voice Quality after Endoscopic Laser Surgery and Radiotherapy for Early Glottic Cancer: Objective Measurements Emphasizing the Voice Handicap Index. Eur. Arch. Otorhinolaryngol. 2008, 265, 543–548. [Google Scholar] [CrossRef]
- Sjogren, E.V.; van Rossum, M.A.; Langeveld, T.P.; Voerman, M.S.; van de Kamp, V.A.; Friebel, M.O.; Wolterbeek, R.; Baatenburg de Jong, R.J. Voice Outcome in T1a Midcord Glottic Carcinoma: Laser Surgery vs Radiotherapy. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 965–972. [Google Scholar] [CrossRef]
- Berania, I.; Dagenais, C.; Moubayed, S.P.; Ayad, T.; Olivier, M.-J.; Guertin, L.; Bissada, E.; Tabet, J.C.; Christopoulos, A. Voice and Functional Outcomes of Transoral Laser Microsurgery for Early Glottic Cancer: Ventricular Fold Resection as a Surrogate. J. Clin. Med. Res. 2015, 7, 632–636. [Google Scholar] [CrossRef][Green Version]
- Bertino, G.; Degiorgi, G.; Tinelli, C.; Cacciola, S.; Occhini, A.; Benazzo, M. CO2 Laser Cordectomy for T1-T2 Glottic Cancer: Oncological and Functional Long-term Results. Eur. Arch. Otorhinolaryngol. 2015, 272, 2389–2395. [Google Scholar] [CrossRef]
- Bajaj, Y.; Uppal, S.; Sharma, R.K.; Grace, A.R.; Howard, D.M.; Nicolaides, A.R.; Coatesworth, A.P. Evaluation of Voice and Quality of Life after Transoral Endoscopic Laser Resection of Early Glottic Carcinoma. J. Laryngol. Otol. 2011, 125, 706–713. [Google Scholar] [CrossRef]
- Keilmann, A.; Napiontek, U.; Engel, C.; Nakarat, T.; Schneider, A.; Mann, W. Long-term Functional Outcome after Unilateral Cordectomy. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 38–46. [Google Scholar] [CrossRef]
- Motta, S.; Cesari, U.; Mesolella, M.; Motta, G. Functional Vocal Results after CO2 Laser Endoscopic Surgery for Glottic Tumours. J. Laryngol. Otol. 2008, 122, 948–951. [Google Scholar] [CrossRef]
- Hamzany, Y.; Crevier-Buchman, L.; Lechien, J.R.; Bachar, G.; Brasnu, D.; Hans, S. Multidimensional Voice Quality Evaluation After Transoral CO2 Laser Cordectomy: A Prospective Study. Ear Nose Throat J. 2021, 100 (Suppl. 1), 27S–32S. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, J.S.; Kim, S.W.; Noh, W.J.; Kim, Y.J.; Oh, D.; Hong, J.C.; Lee, K.D. Voice Outcome According to Surgical Extent of Transoral Laser Microsurgery for T1 Glottic Carcinoma. Laryngoscope 2016, 126, 2051–2056. [Google Scholar] [CrossRef]
- Fink, D.S.; Sibley, H.; Kunduk, M.; Schexnaildre, M.; Kakade, A.; Sutton, C.; McWhorter, A.J. Subjective and Objective Voice Outcomes After Transoral Laser Microsurgery for Early Glottic Cancer. Laryngoscope 2016, 126, 405–407. [Google Scholar] [CrossRef]
- Tomifuji, M.; Araki, K.; Niwa, K.; Miyagawa, Y.; Mizokami, D.; Kitagawa, Y.; Yamashita, T.; Matsunobu, T.; Shiotani, A. Comparison of Voice Quality After Laser Cordectomy with That After Radiotherapy or Chemoradiotherapy for Early Glottic Carcinoma. ORL J. Otorhinolaryngol. Relat. Spec. 2013, 75, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Balzanelli, C.; Mensi, M.C.; Rossini, M.; Antonelli, A.R. Preoperative and Postoperative Voice in Tis-T1 Glottic Cancer Treated by Endoscopic Cordectomy: An Additional Issue for Patient Counseling. Ann. Otol. Rhinol. Laryngol. 2003, 112, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, D.H.; Kim, S.Y.; Park, C.I. Quality of Life and Voice in Patients After Laser Cordectomy for Tis and T1 Glottic Carcinomas. Head Neck 2007, 29, 1010–1016. [Google Scholar] [CrossRef]
- Strieth, S.; Ernst, B.P.; Both, I.; Hirth, D.; Pfisterer, L.N.; Kunzel, J.; Eder, K. Randomized Controlled Single-blinded Clinical Trial of Functional Voice Outcome after Vascular Targeting KTP laser Microsurgery of Early Laryngeal Cancer. Head Neck 2019, 41, 899–907. [Google Scholar] [CrossRef]
- Friedman, A.D.; Hillman, R.E.; Landau-Zemer, T.; Burns, J.A.; Zeitels, S.M. Voice Outcomes for Photoangiolytic KTP Laser Treatment of Early Glottic Cancer. Ann. Otol. Rhinol. Laryngol. 2013, 122, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.E.; Rigby, M.H.; MacLean, M.; Taylor, S.M. ‘How Does That sound?’: Objective and Subjective Voice Outcomes Following CO(2) Laser Resection for Early Glottic Cancer. J. Laryngol. Otol. 2011, 125, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, I.; Huerta, P.; Blanch, J.L.; Fernandez-Planas, A.M.; Jimenez, C.; Bernal-Sprekelsen, M. Voice Quality after CO2 Laser Cordectomy--What Can We Really Expect? Head Neck 2008, 30, 43–49. [Google Scholar] [CrossRef]
- Aichinger, P.; Feichter, F.; Aichstill, B.; Bigenzahn, W.; Schneider-Stickler, B. Inter-device Reliability of DSI Measurement. Logoped. Phoniatr. Vocol. 2012, 37, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hakkesteegt, M.M.; Brocaar, M.P.; Wieringa, M.H.; Feenstra, L. Influence of Age and Gender on the Dysphonia Severity Index. A Study of Normative Values. Folia Phoniatr. Logop. 2006, 58, 264–273. [Google Scholar] [CrossRef]
- Hakkesteegt, M.M.; Brocaar, M.P.; Wieringa, M.H. The Applicability of the Dysphonia Severity Index and the Voice Handicap Index in Evaluating Effects of Voice Therapy and Phonosurgery. J. Voice 2010, 24, 199–205. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Hsu, Y.-B.; Lee, T.-L.; Fu, S.; Wang, L.-M.; Kao, Y.-C. Longitudinal Analysis of Voice Quality in Patients with Early Glottic Cancer after Transoral Laser Microsurgery. Head Neck 2012, 34, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.A.; Har-El, G.; Shapshay, S.; Maune, S.; Zeitels, S.M. Endoscopic Laser Resection of Laryngeal Cancer: Is it Oncologically Safe? Position Statement from the American Broncho-Esophagological Association. Ann. Otol. Rhinol. Laryngol. 2009, 118, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Aluffi Valletti, P.; Taranto, F.; Chiesa, A.; Pia, F.; Valente, G. Impact of Resection Margin Status on Oncological Outcomes after CO2 Laser Cordectomy. Acta Otorhinolaryngol. Ital. 2018, 38, 24–30. [Google Scholar] [PubMed]
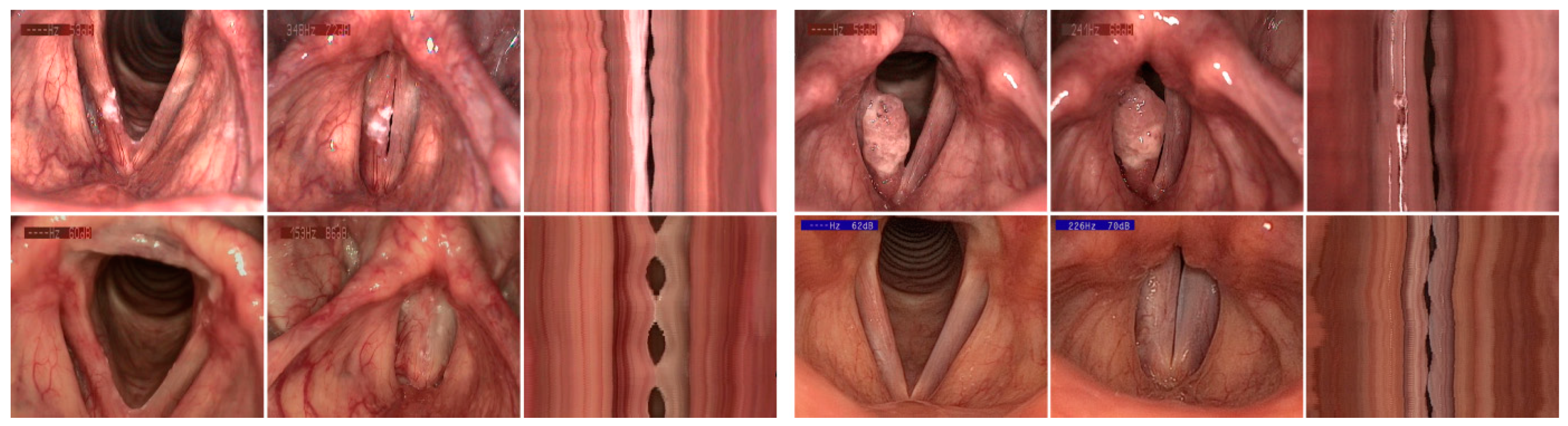
| Number | % | Number | % | |||
|---|---|---|---|---|---|---|
| Gender male female | 43 8 | 84.3% 15.7% | Initial cordectomy (via TOLMS) type I (subepithelial) type II (subligamental) type III (transmuscular) | 24 18 9 | 47.1% 35.3% 17.6% | |
| Age (in years; mean ± SD) | 65 ± 12 | - | Grading of pT1a G1 (well differentiated) G2 (moderately differentiated) G3 (poorly differentiated) | 15 34 2 | 29.4% 66.7% 3.9% | |
| Occurrence of pT1a left vocal fold right vocal fold | 23 28 | 45.1% 54.9% | Follow-up (in months; mean ± SD) | 45 ± 26 | - | |
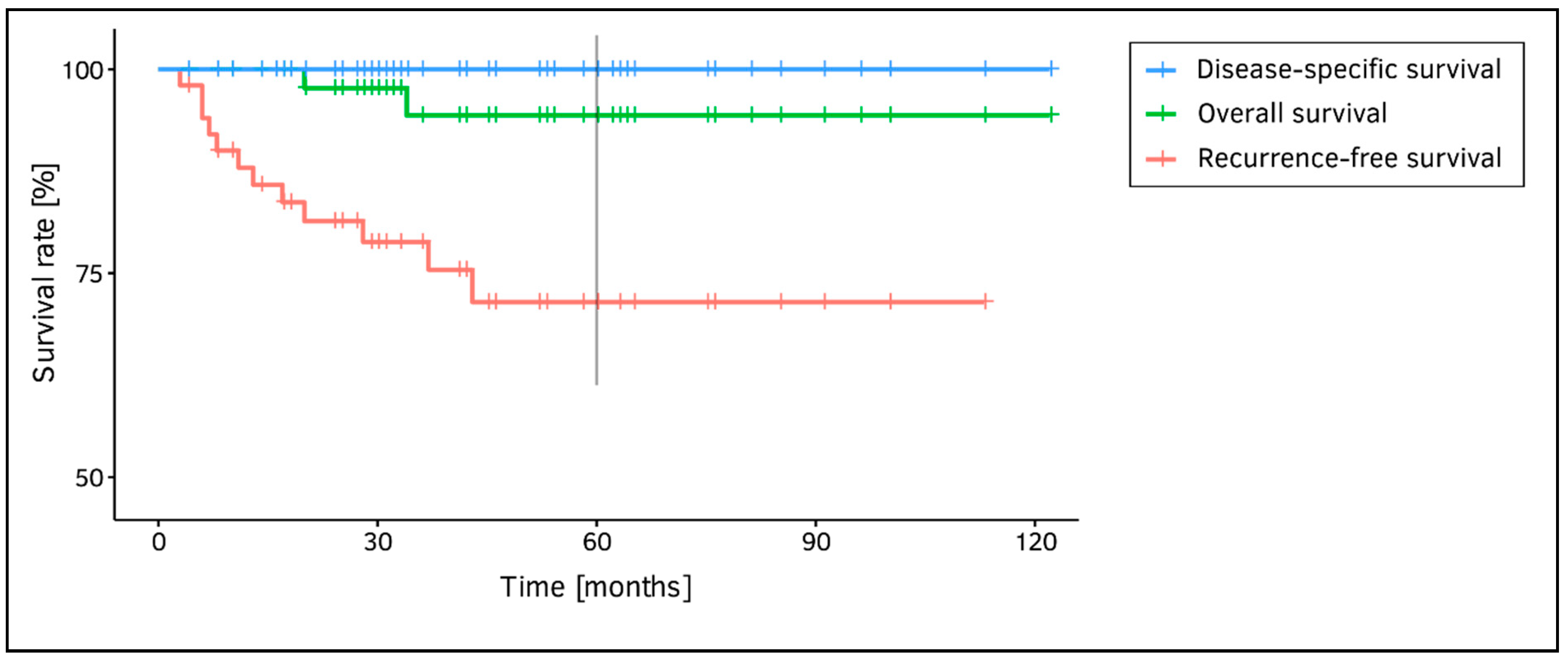
| Vocal fold involvement anterior third middle third posterior third anterior and middle third middle and posterior third entire length | 3 7 1 7 7 26 | 5.9% 13.7% 2.0% 13.7% 13.7% 51.0% | Treatment response local disease control local disease recurrence contralateral secondary pT1a ultimate local disease control with TOLMS alone) larynx preservation | 41 10 2 49 50 | 80.4% 19.6% 3.9% 96.1% 98.0% | |
| Appearance of pT1a hyperkeratotic exophytic ulcerating | 20 29 2 | 39.2% 56.9% 3.9% | Survival disease-specific overall recurrence-free | 51 49 39 | 100.0% 96.1% 76.5% |
| Vocal Measure | Total Group (n = 51) | Type I Cordectomy (n = 24) | Type II Cordectomy (n = 18) | Type III Cordectomy (n = 9) | |
|---|---|---|---|---|---|
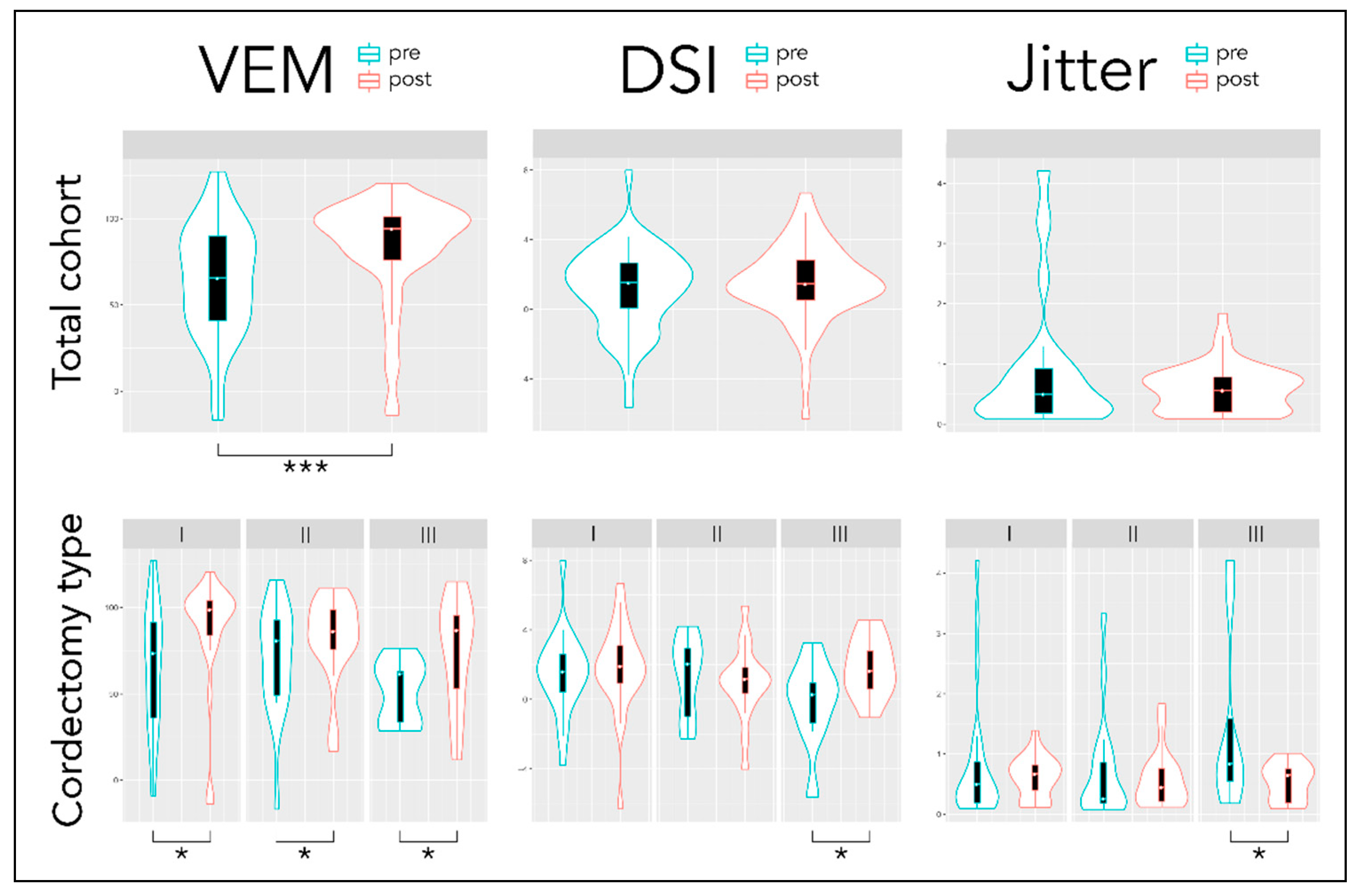
| VEM | Pre Post | 64.4 ± 32.7 82.8 ± 30.5 | 65.4 ± 36.9 86.7 ± 33.5 | 70.3 ± 31.7 81.9 ± 25.4 | 51.0 ± 18.4 74.1 ± 33.2 |
| Diff (CI) | 18.4 (9.0; 29.8) *** | 21.3 (5.1; 37.6) * | 11.6 (−3.2; 32.6) * | 23.1 (−5.7; 52.0) * | |
| DSI | Pre Post | 1.2 ± 2.4 1.5 ± 2.3 | 1.5 ± 2.4 1.8 ± 2.6 | 1.4 ± 2.3 1.0 ± 2.1 | −0.2 ± 2.6 1.8 ± 1.8 |
| Diff (CI) | 0.3 (−0.2; 1.3) | 0.3 (−0.5; 1.9) | −0.4 (−1.4; 0.6) | 2.0 (0.1; 3.9) * | |
| Jitter (%) | Pre Post | 0.9 ± 1.1 0.6 ± 0.4 | 0.8 ± 1.1 0.6 ± 0.3 | 0.7 ± 0.9 0.6 ± 0.5 | 1.5 ± 1.6 0.5 ± 0.3 |
| Diff (CI) | −0.3 (−0.7; −0.02) | −0.2 (−0.7; 0.2) | −0.1 (−0.7; 0.3) | −1.0 (−2.0; 0.1) * | |
| MPT (s) | Pre Post | 13.3 ± 5.6 13.3 ± 6.0 | 14.1 ± 5.2 14.7 ± 6.3 | 12.3 ± 6.6 10.9 ± 5.7 | 13.3 ± 4.5 14.6 ± 4.5 |
| Diff (CI) | −0.01 (−1.9; 1.9) | 0.6 (−2.4; 3.6) | −1.4 (−4.6; 1.7) | 1.3 (−3.6; 6.0) | |
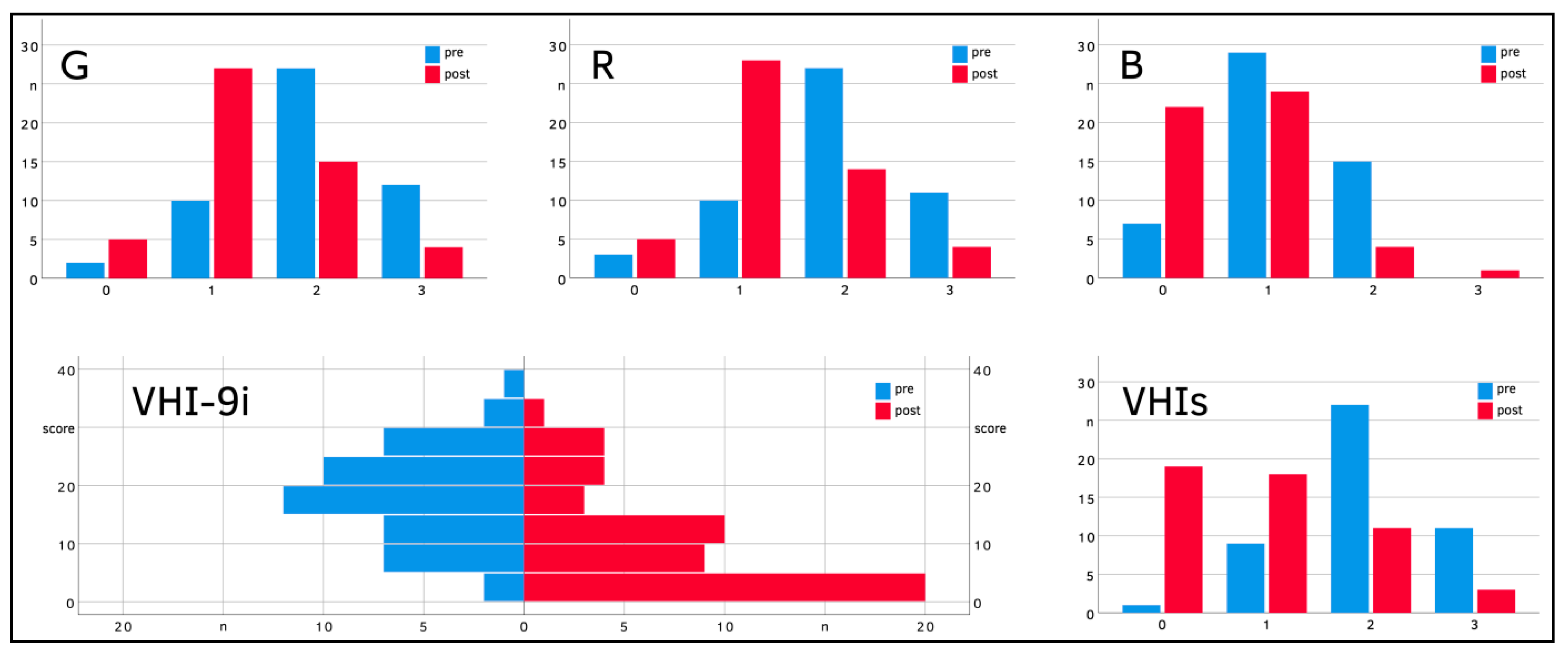
| VHI−9i | Pre Post | 17.7 ± 8.1 9.3 ± 8.8 | 16.6 ± 8.3 10.5 ± 9.0 | 17.1 ± 7.1 7.7 ± 8.7 | 22.1 ± 9.1 9.2 ± 8.8 |
| Diff (CI) | −8.4 (−10.9; −5.6) *** | −6.1 (−10.5; −2.1) ** | −9.4 (−13.1; −4.9) ** | −12.9 (−20.4; −4.3) * | |
| VHIs | Pre Post | 2.0 ± 0.7 1.0 ± 0.9 | 1.9 ± 0.9 1.0 ± 1.0 | 1.9 ± 0.6 0.8 ± 0.9 | 2.4 ± 0.5 1.0 ± 0.9 |
| Diff (CI) | −1.0 (−1.4; −0.8) *** | −0.9 (−1.3; −0.6) *** | −1.1 (−1.7; −0.7) *** | −1.4 (−2.2; −0.6) * | |
| G | Pre Post | 1.9 ± 0.7 1.3 ± 0.7 | 1.5 ± 0.8 1.0 ± 0.8 | 2.2 ± 0.4 1.5 ± 0.6 | 2.2 ± 0.7 1.4 ± 0.6 |
| Diff (CI) | −0.6 (−0.8; −0.4) *** | −0.5 (−0.8; −0.2) ** | −0.7 (−0.9; −0.4) ** | −0.8 (−1.2; −0.2) * | |
| R | Pre Post | 1.8 ± 0.7 1.2 ± 0.7 | 1.5 ± 0.8 1.0 ± 0.8 | 2.1 ± 0.5 1.5 ± 0.6 | 2.0 ± 0.8 1.3 ± 0.6 |
| Diff (CI) | −0.6 (−0.8; −0.4) *** | −0.5 (−0.8; −0.2) ** | −0.6 (−0.9; −0.3) ** | −0.7 (−1.2; −0.1) * | |
| B | Pre Post | 1.0 ± 0.6 0.6 ± 0.6 | 0.8 ± 0.7 0.4 ± 0.6 | 1.2 ± 0.4 0.9 ± 0.5 | 1.4 ± 0.4 0.9 ± 0.7 |
| Diff (CI) | −0.4 (−0.6; −0.2) *** | −0.4 (−0.7; −0.1) ** | −0.3 (−0.6; −0.1) ** | −0.5 (−1.1; 0.1) * | |
| Study | Numbers | Parameters for Evaluation of Vocal Function | Vocal Outcome after Transoral Lasermicrosurgery (TOLMS) | ||
|---|---|---|---|---|---|
| Clinician-Rated Assessment (Subjective) | Patient’s Self-Assessment (Subjective) | Acoustic-Aerodynamic Evaluation (Objective) | |||
| Hamzany et al. (2021) [70] | 27 T1a | GRB | VHI | F0, jitter, shimmer, NHR, MPT | significant subjective improvement, no objective improvement |
| Strieth et al. (2019) [76] | 14 T1a | – | VHI | – | improved voice preservation by KTP-TOLMS (lower VHI scores) compared to CO2-TOLMS (higher VHI scores) |
| Gandhi et al. (2018) [59] | 40 T1a + b (N/S) | GRBAS | VHI | F0, jitter, shimmer, SPI, NHR | excellent vocal outcome (G 0.63, VHI 13); no pretherapeutic data |
| Hong et al. (2018) [61] | 14 T1a + b (N/S) | GRBAS | – | F0, jitter, shimmer, NHR | GRB with mild dysphonia, Jitter 2.37%; no pretherapeutic data |
| Lee et al. (2016) [71] | 50 T1a | GRBAS | VHI | F0, jitter, shimmer, NHR, voice intensity, MPT | G significantly improved; voice quality improved over time in limited ELS resections (I-II) but not in extended cordectomies (III-V) |
| Fink et al. (2016) [72] | 38 T1a | VAS (0–100) | VHI | – | similar or improved voice in limited ELS resections (I-III), VHI improved significantly (VAS n.s.); poorer outcomes in extended resections |
| Kono et al. (2016) [62] | 64 T1a | GRBAS | VHI, V-RQOL | F0, jitter, shimmer, NHR, MPT | mild to moderate impairment (GRB, VHI, jitter), better improvement over time in focused excision compared to defocused vaporization |
| Berania et al. (2015) [65] | 18 T1a | PSS-H&N | VHI-10 | – | favorable functional outcomes (40% mild voice handicap, VHI-10 > 11); no pretherapeutic data |
| Bertino et al. (2015) [66] | 135 T1a | degree of dysphonia (acc. Ricci Maccarini) | – | F0, HNR | mild to slight dysphonia in limited ELS resections (I-II), moderate to severe dysphonia in extended resections (III-V); no pretherapeutic data |
| Laoufi et al. (2014) [57] | 44 T1a | – | VHI, EORTC QLQ-HN35 | – | VHI score mild to moderate impaired (mean 29); no pretherapeutic data |
| Friedman et al. (2013) [77] | 57 T1a | – | V-RQOL | F0, jitter, shimmer, NHR, max. SPL range, max. F0 range, SPL divided by subglottic pressure | significant improvement of subjective (V-RQOL) and most objective (acoustic, aerodynamic) measures |
| Tomifuji et al. (2013) [73] | 33 T1a | GRBAS | VHI | jitter, shimmer, HNR, MPT, MFR | voice quality differs according to the type of cordectomy; no pretherapeutic data |
| van Gogh et al. (2012) [60] | 67 T1a | – | – | F0, jitter, shimmer, NNE | quick voice outcome recovery apart from F0 (remains higher pitched), no significant long-term voice changes |
| Bajaj et al. (2011) [67] | 14 T1a + b (N/S) | GRBAS | VoiSS, UW-QoL | F0, F0 irregularity, CQ range, CQ irregularity | preservation of acceptable vocal function (GRB mild to moderate impaired, low VoiSS score); no pretherapeutic data |
| Keilmann et al. (2011) [68] | 11 T1a | RBH | VHI-12 | F0, jitter, shimmer, MPT, GHD, VRP | discrepancy over time (VHI deteriorated; RBH and objective measures improved); no pretherapeutic data |
| Lester et al. (2011) [78] | 19 T1a + b (N/S) | – | ordinal scale (1–5) | F0, jitter, shimmer, MPT | objective acoustic measures showed no significant changes; deterioration of MPT (13s to 12s) and subjective rating score (3 to 2) |
| Motta et al. (2008) [69] | 49 T1a | – | – | MPT HNR, average voice intensity | outcomes vary in relation to the main site of the pseudo-glottis, vocal compensation without normal voice quality; no pretherapeutic data |
| Núñez Batalla et al. (2008) [63] | 19 T1a | GRBAS | VHI | F0, jitter, shimmer, NNE, MPT | mild to moderate impairment (GRBAS, VHI); no pretherapeutic data |
| Sjögren et al. (2008) [64] | 18 T1a | GRBAS | VHI | F0, jitter, shimmer, intensity, MPT, VC, phonation quotient | mild to moderate voice dysfunction (G, B, VHI) in ca. half of patients; no pretherapeutic data |
| Vilaseca et al. (2008) [79] | 35 T1a | GRBAS | ordinal scale (1–3) | F0, jitter, shimmer, NHR, vocal range, MPT | self-assessed improvement; compared with healthy controls: increase of F0, jitter, shimmer (MPT decrease in extended resections); no pretherapeutic data |
| Roh et al. (2007) [75] | 50 T1a | GRBAS | VHI, EORTC QLQ-HN35 | F0, jitter, shimmer, HNR, MPT, average airflow | improved vocal outcomes, significant in type I and II cordectomies (VHI, G, jitter, shimmer, HNR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Caffier, F.; Nawka, T.; Ermakova, T.; Martin, A.; Mürbe, D.; Caffier, P.P. T1a Glottic Cancer: Advances in Vocal Outcome Assessment after Transoral CO2-Laser Microsurgery Using the VEM. J. Clin. Med. 2021, 10, 1250. https://doi.org/10.3390/jcm10061250
Song W, Caffier F, Nawka T, Ermakova T, Martin A, Mürbe D, Caffier PP. T1a Glottic Cancer: Advances in Vocal Outcome Assessment after Transoral CO2-Laser Microsurgery Using the VEM. Journal of Clinical Medicine. 2021; 10(6):1250. https://doi.org/10.3390/jcm10061250
Chicago/Turabian StyleSong, Wen, Felix Caffier, Tadeus Nawka, Tatiana Ermakova, Alexios Martin, Dirk Mürbe, and Philipp P. Caffier. 2021. "T1a Glottic Cancer: Advances in Vocal Outcome Assessment after Transoral CO2-Laser Microsurgery Using the VEM" Journal of Clinical Medicine 10, no. 6: 1250. https://doi.org/10.3390/jcm10061250
APA StyleSong, W., Caffier, F., Nawka, T., Ermakova, T., Martin, A., Mürbe, D., & Caffier, P. P. (2021). T1a Glottic Cancer: Advances in Vocal Outcome Assessment after Transoral CO2-Laser Microsurgery Using the VEM. Journal of Clinical Medicine, 10(6), 1250. https://doi.org/10.3390/jcm10061250






