Active Travel and Mild Cognitive Impairment among Older Adults from Low- and Middle-Income Countries
Abstract
1. Introduction
2. Methods
2.1. The Survey
2.2. Active Travel
2.3. Mild Cognitive Impairment
2.4. Control Variables
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings
4.2. Interpretation of the Findings
4.3. Public Health and Policy Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 5 March 2021).
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Bohlken, J.; Jacob, L.; Kostev, K. Progression of mild cognitive impairment to dementia in German specialist practices. Dementia 2016, 18, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Harvey, D.; DeCarli, C. Progression of Mild Cognitive Impairment to Dementia in Clinic- vs. Community-Based Cohorts. Arch. Neurol. 2009, 66, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics 2005, 9–14. Available online: https://web.a.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=0016867X&asa=Y&AN=18889206&h=fSVAp2HdpD2AwPPsvBxsm692chWejYvB%2bvWqWalKWF3Y848G4%2fwdUT3i21VKg7BMCzEOSqXPhb8QWN0xQ0v5DA%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d0016867X%26asa%3dY%26AN%3d18889206 (accessed on 18 February 2021).
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Geda, Y.E.; Graff-Radford, N.R.; Petersen, R.C. Physical Exercise as a Preventive or Disease-Modifying Treatment of Dementia and Brain Aging; Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 876–884. [Google Scholar]
- Corbi, G.; Conti, V.; Filippelli, A.; Di Costanzo, A.; Ferrara, N. The Role Of Physical Activity On The Prevention Of Cognitive Impairment. Transl. Med. UniSa 2016, 13, 42–46. [Google Scholar]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Innocenti, A.; Franzoni, F. Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch. Ital. Biol. 2017, 155, 54–62. [Google Scholar] [CrossRef]
- Gallaway, P.J.; Miyake, H.; Buchowski, M.S.; Shimada, M.; Yoshitake, Y.; Kim, A.S.; Hongu, N. Physical activity: A viable way to reduce the risks of mild cognitive impairment, Alzheimer’s disease, and vascular dementia in older adults. Brain Sci. 2017, 7, 22. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Cox, K.; Kurz, A.F. Physical activity and mild cognitive impairment and Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 352–358. [Google Scholar] [CrossRef]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef] [PubMed]
- The Centre for Diet and Activity Research Evidence Brief 4: Walking & Cycling for Transport. Available online: https://www.cedar.iph.cam.ac.uk/resources/evidence/eb-why-active-travel-web/ (accessed on 5 March 2021).
- Vancampfort, D.; Smith, L.; Stubbs, B.; Swinnen, N.; Firth, J.; Schuch, F.B.; Koyanagi, A. Associations between active travel and physical multi-morbidity in six low-and middle-income countries among community-dwelling older adults: A cross-sectional study. PLoS ONE 2018, 13, e0203277. [Google Scholar] [CrossRef]
- World Health Organization Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 5 March 2021).
- Kowal, P.; Chatterji, S.; Naidoo, N.; Biritwum, R.; Fan, W.; Lopez Ridaura, R.; Maximova, T.; Arokiasamy, P.; Phaswana-Mafuya, N.; Williams, S. Data resource profile: The World Health Organization Study on global AGEing and adult health (SAGE). Int. J. Epidemiol. 2012, 41, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef]
- Laverty, A.A.; Palladino, R.; Lee, J.T.; Millett, C. Associations between active travel and weight, blood pressure and diabetes in six middle income countries: A cross-sectional study in older adults. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
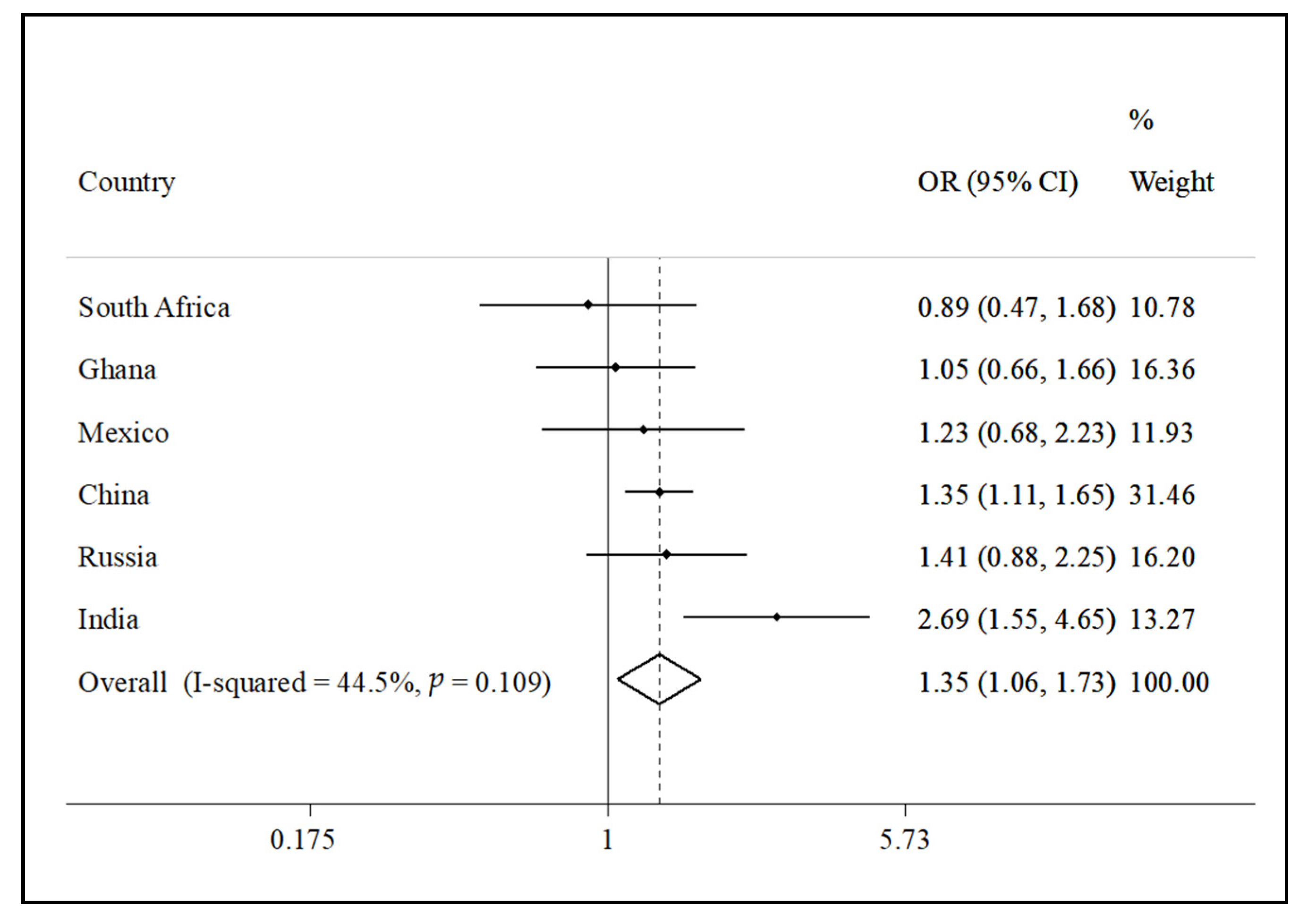
- Koyanagi, A.; Lara, E.; Stubbs, B.; Carvalho, A.F.; Oh, H.; Stickley, A.; Veronese, N.; Vancampfort, D. Chronic physical conditions, multimorbidity, and mild cognitive impairment in low-and middle-income countries. J. Am. Geriatr. Soc. 2018, 66, 721–727. [Google Scholar] [CrossRef]
- Koyanagi, A.; Oh, H.; Vancampfort, D.; Carvalho, A.F.; Veronese, N.; Stubbs, B.; Lara, E. Perceived stress and mild cognitive impairment among 32,715 community-dwelling older adults across six low-and middle-income countries. Gerontology 2019, 65, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The consortium to establish a registry for Alzheimer’s disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale; The Psychological Corporation: New York, NY, USA, 1955. [Google Scholar]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Vancampfort, D.; Stubbs, B.; Lara, E.; Vandenbulcke, M.; Swinnen, N.; Koyanagi, A. Mild cognitive impairment and physical activity in the general population: Findings from six low-and middle-income countries. Exp. Gerontol. 2017, 100, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lara, E.; Koyanagi, A.; Olaya, B.; Lobo, A.; Miret, M.; Tyrovolas, S.; Ayuso-Mateos, J.L.; Haro, J.M. Mild cognitive impairment in a Spanish representative sample: Prevalence and associated factors. Int. J. Geriatr. Psychiatry 2016, 31, 858–867. [Google Scholar] [CrossRef]
- Koyanagi, A.; Stickley, A. The association between sleep problems and psychotic symptoms in the general population: A global perspective. Sleep 2015, 38, 1875–1885. [Google Scholar] [CrossRef]
- Stubbs, B.; Koyanagi, A.; Hallgren, M.; Firth, J.; Richards, J.; Schuch, F.; Rosenbaum, S.; Mugisha, J.; Veronese, N.; Lahti, J. Physical activity and anxiety: A perspective from the World Health Survey. J. Affect. Disord. 2017, 208, 545–552. [Google Scholar] [CrossRef]
- Kessler, R.C.; Üstün, T.B. The world mental health (WMH) survey initiative version of the world health organization (WHO) composite international diagnostic interview (CIDI). Int. J. Methods Psychiatr. Res. 2004, 13, 93–121. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Amieva, H.; Jacqmin-Gadda, H.; Orgogozo, J.; Le Carret, N.; Helmer, C.; Letenneur, L.; Barberger-Gateau, P.; Fabrigoule, C.; Dartigues, J. The 9 year cognitive decline before dementia of the Alzheimer type: A prospective population-based study. Brain 2005, 128, 1093–1101. [Google Scholar] [CrossRef]
- Prince, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.; Karagiannidou, M. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International (ADI): London, UK, 2016. [Google Scholar]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Albert, M.S.; Alonso, A.; Coker, L.H.; Coresh, J.; Davis, S.M.; Deal, J.A.; McKhann, G.M.; Mosley, T.H.; Sharrett, A.R. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017, 74, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Guo, X.; Waern, M.; Östling, S.; Gustafson, D.; Bengtsson, C.; Skoog, I. Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain 2010, 133, 2217–2224. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Veronese, N.; Schofield, P.; Lin, P.; Tseng, P.; Solmi, M.; Thompson, T.; Carvalho, A.F.; Koyanagi, A. Multimorbidity and perceived stress: A population-based cross-sectional study among older adults across six low-and middle-income countries. Maturitas 2018, 107, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Vancampfort, D.; Firth, J.; Schuch, F.B.; Hallgren, M.; Smith, L.; Gardner, B.; Kahl, K.G.; Veronese, N.; Solmi, M. Relationship between sedentary behavior and depression: A mediation analysis of influential factors across the lifespan among 42,469 people in low-and middle-income countries. J. Affect. Disord. 2018, 229, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Lyall, D.M.; Welsh, P.; Anderson, J.; Steell, L.; Guo, Y.; Maldonado, R.; Mackay, D.F.; Pell, J.P.; Sattar, N. Association between active commuting and incident cardiovascular disease, cancer, and mortality: Prospective cohort study. BMJ 2017, 357, j1456. [Google Scholar] [CrossRef]
- Lowenstern, A.; Wang, T.Y. Rethinking Cognitive Impairment in the Management of Older Patients with Cardiovascular Disease. J. Am. Heart Assoc. 2019, 8, e011968. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.J.; Gharaveis, A. Flying solo: A review of the literature on wayfinding for older adults experiencing visual or cognitive decline. Appl. Ergon. 2017, 58, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kremen, W.S.; Jak, A.J.; Panizzon, M.S.; Spoon, K.M.; Franz, C.E.; Thompson, W.K.; Jacobson, K.C.; Vasilopoulos, T.; Vuoksimaa, E.; Xian, H. Early identification and heritability of mild cognitive impairment. Int. J. Epidemiol. 2014, 43, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Petrokofsky, C.; Davis, A. Working Together to Promote Active Travel. A Briefing Document for Local Authorities; Public Health England: London, UK, 2016. [Google Scholar]
- Lindbergh, C.A.; Dishman, R.K.; Miller, L.S. Functional disability in mild cognitive impairment: A systematic review and meta-analysis. Neuropsychol. Rev. 2016, 26, 129–159. [Google Scholar] [CrossRef]
| Age | ||||
|---|---|---|---|---|
| Characteristic | Overall | 50–64 Years | ≥65 Years | |
| (n = 32,715) | (n = 19,092) | (n = 13,623) | ||
| Mild cognitive impairment | No | 85.2 | 86.7 | 82.6 |
| Yes | 14.8 | 13.3 | 17.4 | |
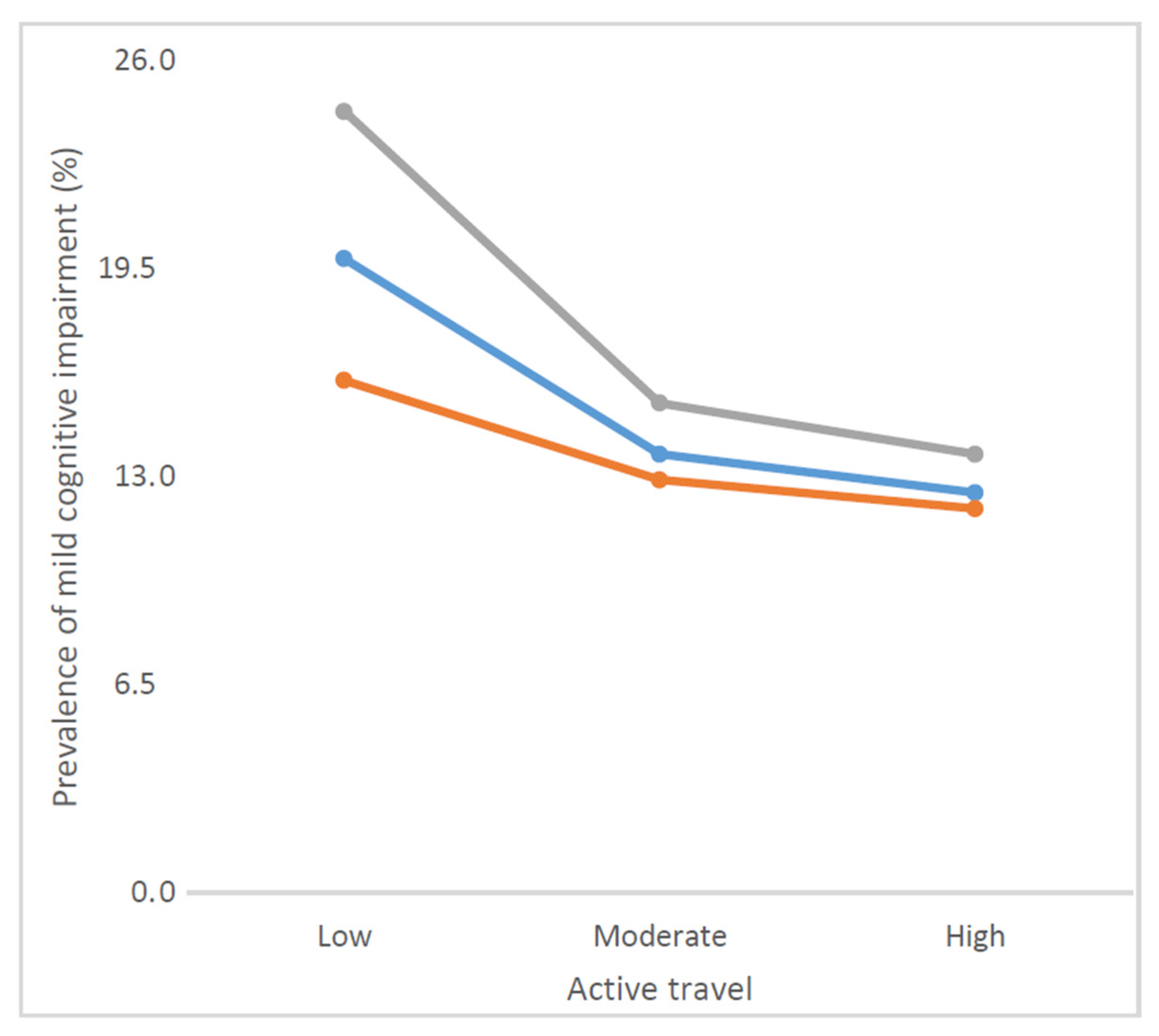
| Active travel | High | 31.5 | 35.2 | 25.4 |
| Moderate | 35.1 | 36.3 | 33.0 | |
| Low | 33.4 | 28.5 | 41.6 | |
| Age (years) | 62.4 (0.2) | 56.3 (0.1) | 72.6 (0.1) | |
| Sex | Male | 47.9 | 49.7 | 45.0 |
| Female | 52.1 | 50.3 | 55.0 | |
| Wealth | Poorest | 17.1 | 14.4 | 21.7 |
| Poorer | 19.0 | 17.7 | 21.0 | |
| Middle | 19.5 | 19.0 | 20.4 | |
| Richer | 21.3 | 23.6 | 17.5 | |
| Richest | 23.1 | 25.3 | 19.4 | |
| Education (years) | 6.0 (0.2) | 6.5 (0.2) | 5.2 (0.2) | |
| Setting | Rural | 53.8 | 56.6 | 49.4 |
| Urban | 46.2 | 43.4 | 50.6 | |
| Alcohol consumption | No | 81.3 | 78.4 | 86.1 |
| Yes | 18.7 | 21.6 | 13.9 | |
| Smoking | Never | 58.6 | 56.3 | 62.2 |
| Current | 34.9 | 38.3 | 29.3 | |
| Past | 6.6 | 5.4 | 8.5 | |
| Sleep problems | No | 91.3 | 93.4 | 87.8 |
| Yes | 8.7 | 6.6 | 12.2 | |
| Anxiety | No | 91.9 | 92.9 | 90.3 |
| Yes | 8.1 | 7.1 | 9.7 | |
| Depression | No | 94.0 | 94.2 | 93.5 |
| Yes | 6.0 | 5.8 | 6.5 | |
| Diabetes | No | 93.2 | 94.2 | 91.4 |
| Yes | 6.8 | 5.8 | 8.6 | |
| Hypertension | No | 45.0 | 50.0 | 36.6 |
| Yes | 55.0 | 50.0 | 63.4 | |
| Stroke | No | 97.0 | 97.9 | 95.4 |
| Yes | 3.0 | 2.1 | 4.6 | |
| Obesity | No | 88.5 | 87.9 | 89.6 |
| Yes | 11.5 | 12.1 | 10.4 | |
| Work physical activity | ≤150 min/week | 40.2 | 33.0 | 52.7 |
| >150 min/week | 59.8 | 67.0 | 47.3 | |
| Leisure physical activity | ≤150 min/week | 89.8 | 89.2 | 90.7 |
| >150 min/week | 10.2 | 10.8 | 9.3 | |
| Overall | Age 50–64 Years | Age ≥65 Years | |||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | ||
| Active travel | High | 1.00 | 1.00 | 1.00 | |||
| Moderate | 0.97 | (0.84,1.13) | 0.95 | (0.79,1.14) | 1.04 | (0.82,1.31) | |
| Low | 1.33 * | (1.14,1.54) | 1.07 | (0.89,1.30) | 1.70 * | (1.32,2.19) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, L.; Veronese, N.; López-Sánchez, G.F.; Yang, L.; Pizzol, D.; Butler, L.T.; Barnett, Y.; Felez-Nobrega, M.; Jacob, L.; Shin, J.I.; et al. Active Travel and Mild Cognitive Impairment among Older Adults from Low- and Middle-Income Countries. J. Clin. Med. 2021, 10, 1243. https://doi.org/10.3390/jcm10061243
Smith L, Veronese N, López-Sánchez GF, Yang L, Pizzol D, Butler LT, Barnett Y, Felez-Nobrega M, Jacob L, Shin JI, et al. Active Travel and Mild Cognitive Impairment among Older Adults from Low- and Middle-Income Countries. Journal of Clinical Medicine. 2021; 10(6):1243. https://doi.org/10.3390/jcm10061243
Chicago/Turabian StyleSmith, Lee, Nicola Veronese, Guillermo F. López-Sánchez, Lin Yang, Damiano Pizzol, Laurie T. Butler, Yvonne Barnett, Mireia Felez-Nobrega, Louis Jacob, Jae Il Shin, and et al. 2021. "Active Travel and Mild Cognitive Impairment among Older Adults from Low- and Middle-Income Countries" Journal of Clinical Medicine 10, no. 6: 1243. https://doi.org/10.3390/jcm10061243
APA StyleSmith, L., Veronese, N., López-Sánchez, G. F., Yang, L., Pizzol, D., Butler, L. T., Barnett, Y., Felez-Nobrega, M., Jacob, L., Shin, J. I., Tully, M. A., Gorely, T., Oh, H., & Koyanagi, A. (2021). Active Travel and Mild Cognitive Impairment among Older Adults from Low- and Middle-Income Countries. Journal of Clinical Medicine, 10(6), 1243. https://doi.org/10.3390/jcm10061243












