Clinical Evidence behind Stereotactic Radiotherapy for the Treatment of Ventricular Tachycardia (STAR)—A Comprehensive Review
Abstract
1. Introduction
2. Materials & Methods
3. Results
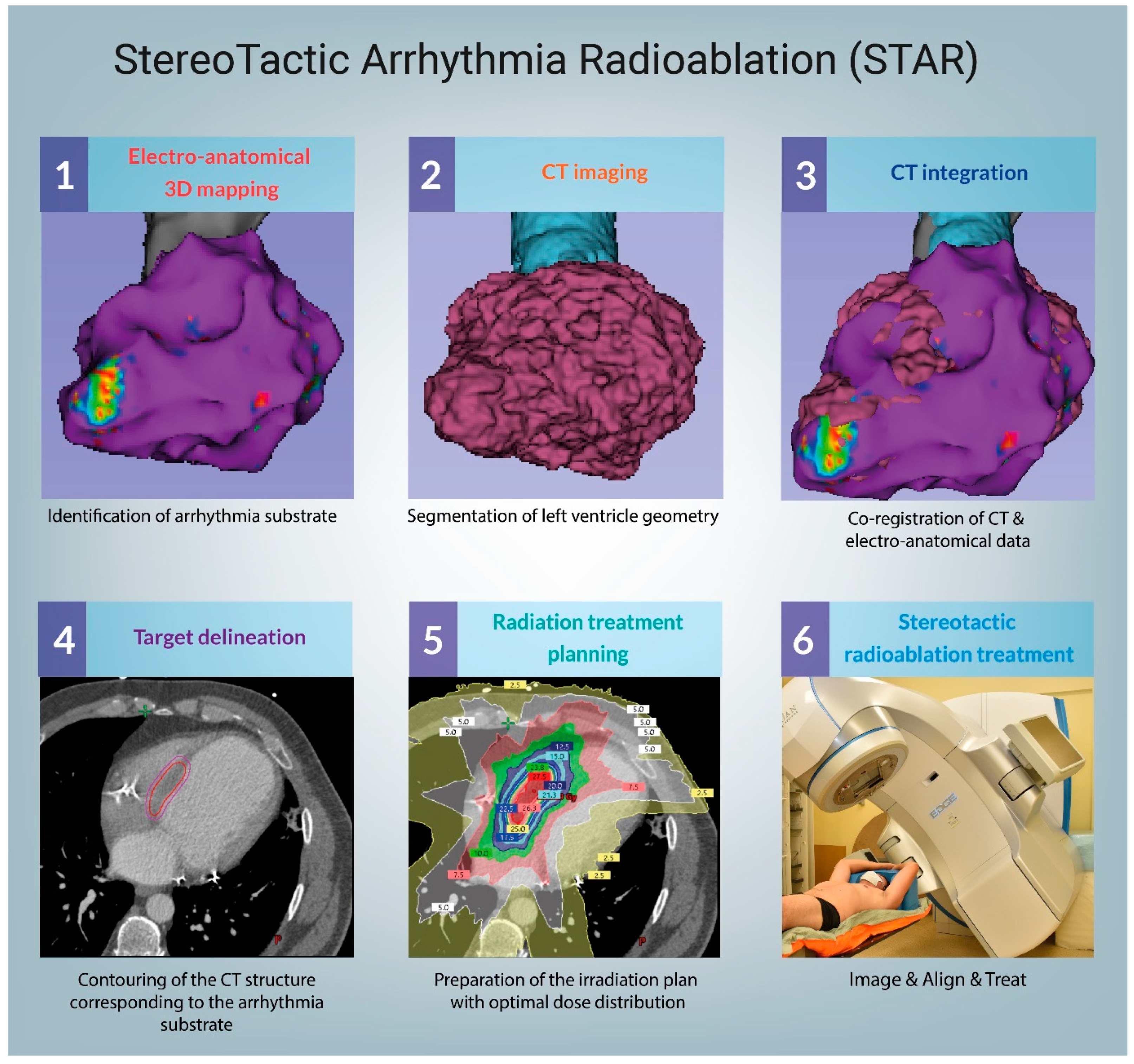
3.1. Technical Considerations
3.2. Mechanisms of STAR
3.3. Clinical Data—From the First Case Report to Clinical Series and Prospective Trials
3.4. Case Reports
3.5. STARting to Think outside of the Box
3.6. Ongoing Clinical Trials
4. Discussion
4.1. Target Volume Delineation
4.2. C-Arm or Cyber Knife?
4.3. The Risk Profile—Early Toxicity
4.4. Late Toxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tang, P.T.; Shenasa, M.; Boyle, N.G. Ventricular arrhythmias and sudden cardiac death. Card. Electrophysiol. Clin. 2017, 9, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.T.; Schilling, R.J. Sudden cardiac death and arrhythmias. Arrhythmia Electrophysiol. Rev. 2018, 7, 111. [Google Scholar] [CrossRef]
- Shivkumar, K. Catheter ablation of ventricular arrhythmias. N. Engl. J. Med. 2019, 380, 1555–1564. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm 2018, 15, e190–e252. [Google Scholar]
- Dukkipati, S.R.; Koruth, J.S.; Choudry, S.; Miller, M.A.; Whang, W.; Reddy, V.Y. Catheter ablation of ventricular tachycardia in structural heart disease: Indications, strategies, and outcomes-Part II. J. Am. Coll. Cardiol. 2017, 70, 2924–2941. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Armenta, J.; Soto-Iglesias, D.; Silva, E.; Penela, D.; Jáuregui, B.; Linhart, M.; Bisbal, F.; Acosta, J.; Fernandez, M.; Borras, R.; et al. Safety and outcomes of ventricular tachycardia substrate ablation during sinus rhythm: A prospective multicenter registry. JACC Clin. Electrophysiol. 2020. [Google Scholar] [CrossRef]
- Santangeli, P.; Frankel, D.S.; Tung, R.; Vaseghi, M.; Sauer, W.H.; Tzou, W.S.; Mathuria, N.; Nakahara, S.; Dickfeldt, T.M.; Lakkireddy, D.; et al. Early mortality after catheter ablation of ventricular tachycardia in patients with structural heart disease. J. Am. Coll. Cardiol. 2017, 69, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, C.; Kolte, D.; Harikrishnan, P.; Khera, S.; Aronow, W.S.; Mujib, M.; Mellana, W.M.; Eugenio, P.; Lessner, S.; Ferrick, A.; et al. Catheter ablation of postinfarction ventricular tachycardia: Ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014. [Google Scholar] [CrossRef]
- Arenal, Á.; Hernández, J.; Calvo, D.; Ceballos, C.; Atéa, L.; Datino, T.; Atienza, F.; González-Torrecilla, E.; Eídelman, G.; Miracle, Á.; et al. Safety, long-term results, and predictors of recurrence after complete endocardial ventricular tachycardia substrate ablation in patients with previous myocardial infarction. Am. J. Cardiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef]
- Robinson, C.G.; Samson, P.P.; Moore, K.M.S.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Lang, A.; Cooper, D.H.; Faddis, M.; et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation 2019, 139, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, R.; Cvek, J.; Knybel, L.; Jiravsky, O.; Molenda, L.; Kodaj, M.; Fiala, M.; Peichl, P.; Feltl, D.; Januška, J.; et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. EP Eur. 2019, 21, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Dickfeld, T.; Tian, J.; Ahmad, G.; Jimenez, A.; Turgeman, A.; Kuk, R.; Peters, M.; Saliaris, A.; Saba, M.; Shorofsky, S.; et al. MRI-guided ventricular tachycardia ablation integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter- defibrillators. Circ. Arrhythmia Electrophysiol. 2011. [Google Scholar] [CrossRef]
- Cochet, H.; Komatsu, Y.; Sacher, F.; Jadidi, A.S.; Scherr, D.; Riffaud, M.; Derval, N.; Shah, A.; Roten, L.; Pascale, P.; et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: A pilot study. J. Cardiovasc. Electrophysiol. 2013. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Ciconte, G.; Manguso, F.; Vicedomini, G.; Mecarocci, V.; Conti, M.; Giannelli, L.; Pozzi, P.; Borrelli, V.; Menicanti, L.; et al. Assessing the malignant ventricular arrhythmic substrate in patients with brugada syndrome. J. Am. Coll. Cardiol. 2018. [Google Scholar] [CrossRef]
- Talib, A.K.; Takagi, M.; Shimane, A.; Nakano, M.; Hayashi, T.; Okajima, K.; Kentaro, M.; Fukada, K.; Kowase, S.; Kurosaki, K.; et al. Efficacy of endocardial ablation of drug-resistant ventricular fibrillation in brugada syndrome. Circ. Arrhythma Electrophysiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nogami, A.; Kurosaki, K.; Hanaki, Y.; Komatsu, Y.; Fukamizu, S.; Morishima, I.; Kaitani, K.; Nishiuchi, S.; Talib, A.K.; et al. Radiofrequency catheter ablation of ventricular tachycardia in patients with hypertrophic cardiomyopathy and apical aneurysm. JACC Clin. Electrophysiol. 2018. [Google Scholar] [CrossRef]
- Bogun, F.M.; Desjardins, B.; Good, E.; Gupta, S.; Crawford, T.; Oral, H.; Ebinger, M.; Pelosi, F.; Chugh, A.; Jongnarangsin, K.; et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy. utility for identifying the ventricular arrhythmia substrate. J. Am. Coll. Cardiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Berruezo, A.; Ortiz-Pérez, J.T.; Silva, E.; Mont, L.; Borràs, R.; De Caralt, T.M.; Perea, R.J.; Fernández-Armenta, J.; Zeljko, H.; et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ. Arrhythmia Electrophysiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Desjardins, B.; Baman, T.; Ilg, K.; Good, E.; Crawford, T.; Oral, H.; Pelosi, F.; Chugh, A.; Morady, F.; et al. Delayed-enhanced MR scar imaging and intraprocedural registration into an electroanatomical mapping system in post-infarction patients. JACC Cardiovasc. Imaging 2012. [Google Scholar] [CrossRef]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019. [Google Scholar] [CrossRef] [PubMed]
- Cvek, J.; Neuwirth, R.; Knybel, L.; Molenda, L.; Otahal, B.; Pindor, J.; Murárová, M.; Kodaj, M.; Fiala, M.; Branny, M.; et al. Cardiac radiosurgery for malignant ventricular tachycardia. Cureus 2014. [Google Scholar] [CrossRef]
- Loo, B.W.; Soltys, S.G.; Wang, L.; Lo, A.; Fahimian, B.P.; Iagaru, A.; Norton, L.; Shan, X.; Gardner, E.; Fogarty, T.; et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ. Arrhythmia Electrophysiol. 2015, 8, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, Y. Stereotactic cardiac radiation to control ventricular tachycardia and fibrillation storm in a patient with apical hypertrophic cardiomyopathy at burnout stage: Case report. J. Korean Med. Sci. 2020, 35. [Google Scholar] [CrossRef]
- Jumeau, R.; Ozsahin, M.; Schwitter, J.; Vallet, V.; Duclos, F.; Zeverino, M.; Moeckli, R.; Pruvot, E.; Bourhis, J. Rescue procedure for an electrical storm using robotic non-invasive cardiac radio-ablation. Radiother. Oncol. 2018, 128, 189–191. [Google Scholar] [CrossRef]
- Haskova, J.; Peichl, P.; Pirk, J.; Cvek, J.; Neuwirth, R.; Kautzner, J. Stereotactic radiosurgery as a treatment for recurrent ventricular tachycardia associated with cardiac fibroma. HeartRhythm Case Rep. 2019, 5, 44–47. [Google Scholar] [CrossRef]
- Scholz, E.P.; Seidensaal, K.; Naumann, P.; André, F.; Katus, H.A.; Debus, J. Risen from the dead: Cardiac stereotactic ablative radiotherapy as last rescue in a patient with refractory ventricular fibrillation storm. HeartRhythm Case Rep. 2019, 5, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Huang, L.; Tan, H.; Zhang, H.; Mei, J.; Shi, H.; Jiang, C.; Tan, C.; Zheng, J.; Liu, X. Stereotactic body radiation therapy for refractory ventricular tachycardia secondary to cardiac lipoma: A case report. Pacing Clin. Electrophysiol. 2019, 42, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Martí-Almor, J.; Jiménez-López, J.; Rodríguez de Dios, N.; Tizón, H.; Vallés, E.; Algara, M. Noninvasive ablation of ventricular tachycardia with stereotactic radiotherapy in a patient with arrhythmogenic right ventricular cardiomyopathy. Rev. Esp. Cardiol. (Engl. Ed). 2019. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, A.; Downar, E.; Chauhan, V.S.; Lindsay, P.; Nair, K.; Ha, A.; Hope, A.; Nanthakumar, K. Electroanatomical mapping–guided stereotactic radiotherapy for right ventricular tachycardia storm. HeartRhythm Case Rep. 2019, 5, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Krug, D.; Blanck, O.; Demming, T.; Dottermusch, M.; Koch, K.; Hirt, M.; Kotzott, L.; Zaman, A.; Eidinger, L.; Siebert, F.A.; et al. Stereotactic body radiotherapy for ventricular tachycardia (cardiac radiosurgery): First-in-patient treatment in Germany. Strahlenther. Onkol. 2020, 196, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mayinger, M.; Kovacs, B.; Tanadini-Lang, S.; Ehrbar, S.; Wilke, L.; Chamberlain, M.; Moreira, A.; Weitkamp, N.; Brunckhorst, C.; Duru, F.; et al. First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Radiother. Oncol. 2020. [Google Scholar] [CrossRef]
- Lloyd, M.S.; Wight, J.; Schneider, F.; Hoskins, M.; Attia, T.; Escott, C.; Lerakis, S.; Higgins, K.A. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm 2019. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.; Hayase, J.; Hu, P.; Cao, M.; Deng, J.; Ajijola, O.; Do, D.; Vaseghi, M.; Buch, E.; Khakpour, H.; et al. Non-invasive stereotactic body radiation therapy for refractory ventricular arrhythmias: An institutional experience. J. Interv. Card. Electrophysiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Rivera, D.; Burkhardt, J.D.; Pollard, B.; Gardner, E.; Maguire, P.; Zei, P.C.; Natale, A.; Al-Ahmad, A. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm 2020. [Google Scholar] [CrossRef]
- Jáuregui, B.; Soto-Iglesias, D.; Zucchelli, G.; Penela, D.; Ordóñez, A.; Terés, C.; Chauca, A.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; et al. Arrhythmogenic substrate detection in chronic ischaemic patients undergoing ventricular tachycardia ablation using multidetector cardiac computed tomography: Compared evaluation with cardiac magnetic resonance. EP Eur. 2021. [Google Scholar] [CrossRef]
- Brett, C.; Cook, J.; Aboud, A.A.; Karim, R.; Stevenson, W.; Shinohara, E.T. Novel workflow for conversion of catheter-based electroanatomic mapping to DICOM imaging for cardiac radiation ablation of ventricular tachycardia. Int. J. Radiat. Oncol. 2020. [Google Scholar] [CrossRef]
- Hohmann, S.; Henkenberens, C.; Zormpas, C.; Christiansen, H.; Bauersachs, J.; Duncker, D.; Veltmann, C. A novel open-source software-based high-precision workflow for target definition in cardiac radioablation. J. Cardiovasc. Electrophysiol. 2020. [Google Scholar] [CrossRef]
- Wang, L.; Fahimian, B.; Soltys, S.G.; Zei, P.; Lo, A.; Gardner, E.A.; Maguire, P.J.; Loo, B.W., Jr. Stereotactic arrhythmia radioablation (STAR) of ventricular tachycardia: A treatment planning study. Cureus 2016, 8. [Google Scholar] [CrossRef]
- Lydiard, S.; Hugo, G.; O’Brien, R.; Blanck, O.; Keall, P. A review of cardiac radioablation (CR) for arrhythmias: Procedures, technology and future opportunities. Int. J. Radiat. Oncol. 2020. [Google Scholar] [CrossRef]
- Sharma, A.; Wong, D.; Weidlich, G.; Fogarty, T.; Jack, A.; Sumanaweera, T.; Maguire, P. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm 2010, 7, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Maguire, P.J.; Gardner, E.; Jack, A.B.; Zei, P.; Al-Ahmad, A.; Fajardo, L.; Ladich, E.; Takeda, P. Cardiac Radiosurgery (CyberHeartTM) for treatment of arrhythmia: Physiologic and histopathologic correlation in the porcine model. Cureus 2012. [Google Scholar] [CrossRef]
- Zei, P.C.; Mak, R. Noninvasive stereotactic radioablation for ventricular tachycardia. Circulation 2019, 139, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, L.F.; Stewart, J.R. Experimental radiation-induced heart disease. I. Light microscopic studies. Am. J. Pathol. 1970, 59, 299–316. [Google Scholar]
- Kiani, S.; Kutob, L.; Schneider, F.; Higgins, K.A.; Lloyd, M.S. Histopathologic and ultrastructural findings in human myocardium after stereotactic body radiation therapy for recalcitrant ventricular tachycardia. Circ. Arrhythmia Electrophysiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Blanck, O.; Bode, F.; Gebhard, M.; Hunold, P.; Brandt, S.; Bruder, R.; Grossherr, M.; Vonthein, R.; Rades, D.; Dunst, J. Dose-escalation study for cardiac radiosurgery in a porcine model. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 590–598. [Google Scholar] [CrossRef]
- Robinson, C.G.; Samson, P.; Moore, K.M.S.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Cooper, D.H.; Faddis, M.; Noheria, A.; et al. Longer term results from a Phase I/II study of ep-guided noninvasive cardiac radioablation for treatment of ventricular tachycardia (ENCORE-VT). Int. J. Radiat. Oncol. 2019, 105, 682. [Google Scholar] [CrossRef]
- Monroy, E.; Azpiri, J.; De La Peña, C.; Cardona, C.; Hinojosa, M.; Zamarripa, R.; Assad, J. Late gadolinium enhancement cardiac magnetic resonance imaging post Robotic Radiosurgical Pulmonary Vein Isolation (RRPVI). First case in the world. Cureus 2016, 8. [Google Scholar] [CrossRef]
- Blanck, O.; Buergy, D.; Vens, M.; Eidinger, L.; Zaman, A.; Krug, D.; Rudic, B.; Boda-Heggemann, J.; Giordano, F.A.; Boldt, L.H.; et al. Radiosurgery for ventricular tachycardia: Preclinical and clinical evidence and study design for a German multi-center multi-platform feasibility trial (RAVENTA). Clin. Res. Cardiol. 2020, 109, 1319–1332. [Google Scholar] [CrossRef]
- Boda-Heggemann, J.; Blanck, O.; Mehrhof, F.; Ernst, F.; Buergy, D.; Fleckenstein, J.; Tülümen, E.; Krug, D.; Siebert, F.-A.; Zaman, A.; et al. Interdisciplinary clinical target volume generation for cardiac radioablation: Multi-center benchmarking for the RAdiosurgery for VENtricular TAchycardia (RAVENTA) trial. Int. J. Radiat. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.G.; Kashani, R.; Bradley, J.D.; Roach, M.C.; Mutic, S.; Schill, M.; Cuculich, P.S. Noninvasive stereotactic cardiac ablation for recurrent Ventricular Tachycardia (VT): Technical considerations and early clinical experience. Int. J. Radiat. Oncol. 2016. [Google Scholar] [CrossRef]


| Date | 1st Author; Type | #, Irradiation Method | Median Age | Etiology; Mean LVEF (%) | Median Follow-Up (Months) | Description of Treatment Outcome and Toxicity |
|---|---|---|---|---|---|---|
| July 2014 | Cvek [23]; Case report | 1; CK | 72 | Dilated cardiomyopathy; 25% | 4 |
|
| ||||||
| June 2015 | Loo [24]; Case report | 1; CK | 71 | Ischemic; 24% | 9 |
|
| ||||||
| December 2017 | Cuculich [10]; Case series | 5; C-arm | 62 | Mostly non-ischemic (60%); 23% | 12 |
|
| ||||||
| November 2018 | Robinson [11]; Clinical trial | 19; C-arm | 66 | Mostly ischemic (58%); 25% ^ | 13 |
|
| ||||||
| December 2018 | Neuwirth [12]; Case series | 10; CK | 64 | Mostly ischemic (80%); 26.5% | 28 |
|
| ||||||
| September 2019 | Lloyd [34]; Case series | 10; C-arm | 61 | Mostly nonischemic (60%); N/A | 6 * |
|
| ||||||
| March 2020 | Gianni [36]; Clinical trial | 5; CK | 61 | Mostly ischemic (80%); 34% | 12 * |
|
| ||||||
| August 2020 | Chin [35]; Case series | 8; C-arm | 74 | Ischemic/nonischemic (even); 21% | 7.8 |
|
|
| Date | 1st Author | Age; VT Type | Etiology | Follow-Up | Irradiation Method | Description of Treatment Outcome and Toxicity |
|---|---|---|---|---|---|---|
| May 2018 | Jumeau [26] | 75; Incessant VT (polymorphic) | Severe dilated cardiomyopathy | 4 months | CK | Free from VT up to 4th month after STAR |
| October 2018 | Haskova [27] | 34; Recurrent VT (monomorphic) | Cardiac fibroma | 8 months | CK | VT gradually subsided within 8 months after STAR |
| March 2019 | Scholz [28] | 53; Ventricular fibrillation | Ischemic | 60 days | C-arm | Cessation of arrhythmic episodes in 2 weeks and no recurrence within 60 days |
| June 2019 | Zeng [29] | 29; Recurrent VT (two morphologies) | Cardiac Lymphoma | 4 months | CK | Free from VT up to 4th month after STAR |
| July 2019 | Marti-Almor [30] | 64; Incessant VT (monomorphic) | Right ventricular cardiomyopathy | 4 months | C-arm | Free from VT up to 4th month after STAR |
| September 2019 | Bhaskaran [31] | 34; VT storm | Unknown | 60 days | C-arm | Cessation of arrhythmic episodes in 6 days and no recurrence within 60 days |
| Octobr 2019 | Krug [32] | 78; Recurrent VT (monomorphic) | Dilated cardiomyopathy | 57 days | C-arm | Partial VT burden reduction. The patient developed sepsis-associated cardiac circulatory failure which led to death 57 days after treatment |
| February 2020 | Mayinger [33] | 71; Recurrent VT (polymorphic) | Nonischemic | 3 months | MR-linac | Immediate aggravation of the clinical VT (48 h) followed by cessation of VT for the rest of the FU |
| July 2020 | Park [25] | 76; Recurrent VT (monomorphic) | apical hypertrophic cardiomyopathy | 6 months | C-arm | Despite two occurrences of sustained VT at 6 and 8 weeks, patient remained free from ICD shocks up to 6th month after STAR. |
| Date of Start | Full Name of the Trial | Country of Origin | Clinical Trial Identifier | Status | Planned Number of Participants |
|---|---|---|---|---|---|
| February 2015 | CyberHeart’s Cardiac Arrhythmia Ablation Treatment: Patients with Refractory Ventricular Tachycardia | USA | NCT02661048 | Active, not recruiting | 5 |
| Primary objectives completed | |||||
| Results published | |||||
| July 2016 | Phase I/II Study of EP-guided Noninvasive Cardiac Radioablation for Treatment of Ventricular Tachycardia | USA | NCT02919618 | Active, not recruiting | 19 |
| Primary objectives completed | |||||
| Results published | |||||
| August 2018 | Phase I/II Study of 4-D Navigated NonInvasive Radiosurgical Ablation of Ventricular Tachycardia | Czech Republic | NCT03601832 | Active, recruiting | 10 |
| August 2018 | STereotactic Ablative Radiosurgery of Recurrent Ventricular Tachycardia in Structural Heart Disease | Czech Republic | NCT03819504 | Withdrawn | 50 |
| September 2019 | STereotactic RadioAblation by Multimodal Imaging for VT | Italy | NCT04066517 | Active, recruiting | 15 |
| September 2019 | Minimally Invasive Arrhythmia Treatment with External Radiation Therapy for Intractable Ventricular Tachycardia | Japan | jRCTs032190041 | Active, recruiting | 3 |
| December 2019 | Radiosurgery for the Treatment of Refractory Ventricular Extrasystoles and Tachycardias | Germany | NCT03867747 | Active, recruiting | 20 |
| January 2020 | Stereotactic Arrhythmia Radioablation for Ventricular Tachycardia Management | Canada | NCT04065802 | Active, recruiting | 20 |
| August 2020 | Cohort Study—SBRT for VT Radioablation | Canada | NCT04162171 | Not yet recruiting | 12 |
| October 2020 | STereotactic Ablative Radiosurgery of Recurrent Ventricular Tachycardia in Structural Heart Disease | Czech Republic | NCT04612140 | Active, recruiting | 100 |
| September 2020 | Stereotactic Management of Arrhythmia—Radiosurgery in Treatment of Ventricular Tachycardia | Poland | NCT04642963 | Active, recruiting | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miszczyk, M.; Jadczyk, T.; Gołba, K.; Wojakowski, W.; Wita, K.; Bednarek, J.; Blamek, S. Clinical Evidence behind Stereotactic Radiotherapy for the Treatment of Ventricular Tachycardia (STAR)—A Comprehensive Review. J. Clin. Med. 2021, 10, 1238. https://doi.org/10.3390/jcm10061238
Miszczyk M, Jadczyk T, Gołba K, Wojakowski W, Wita K, Bednarek J, Blamek S. Clinical Evidence behind Stereotactic Radiotherapy for the Treatment of Ventricular Tachycardia (STAR)—A Comprehensive Review. Journal of Clinical Medicine. 2021; 10(6):1238. https://doi.org/10.3390/jcm10061238
Chicago/Turabian StyleMiszczyk, Marcin, Tomasz Jadczyk, Krzysztof Gołba, Wojciech Wojakowski, Krystian Wita, Jacek Bednarek, and Sławomir Blamek. 2021. "Clinical Evidence behind Stereotactic Radiotherapy for the Treatment of Ventricular Tachycardia (STAR)—A Comprehensive Review" Journal of Clinical Medicine 10, no. 6: 1238. https://doi.org/10.3390/jcm10061238
APA StyleMiszczyk, M., Jadczyk, T., Gołba, K., Wojakowski, W., Wita, K., Bednarek, J., & Blamek, S. (2021). Clinical Evidence behind Stereotactic Radiotherapy for the Treatment of Ventricular Tachycardia (STAR)—A Comprehensive Review. Journal of Clinical Medicine, 10(6), 1238. https://doi.org/10.3390/jcm10061238






