Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold
Abstract
1. Introduction
2. Patients and Methods
3. Statistical Aanalysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Abdollah, F.; Sun, M.; Briganti, A.; Thuret, R.; Schmitges, J.; Gallina, A.; Suardi, N.; Capitanio, U.; Salonia, A.; Shariat, S.F.; et al. Critical assessment of the European Association of Urology guideline indications for pelvic lymph node dissection at radical prostatectomy. BJU Int. 2011, 108, 1769–1775. [Google Scholar] [CrossRef]
- Hoshi, S.; Hayashi, N.; Kurota, Y.; Hoshi, K.; Muto, A.; Sugano, O.; Numahata, K.; Bilim, V.; Sasagawa, I.; Ohta, S. Comparison of semi-extended and standard lymph node dissection in radical prostatectomy: A single-institute experience. Mol. Clin. Oncol. 2015, 3, 1085–1087. [Google Scholar] [CrossRef][Green Version]
- Fossati, N.; Willemse, P.-P.M.; Broeck, T.V.D.; Bergh, R.C.V.D.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, L.; Lerut, E.; Haustermans, K.; Deroose, C.M.; Oyen, R.; Isebaert, S.; Budiharto, T.; Ameye, F.; Mottaghy, F.M.; Bogaerts, K.; et al. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol. Oncol. 2015, 33, 109.e23-31. [Google Scholar] [CrossRef]
- Wu, H.; Xu, T.; Wang, X.; Yu, Y.-B.; Fan, Z.-Y.; Li, D.-X.; Luo, L.; Yang, X.-C.; Jiao, W.; Niu, H.-T. Diagnostic Performance of 68Gallium Labelled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Staging the Prostate Cancer with Intermediate or High Risk Prior to Radical Prostatectomy: A Systematic Review and Meta-analysis. World J. Men’s Health 2020, 38, 208. [Google Scholar]
- Heidenreich, A. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymphadenectomy: Optimizing a Risk-Adapted Surgical Approach. Eur. Urol. 2012, 61, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.V.; Trock, B.J.; Humphreys, E.B.; Mangold, L.A.; Walsh, P.C.; Epstein, J.I.; Partin, A.W. Updated Nomogram to Predict Pathologic Stage of Prostate Cancer Given Prostate-Specific Antigen Level, Clinical Stage, and Biopsy Gleason Score (Partin Tables) Based on Cases from 2000 to 2005. Urology 2007, 69, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Fossati, N.; Zaffuto, E.; Bandini, M.; Dell’Oglio, P.; Bravi, C.A.; Fallara, G.; Pellegrino, F.; Nocera, L.; Karakiewicz, P.I.; et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. Eur. Urol. 2017, 72, 632–640. [Google Scholar] [CrossRef]
- Hansen, J.; Rink, M.; Bianchi, M.; Kluth, L.A.; Tian, Z.; Ahyai, S.A.; Shariat, S.F.; Briganti, A.; Steuber, T.; Fisch, M.; et al. External validation of the updated briganti nomogram to predict lymph node invasion in prostate cancer patients undergoing extended lymph node dissection. Prostate 2012, 73, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cagiannos, I.; Karakiewicz, P.; Eastham, J.A.; Ohori, M.; Rabbani, F.; Gerigk, C.; Reuter, V.; Graefen, M.; Hammerer, P.G.; Erbersdobler, A.; et al. A Preoperative Nomogram Identifying Decreased Risk of Positive Pelvic Lymph Nodes in Patients With Prostate Cancer. J. Urol. 2003, 170, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Partin, A.W.; A Mangold, L.; Lamm, D.M.; Walsh, P.C.; I Epstein, J.; Pearson, J.D. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology 2001, 58, 843–848. [Google Scholar] [CrossRef]
- Godoy, G.; Chong, K.T.; Cronin, A.; Vickers, A.; Laudone, V.; Touijer, K.; Guillonneau, B.; Eastham, J.A.; Scardino, P.T.; Coleman, J.A. Extent of Pelvic Lymph Node Dissection and the Impact of Standard Template Dissection on Nomogram Prediction of Lymph Node Involvement. Eur. Urol. 2011, 60, 195–201. [Google Scholar] [CrossRef]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Milonas, D.; Venclovas, Z.; Muilwijk, T.; Jievaltas, M.; Joniau, S. External validation of Memorial Sloan Kettering Cancer Center nomogram and prediction of optimal candidate for lymph node dissection in clinically localized prostate cancer. Central Eur. J. Urol. 2020, 73, 19–25. [Google Scholar]
- Schmitges, J.; Karakiewicz, P.; Sun, M.; Abdollah, F.; Budäus, L.; Isbarn, H.; Bianchi, M.; Trinh, Q.-D.; Schlomm, T.; Chun, F.; et al. Predicting the risk of lymph node invasion during radical prostatectomy using the European association of urology guideline nomogram: A validation study. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 624–629. [Google Scholar] [CrossRef]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B.; Egevad, L.L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Briganti 2012 Nomogram: “Prediction of Lymph Node Involvement in patients w—Evidencio”. Available online: https://www.evidencio.com/models/show/670 (accessed on 21 October 2020).
- Prostate Cancer Nomograms: Pre-Radical Prostatectomy | Memorial Sloan Kettering Cancer Center. Available online: https://www.mskcc.org/nomograms/prostate/pre_op (accessed on 21 October 2020).
- Mattei, A.; Fuechsel, F.G.; Dhar, N.B.; Warncke, S.H.; Thalmann, G.N.; Krause, T.; Studer, U.E. The Template of the Primary Lymphatic Landing Sites of the Prostate Should Be Revisited: Results of a Multimodality Mapping Study. Eur. Urol. 2008, 53, 118–125. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Pierorazio, P.M.; Gorin, M.A.; Allaf, M.E.; Carter, H.B.; Walsh, P.C. Anatomic Extent of Pelvic Lymph Node Dissection: Impact on Long-term Cancer-specific Outcomes in Men With Positive Lymph Nodes at Time of Radical Prostatectomy. Urology 2013, 82, 653–659. [Google Scholar] [CrossRef]
- Schiavina, R.; Bertaccini, A.; Franceschelli, A.; Manferrari, F.; Vagnoni, V.; Borghesi, M.; Morselli-Labate, A.M.; Martorana, G. The impact of the extent of lymph-node dissection on biochemical relapse after radical prostatectomy in node-negative patients. Anticancer Res. 2010, 30, 2297. [Google Scholar]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Bandini, M.; Marchioni, M.; Pompe, R.S.; Tian, Z.; Gandaglia, G.; Fossati, N.; Abdollah, F.; Graefen, M.; Montorsi, F.; Saad, F.; et al. First North American validation and head-to-head comparison of four preoperative nomograms for prediction of lymph node invasion before radical prostatectomy. BJU Int. 2017, 121, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Turo, R.; Forster, J.A.; West, R.M.; Prescott, S.; Paul, A.B.; Cross, W.R. Do prostate cancer nomograms give accurate information when applied to European patients? Scand. J. Urol. 2015, 49, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hueting, T.A.; Cornel, E.B.; Somford, D.M.; Jansen, H.; Van Basten, J.-P.A.; Pleijhuis, R.G.; Korthorst, R.A.; Van Der Palen, J.A.; Koffijberg, H. External Validation of Models Predicting the Probability of Lymph Node Involvement in Prostate Cancer Patients. Eur. Urol. Oncol. 2018, 1, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Hinev, A.I.; Anakievski, D.; Kolev, N.H.; Hadjiev, V.I. Validation of Nomograms Predicting Lymph Node Involvement in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection. Urol. Int. 2014, 92, 300–305. [Google Scholar] [CrossRef]
- De Reijke, T.M.; Coenen, J.L.L.M.; Gietema, J.A. Dutch Guideline on Prostate Cancer; Dutch Association of Urology: Rotterdam, The Netherlands, 2016. [Google Scholar]
- Venclovas, Z.; Jievaltas, M.; Milonas, D. Significance of Time Until PSA Recurrence After Radical Prostatectomy Without Neo- or Adjuvant Treatment to Clinical Progression and Cancer-Related Death in High-Risk Prostate Cancer Patients. Front. Oncol. 2019, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Sagalovich, D.; Calaway, A.; Srivastava, A.; Sooriakumaran, P.; Tewari, A.K. Assessment of required nodal yield in a high risk cohort undergoing extended pelvic lymphadenectomy in robotic-assisted radical prostatectomy and its impact on functional outcomes. BJU Int. 2012, 111, 85–94. [Google Scholar] [CrossRef] [PubMed]
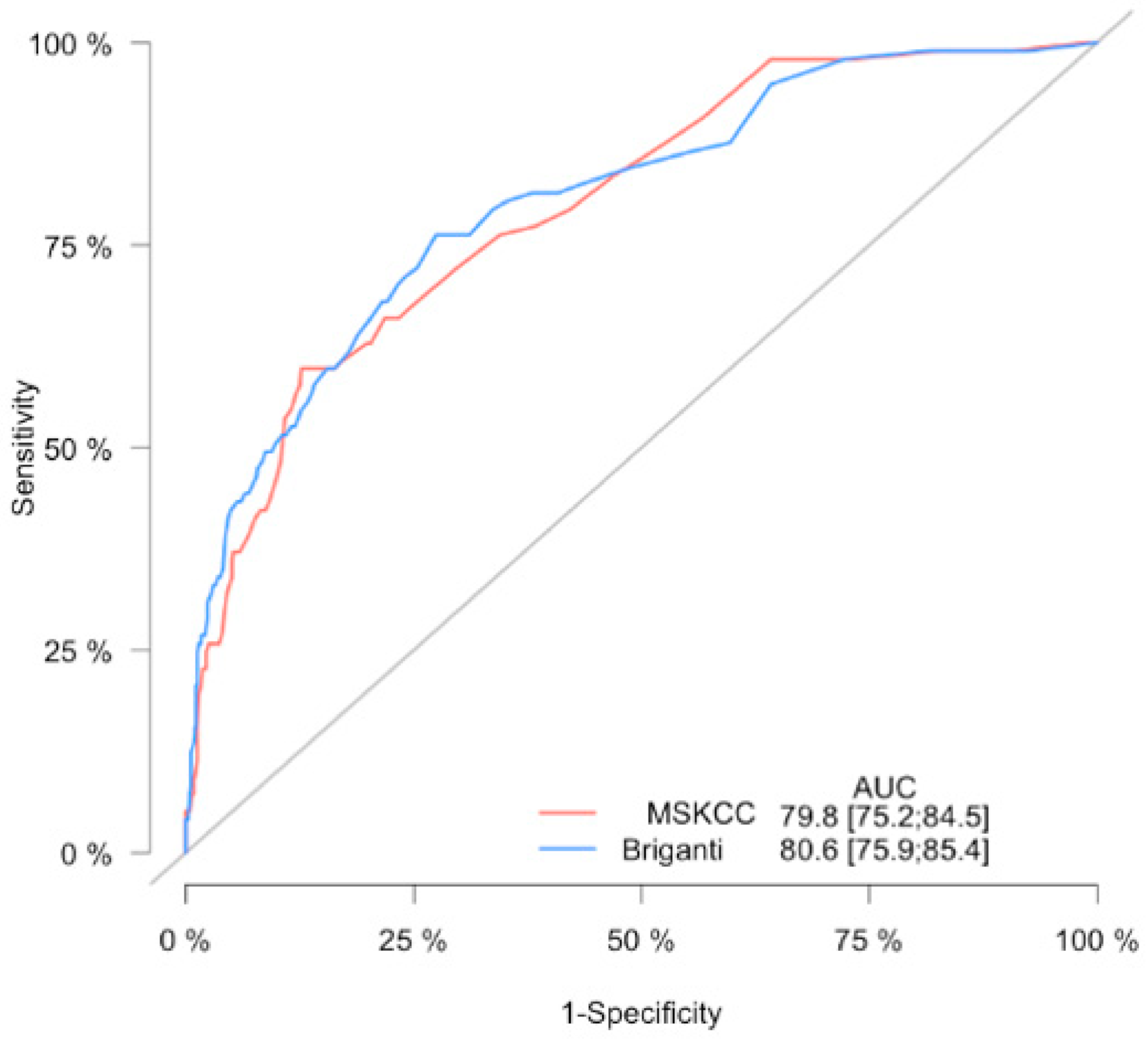
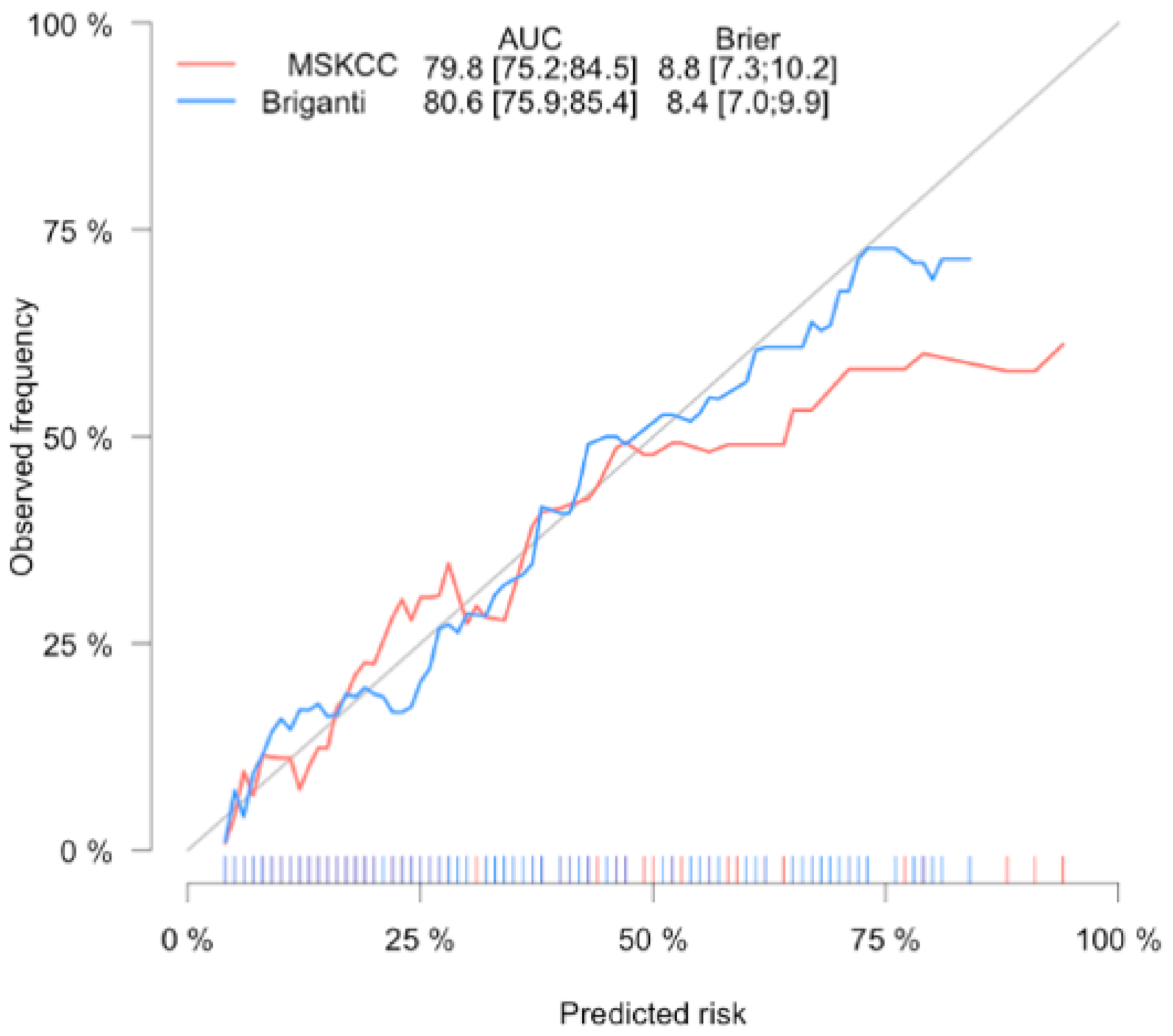
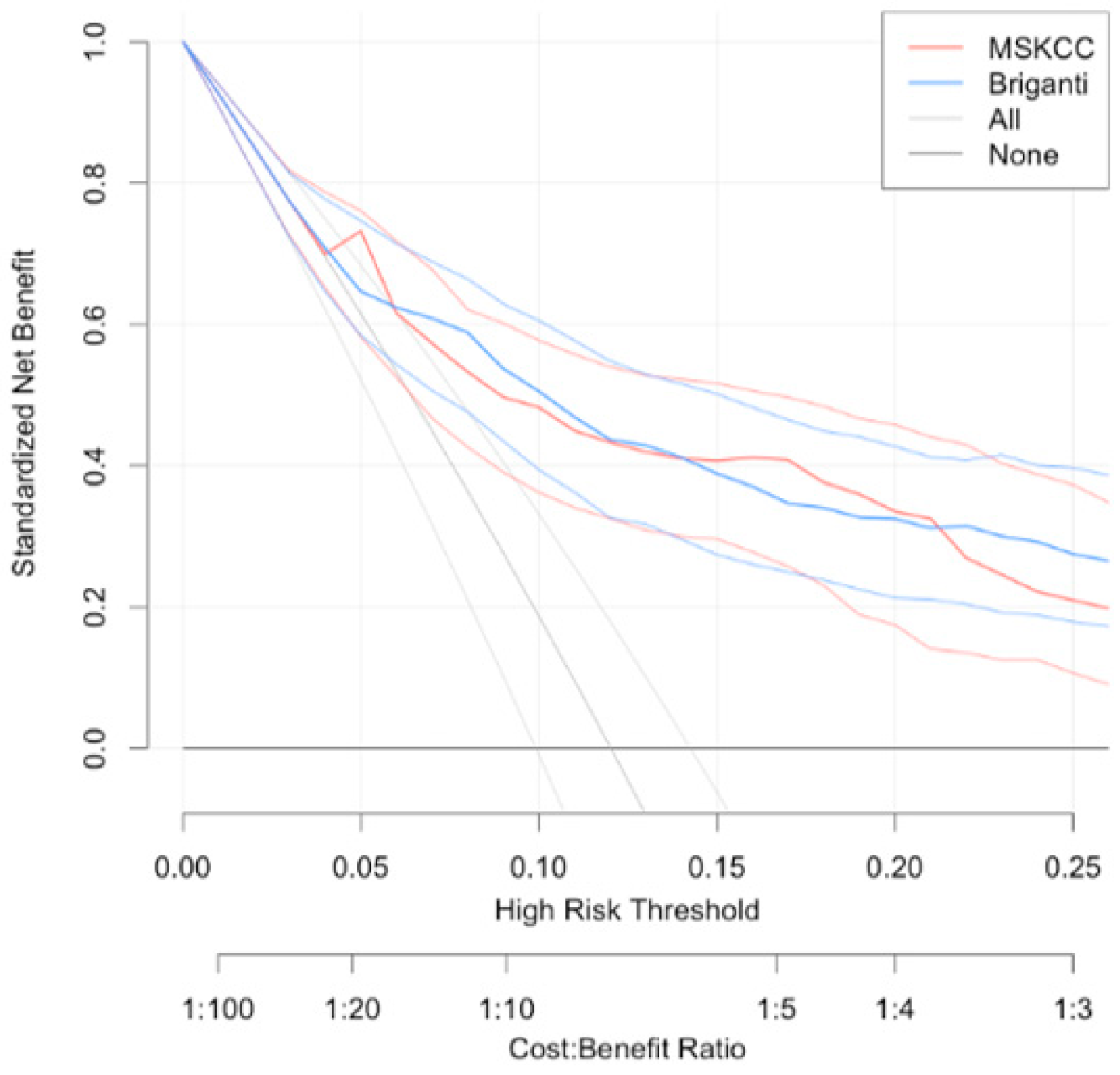
| Parameter | pN0 (n = 710) | pN1 (n = 97) | p Value | All (n = 807) |
|---|---|---|---|---|
| Age (yr): median, (IQR) | 65 (60–69) | 64 (57–67.5) | 0.034 | 65 (60–69) |
| PSA (ng/mL): median, (IQR) | 10.1 (6.28–14.23) | 12.4 (8.27–19.85) | <0.001 | 10.3 (6.5–14.7) |
| Clinical stage: n, (%) | <0.001 | |||
| cT1 | 101 (14.2) | 3 (3.1) | 104 (12.9) | |
| cT2 | 444 (62.5) | 34 (35.1) | 478 (59.2) | |
| cT3 | 165 (23.3) | 60 (61.8) | 225 (27.9) | |
| Biopsy Gleason Score: n, (%) | <0.001 | |||
| 6 | 286 (40.3) | 11 (11.3) | 297 (36.8) | |
| 3 + 4 | 258 (36.3) | 25 (25.8) | 283 (35.1) | |
| 4 + 3 | 57 (8.0) | 20 (20.6) | 77 (9.5) | |
| 8 | 76 (10.7) | 22 (22.7) | 98 (12.1) | |
| 9–10 | 33 (4.6) | 19 (19.6) | 52 (6.4) | |
| % of positive cores: median, (IQR) | 40 (25–62) | 62 (38–88) | <0.001 | 50 (25–64.5) |
| Pathological Gleason Score: n, (%) | <0.001 | |||
| 6 | 136 (19.2) | 1 (1.0) | 137 (16.9) | |
| 3 + 4 | 334 (47.0) | 13 (13.4) | 347 (43) | |
| 4 + 3 | 112 (15.8) | 19 (19.6) | 131 (16.2) | |
| 8 | 60 (8.5) | 15 (15.5) | 75 (9.3) | |
| 9–10 | 68 (9.6) | 49 (50.5) | 117 (14.5) | |
| Pathologic stage: n, (%) | <0.001 | |||
| pT2 | 350 (49.3) | 6 (6.2) | 356 (44.3) | |
| pT3a | 278 (39.2) | 29 (29.9) | 307 (38) | |
| pT3b | 82 (11.5) | 60 (61.9) | 142 (17.6) | |
| pT4 | 0 | 2 (2.1) | 2 (0.2) | |
| No, of LN removed: median, (IQR) | 6 (4–10) | 11 (8–18) | <0.001 | 7 (4–11) |
| Positive surgical margin: n, (%) | 267 (37.6) | 60 (61.9) | <0.001 | 327 (40.5) |
| MSKCC: median, (IQR) | 8 (4–16) | 32 (12–48.5) | <0.001 | 9 (5–20) |
| Briganti: median, (IQR) | 7 (3–17) | 37 (16–67.5) | <0.001 | 8 (4–22) |
| Cut-off | Patients in Whom PLND is Not Recommended According to the Cut-Off (below Cut-Off) | Missing % | Patients below Cut-Off without Histologic LNI | Patients below Cut-Off with Histologic LNI | Patients above Cut-Off without Histologic LNI | Patients above Cut-Off with Histologic LNI | NPV |
|---|---|---|---|---|---|---|---|
| 1 | 54 (6.7) | 0 | 54 (7.61) | 0 (0) | 656 (92.39) | 97 (100) | 100 |
| 2 | 132 (16.4) | 0.76 | 131 (18.45) | 1 (1.03) | 579 (81.55) | 96 (98.97) | 99.24 |
| 3 | 199 (24.7) | 1 | 197 (27.75) | 2 (2.06) | 513 (72.25) | 95 (97.94) | 99 |
| 4 | 259 (32.1) | 1.93 | 254 (35.77) | 5 (5.15) | 456 (64.23) | 92 (94.85) | 98.07 |
| 5 | 298 (36.9) | 4.03 | 286 (40.28) | 12 (12.37) | 424 (59.72) | 85 (87.63) | 95.97 |
| 6 | 328 (40.6) | 3.96 | 315 (44.37) | 13 (13.4) | 395 (55.63) | 84 (86.6) | 96.04 |
| 7 | 379 (47) | 3.96 | 364 (51.27) | 15 (15.46) | 346 (48.73) | 82 (84.54) | 96.04 |
| 8 | 420 (52) | 4.05 | 403 (56.76) | 17 (17.53) | 307 (43.24) | 80 (82.47) | 95.95 |
| 9 | 438 (54.3) | 4.11 | 420 (59.15) | 18 (18.56) | 290 (40.85) | 79 (81.44) | 95.89 |
| 10 | 458 (56.8) | 3.93 | 440 (61.97) | 18 (18.56) | 270 (38.03) | 79 (81.44) | 96.07 |
| 15 | 538 (66.7) | 4.28 | 515 (72.54) | 23 (23.71) | 195 (27.46) | 74 (76.29) | 95.72 |
| Cut-off | Patients in whom PLND Is not Recommended According to the cut-off (below cut-off) | Missing % | Patients below Cut-off without Histologic LNI | Patients below Cut-off with Histologic LNI | Patients above Cut-off without Histologic LNI | Patients above Cut-off with Histologic LNI | NPV |
|---|---|---|---|---|---|---|---|
| 1 | 10 (1.2) | 0 | 10 (1.4) | 0 (0) | 700 (98.6) | 97 (100) | 100 |
| 2 | 69 (8.6) | 1.4 | 68 (9.6) | 1 (1.03) | 642 (90.4) | 96 (98.97) | 98.6 |
| 3 | 123 (15.2) | 0.8 | 122 (17.2) | 1 (1.03) | 588 (82.8) | 96 (98.97) | 99.2 |
| 4 | 190 (23.5) | 1.05 | 188 (24.48) | 2 (2.06) | 522 (73.52) | 95 (97.94) | 98.95 |
| 5 | 256 (31.7) | 0.78 | 254 (35.77) | 2 (2.06) | 456 (64.22) | 95 (97.94) | 99.22 |
| 6 | 316 (39.2) | 2.85 | 307 (43.24) | 9 (9.28) | 403 (56.76) | 88 (90.72) | 97.15 |
| 7 | 359 (44.5) | 3.62 | 346 (48.73) | 13 (13.4) | 364 (51.27) | 84 (86.6) | 96.38 |
| 8 | 393 (48.7) | 4.07 | 377 (53.1) | 16 (16.49) | 333 (46.9) | 81 (83.51) | 95.93 |
| 9 | 431 (53.4) | 4.64 | 411 (57.89) | 20 (20.62) | 299 (42.11) | 77 (79.38) | 95.36 |
| 10 | 460 (57) | 4.78 | 438 (61.69) | 22 (22.68) | 272 (38.31) | 75 (77.32) | 95.22 |
| 15 | 561 (69.5) | 5.53 | 530 (74.65) | 31 (31.96) | 180 (25.35) | 66 (68.04) | 94.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venclovas, Z.; Muilwijk, T.; Matjosaitis, A.J.; Jievaltas, M.; Joniau, S.; Milonas, D. Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold. J. Clin. Med. 2021, 10, 999. https://doi.org/10.3390/jcm10050999
Venclovas Z, Muilwijk T, Matjosaitis AJ, Jievaltas M, Joniau S, Milonas D. Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold. Journal of Clinical Medicine. 2021; 10(5):999. https://doi.org/10.3390/jcm10050999
Chicago/Turabian StyleVenclovas, Zilvinas, Tim Muilwijk, Aivaras J. Matjosaitis, Mindaugas Jievaltas, Steven Joniau, and Daimantas Milonas. 2021. "Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold" Journal of Clinical Medicine 10, no. 5: 999. https://doi.org/10.3390/jcm10050999
APA StyleVenclovas, Z., Muilwijk, T., Matjosaitis, A. J., Jievaltas, M., Joniau, S., & Milonas, D. (2021). Head-to-Head Comparison of Two Nomograms Predicting Probability of Lymph Node Invasion in Prostate Cancer and the Therapeutic Impact of Higher Nomogram Threshold. Journal of Clinical Medicine, 10(5), 999. https://doi.org/10.3390/jcm10050999






