Maternal and Cord Blood Hemoglobin as Determinants of Placental Weight: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
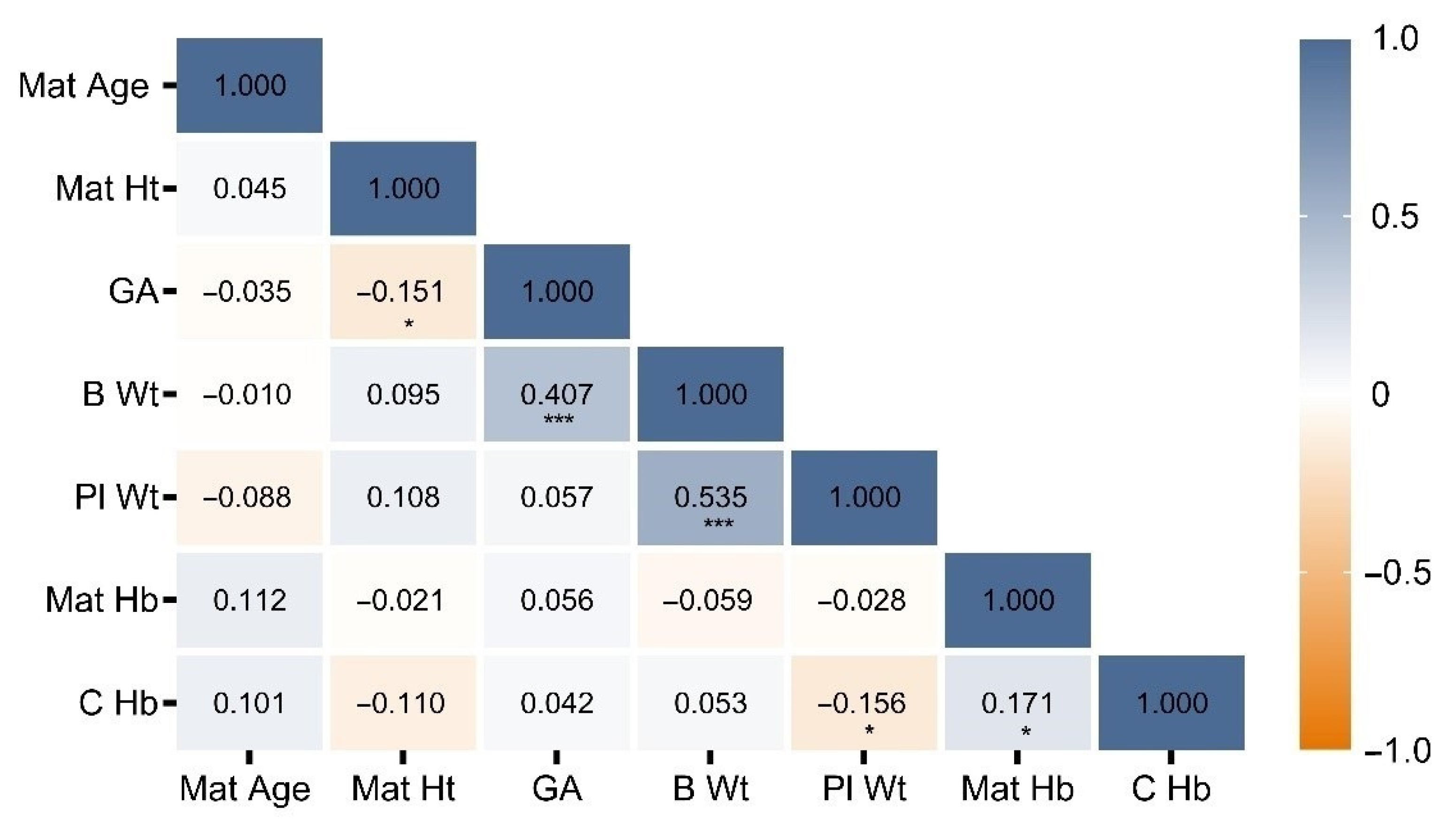
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sibley, C.P.; Brownbill, P.; Dilworth, M.; Glazier, J.D. Review: Adaptation in placental nutrient supply to meet fetal growth demand: Implications for programming. Placenta 2010, 31, S70–S74. [Google Scholar] [CrossRef]
- Heinonen, S.; Taipale, P.; Saarikoski, S. Weights of placentae from small-for-gestational age infants revisited. Placenta 2001, 22, 399–404. [Google Scholar] [CrossRef]
- Williams, L.A.; Evans, S.F.; Newnham, J.P. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ 1997, 314, 1864–1868. [Google Scholar] [CrossRef]
- McNamara, H.; Hutcheon, J.A.; Platt, R.W.; Benjamin, A.; Kramer, M.S. Risk factors for high and low placental weight. Paediatr. Perinat. Epidemiol. 2014, 28, 97–105. [Google Scholar] [CrossRef]
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin. Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef]
- Woodman, A.G.; Care, A.S.; Mansour, Y.; Cherak, S.J.; Panahi, S.; Gragasin, F.S.; Bourque, S.L. Modest and Severe Maternal Iron Deficiency in Pregnancy are Associated with Fetal Anaemia and Organ-Specific Hypoxia in Rats. Sci. Rep. 2017, 7, 46573. [Google Scholar] [CrossRef]
- Woodman, A.G.; Mah, R.; Keddie, D.; Noble, R.M.N.; Panahi, S.; Gragasin, F.S.; Lemieux, H.; Bourque, S.L. Prenatal iron deficiency causes sex-dependent mitochondrial dysfunction and oxidative stress in fetal rat kidneys and liver. FASEB J. 2018, 32, 3254–3263. [Google Scholar] [CrossRef]
- Auinger, W.; Zeibekis, N. The influence of anemia on the weight of child and placenta. Geburtshilfe Frauenheilkd. 1977, 37, 589–592. [Google Scholar]
- Godfrey, K.M.; Redman, C.W.; Barker, D.J.; Osmond, C. The effect of maternal anaemia and iron deficiency on the ratio of fetal weight to placental weight. Br. J. Obstet. Gynaecol. 1991, 98, 886–891. [Google Scholar] [CrossRef]
- Larsen, S.; Bjelland, E.K.; Haavaldsen, C.; Eskild, A. Placental weight in pregnancies with high or low hemoglobin concentrations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 48–52. [Google Scholar] [CrossRef]
- Lao, T.T.; Wong, W.M. Placental ratio--its relationship with mild maternal anaemia. Placenta 1997, 18, 593–596. [Google Scholar] [CrossRef]
- Lelic, M.; Bogdanovic, G.; Ramic, S.; Brkicevic, E. Influence of maternal anemia during pregnancy on placenta and newborns. Med. Arch. 2014, 68, 184–187. [Google Scholar] [CrossRef]
- Kadyrov, M.; Kosanke, G.; Kingdom, J.; Kaufmann, P. Increased fetoplacental angiogenesis during first trimester in anaemic women. Lancet 1998, 352, 1747–1749. [Google Scholar] [CrossRef]
- Agboola, A. Effect of type and duration of anemia on placental weight and villous histology. J. Natl. Med. Assoc. 1979, 71, 1067–1069. [Google Scholar]
- Stoz, F.; Schultz, R.; Kohne, E.; Schuhmann, R.A. Correlation between maternal hemoglobin levels and placental morphology and findings in newborn infants. Z. Geburtshilfe Perinatol. 1987, 191, 81–84. [Google Scholar]
- Singla, P.N.; Chand, S.; Khanna, S.; Agarwal, K.N. Effect of maternal anaemia on the placenta and the newborn infant. Acta Paediatr. Scand. 1978, 67, 645–648. [Google Scholar] [CrossRef]
- Marwah, P.; Singla, P.N.; Krishna, M.; Agarwal, K.N. Effect of pregnancy anaemia on cellular growth in the human placenta. Acta Paediatr. Scand. 1979, 68, 899–901. [Google Scholar] [CrossRef]
- Sanni, O.B.; Chambers, T.; Li, J.H.; Rowe, S.; Woodman, A.G.; Ospina, M.B.; Bourque, S.L. A systematic review and meta-analysis of the correlation between maternal and neonatal iron status and haematologic indices. E Clin. Clin. 2020, 27, 100555. [Google Scholar] [CrossRef]
- Csorba, R.; Soliman, A.A.; Wieg, C.; Tsikouras, P.; Rath, W.; von Tempelhoff, G.F. Correlation of rheological parameters in maternal and fetal blood at term. J. Matern. Fetal. Neonatal Med. 2015, 28, 969–976. [Google Scholar] [CrossRef]
- Timilsina, S.; Karki, S.; Gautam, A.; Bhusal, P.; Paudel, G.; Sharma, D. Correlation between maternal and umbilical cord blood in pregnant women of Pokhara Valley: A cross sectional study. BMC Pregnancy Childbirth 2018, 18. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, N.; Srivastava, R.; Kumar, A. Maternal and Cord Blood Hepcidin Concentrations in Severe Iron Deficiency Anemia. Pediatr. Neonatol. 2016, 57, 413–419. [Google Scholar] [CrossRef][Green Version]
- Carroll, P.D.; Nankervis, C.A.; Iams, J.; Kelleher, K. Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J. Perinatol. 2012, 32, 97–102. [Google Scholar] [CrossRef]
- Qaiser, D.H.; Sandila, M.P.; Omair, A.; Ghori, G.M. Correlation of routine haematological parameters between normal maternal blood and the cord blood of healthy newborns in selected hospitals of Karachi. J. Coll. Physicians Surg. Pak. 2013, 23, 128–131. [Google Scholar]
- Bratlid, D.; Moe, P.J. Hemoglobin and serum ferritin levels in mothers and infants at birth. Eur. J. Pediatr. 1980, 134, 125–127. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition In-formation System 2011, (WHO/NMH/NHD/MNM/11.1). Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 20 January 2021).
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Pena-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health. 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Gomez, M.F.; Field, C.J.; Olstad, D.L.; Loehr, S.; Ramage, S.; McCargar, L.J.; APrON Study Team. Use of micronutrient supplements among pregnant women in Alberta: Results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Matern. Child. Nutr. 2015, 11, 497–510. [Google Scholar] [CrossRef]
- Parker, M.; Han, Z.; Abu-Haydar, E.; Matsiko, E.; Iyakaremye, D.; Tuyisenge, L.; Magaret, A.; Lyambabaje, A. An evaluation of hemoglobin measurement tools and their accuracy and reliability when screening for child anemia in Rwanda: A randomized study. PLoS ONE 2018, 13, e0187663. [Google Scholar] [CrossRef]
- Hutcheon, J.A.; McNamara, H.; Platt, R.W.; Benjamin, A.; Kramer, M.S. Placental weight for gestational age and adverse perinatal outcomes. Obstet. Gynecology 2012, 119, 1251–1258. [Google Scholar] [CrossRef]
- Lao, T.T.; Tam, K.F. Placental ratio and anemia in third-trimester pregnancy. J. Reprod. Med. 2000, 45, 923–928. [Google Scholar]
- Burton, G.J.; Jauniaux, E.; Murray, A.J. Oxygen and placental development; parallels and differences with tumour biology. Placenta 2017, 56, 14–18. [Google Scholar] [CrossRef]
- Murray, A.J. Oxygen delivery and fetal-placental growth: Beyond a question of supply and demand? Placenta 2012, 33 (Suppl. 2), e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Cui, W.; Rosario, G.X.; Scott, R.L.; Dhakal, P.; Renaud, S.J.; Tachibana, M.; Rumi, M.A.; Mason, C.W.; Krieg, A.J.; et al. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc. Natl. Acad. Sci. USA 2016, 113, E7212–E7221. [Google Scholar] [CrossRef] [PubMed]
- Sferruzzi-Perri, A.N.; Higgins, J.S.; Vaughan, O.R.; Murray, A.J.; Fowden, A.L. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc. Natl. Acad. Sci. USA 2019, 116, 1621–1626. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Camm, E.J.; Sferruzzi-Perri, A.N.; Ashmore, T.J.; Yung, H.W.; Cindrova-Davies, T.; Spiroski, A.M.; Sutherland, M.R.; Logan, A.; Austin-Williams, S.; et al. Placental Adaptation to Early-Onset Hypoxic Pregnancy and Mitochondria-Targeted Antioxidant Therapy in a Rodent Model. Am. J. Pathol. 2018, 188, 2704–2716. [Google Scholar] [CrossRef]
- Tissot van Patot, M.C.; Murray, A.J.; Beckey, V.; Cindrova-Davies, T.; Johns, J.; Zwerdlinger, L.; Jauniaux, E.; Burton, G.J.; Serkova, N.J. Human placental metabolic adaptation to chronic hypoxia, high altitude: Hypoxic preconditioning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R166–R172. [Google Scholar] [CrossRef]
- Zamudio, S. The placenta at high altitude. High Alt. Med. Biol. 2003, 4, 171–191. [Google Scholar] [CrossRef]
- Laflamme, E.M. Maternal hemoglobin concentration and pregnancy outcome: A study of the effects of elevation in el alto, bolivia. Mcgill J. Med. 2011, 13, 47. [Google Scholar]
- Postigo, L.; Heredia, G.; Illsley, N.P.; Torricos, T.; Dolan, C.; Echalar, L.; Tellez, W.; Maldonado, I.; Brimacombe, M.; Balanza, E.; et al. Where the O2 goes to: Preservation of human fetal oxygen delivery and consumption at high altitude. J. Physiol. 2009, 587, 693–708. [Google Scholar] [CrossRef]
- Huang, S.T.; Vo, K.C.; Lyell, D.J.; Faessen, G.H.; Tulac, S.; Tibshirani, R.; Giaccia, A.J.; Giudice, L.C. Developmental response to hypoxia. FASEB J. 2004, 18, 1348–1365. [Google Scholar] [CrossRef]
- Higgins, J.S.; Vaughan, O.R.; Fernandez de Liger, E.; Fowden, A.L.; Sferruzzi-Perri, A.N. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J. Physiol. 2016, 594, 1341–1356. [Google Scholar] [CrossRef]
- Rosario, G.X.; Konno, T.; Soares, M.J. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev. Biol. 2008, 314, 362–375. [Google Scholar] [CrossRef]
- Gambling, L.; Lang, C.; McArdle, H.J. Fetal regulation of iron transport during pregnancy. Am. J. Clin. Nutr. 2011, 94, 1903S–1907S. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Kolahi, K.; Pierce, M.; Valent, A.; Drake, R.; Louey, S. Biological features of placental programming. Placenta 2016, 48 (Suppl. 1), S47–S53. [Google Scholar] [CrossRef]
- Christians, J.K.; Grynspan, D.; Greenwood, S.L.; Dilworth, M.R. The problem with using the birthweight:placental weight ratio as a measure of placental efficiency. Placenta 2018, 68, 52–58. [Google Scholar] [CrossRef]
- Hayward, C.E.; Lean, S.; Sibley, C.P.; Jones, R.L.; Wareing, M.; Greenwood, S.L.; Dilworth, M.R. Placental Adaptation: What Can We Learn from Birthweight:Placental Weight Ratio? Front. Physiol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Janoudi, G.; Kelly, S.; Yasseen, A.; Hamam, H.; Moretti, F.; Walker, M. Factors Associated With Increased Rates of Caesarean Section in Women of Advanced Maternal Age. J. Obstet. Gynaecol. Can. 2015, 37, 517–526. [Google Scholar] [CrossRef]
- Rydahl, E.; Declercq, E.; Juhl, M.; Maimburg, R.D. Cesarean section on a rise-Does advanced maternal age explain the increase? A population register-based study. PLoS ONE 2019, 14, e0210655. [Google Scholar] [CrossRef]
- Leary, S.D.; Godfrey, K.M.; Greenaway, L.J.; Davill, V.A.; Fall, C.H. Contribution of the umbilical cord and membranes to untrimmed placental weight. Placenta 2003, 24, 276–278. [Google Scholar] [CrossRef]
- Ogawa, M.; Matsuda, Y.; Nakai, A.; Hayashi, M.; Sato, S.; Matsubara, S. Standard curves of placental weight and fe-tal/placental weight ratio in Japanese population: Difference according to the delivery mode, fetal sex, or maternal parity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 225–231. [Google Scholar] [CrossRef]
| Variable | Mean (Range) | SD |
|---|---|---|
| Maternal age (y) | 32.5 (20.0–45.5) | 5.1 |
| Maternal height at delivery (cm) | 163 (118–185) | 8 |
| Maternal Hb (g/L) | 111.5 (75.0–146.0) | 14.0 |
| ASA (n, %) | ||
| Level 1 | 84 (46%) | |
| Level 2 | 90 (49%) | |
| Level 3 | 8 (4%) | |
| Cord blood Hb (g/L) | 142.3 (100.0–191.0) | 15.9 |
| Birth weight (g) | 3458 (2400–5080) | 505 |
| Gestational age (wk) | 38.6 (35.0–41.0) | 1.0 |
| Placental weight (g) | 772.3 (395.0–1500.0) | 188.4 |
| Infant sex (n, %) | ||
| Male | 85 (46%) | |
| Female | 94 (54%) | |
| APGAR Score | ||
| 1 min | 9.0 (4.0–10.0) | 1.1 |
| 5 min | 9.0 (5.0–10.0) | 0.5 |
| Variable | β | SE | 95% CI | p |
|---|---|---|---|---|
| Birth weight | 0.224 | 0.025 | 0.173, 0.275 | <0.001 |
| Cord blood Hb | −2.444 | 0.741 | −3.908, −0.982 | 0.001 |
| Gestational age | −39.745 | 13.869 | −67.121, −12.370 | 0.005 |
| ASA | 37.876 | 20.490 | −2.568, 78.321 | 0.066 |
| APGAR (5 min) | 36.726 | 21.994 | −6.686, 80.139 | 0.097 |
| Maternal age | −2.330 | 2.275 | −6.822, 2.161 | 0.307 |
| Maternal Hb | 0.660 | 0.831 | −0.980, 2.300 | 0.428 |
| Sex of newborn | −0.948 | 22.966 | −46.279, 44.383 | 0.967 |
| Maternal height | −0.018 | 1.480 | −2.939, 2.903 | 0.990 |
| Variable | β | SE | 95% CI | p |
|---|---|---|---|---|
| Gestational age | 217.108 | 29.886 | 158.116, 276.100 | <0.001 |
| Placental weight | 1.168 | 0.163 | 0.845, 1.490 | <0.001 |
| Maternal height | 6.155 | 3.520 | −0.794, 13.104 | 0.082 |
| Cord blood Hb | 3.152 | 1.840 | −0.480, 6.784 | 0.088 |
| Maternal Hb | −3.353 | 1.978 | −7.260, 0.552 | 0.092 |
| Sex of newborn | −59.904 | 55.366 | −169.193, 49.385 | 0.281 |
| Maternal age | 4.844 | 5.461 | −5.935, 15.624 | 0.376 |
| APGAR (5 min) | −43.964 | 53.043 | −148.668, 60.740 | 0.408 |
| ASA | 1.493 | 52.427 | −101.994, 104.980 | 0.977 |
| Variable | β | SE | 95% CI | p |
|---|---|---|---|---|
| Cord blood Hb | 0.015 | 0.004 | 0.007, 0.024 | 0.001 |
| Gestational age | 0.247 | 0.074 | 0.101, 0.393 | 0.001 |
| APGAR (5 min) | −0.168 | 0.131 | −0.426, 0.091 | 0.091 |
| Maternal age | 0.017 | 0.014 | −0.010, 0.044 | 0.207 |
| ASA | −0.139 | 0.130 | −0.395, 0.117 | 0.286 |
| Maternal Hb | −0.002 | 0.005 | −0.012, 0.007 | 0.631 |
| Sex of newborn | 0.023 | 0.137 | −0.248, 0.295 | 0.864 |
| Maternal height | 0.001 | 0.009 | −0.016, 0.018 | 0.885 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gragasin, F.S.; Ospina, M.B.; Serrano-Lomelin, J.; Kim, S.H.; Kokotilo, M.; Woodman, A.G.; Renaud, S.J.; Bourque, S.L. Maternal and Cord Blood Hemoglobin as Determinants of Placental Weight: A Cross-Sectional Study. J. Clin. Med. 2021, 10, 997. https://doi.org/10.3390/jcm10050997
Gragasin FS, Ospina MB, Serrano-Lomelin J, Kim SH, Kokotilo M, Woodman AG, Renaud SJ, Bourque SL. Maternal and Cord Blood Hemoglobin as Determinants of Placental Weight: A Cross-Sectional Study. Journal of Clinical Medicine. 2021; 10(5):997. https://doi.org/10.3390/jcm10050997
Chicago/Turabian StyleGragasin, Ferrante S., Maria B. Ospina, Jesus Serrano-Lomelin, Su Hwan Kim, Matthew Kokotilo, Andrew G. Woodman, Stephen J. Renaud, and Stephane L. Bourque. 2021. "Maternal and Cord Blood Hemoglobin as Determinants of Placental Weight: A Cross-Sectional Study" Journal of Clinical Medicine 10, no. 5: 997. https://doi.org/10.3390/jcm10050997
APA StyleGragasin, F. S., Ospina, M. B., Serrano-Lomelin, J., Kim, S. H., Kokotilo, M., Woodman, A. G., Renaud, S. J., & Bourque, S. L. (2021). Maternal and Cord Blood Hemoglobin as Determinants of Placental Weight: A Cross-Sectional Study. Journal of Clinical Medicine, 10(5), 997. https://doi.org/10.3390/jcm10050997







