Differences in Knee Shape between ACL Injured and Non-Injured: A Matched Case-Control Study of 168 Patients
Abstract
1. Introduction
2. Experimental Section
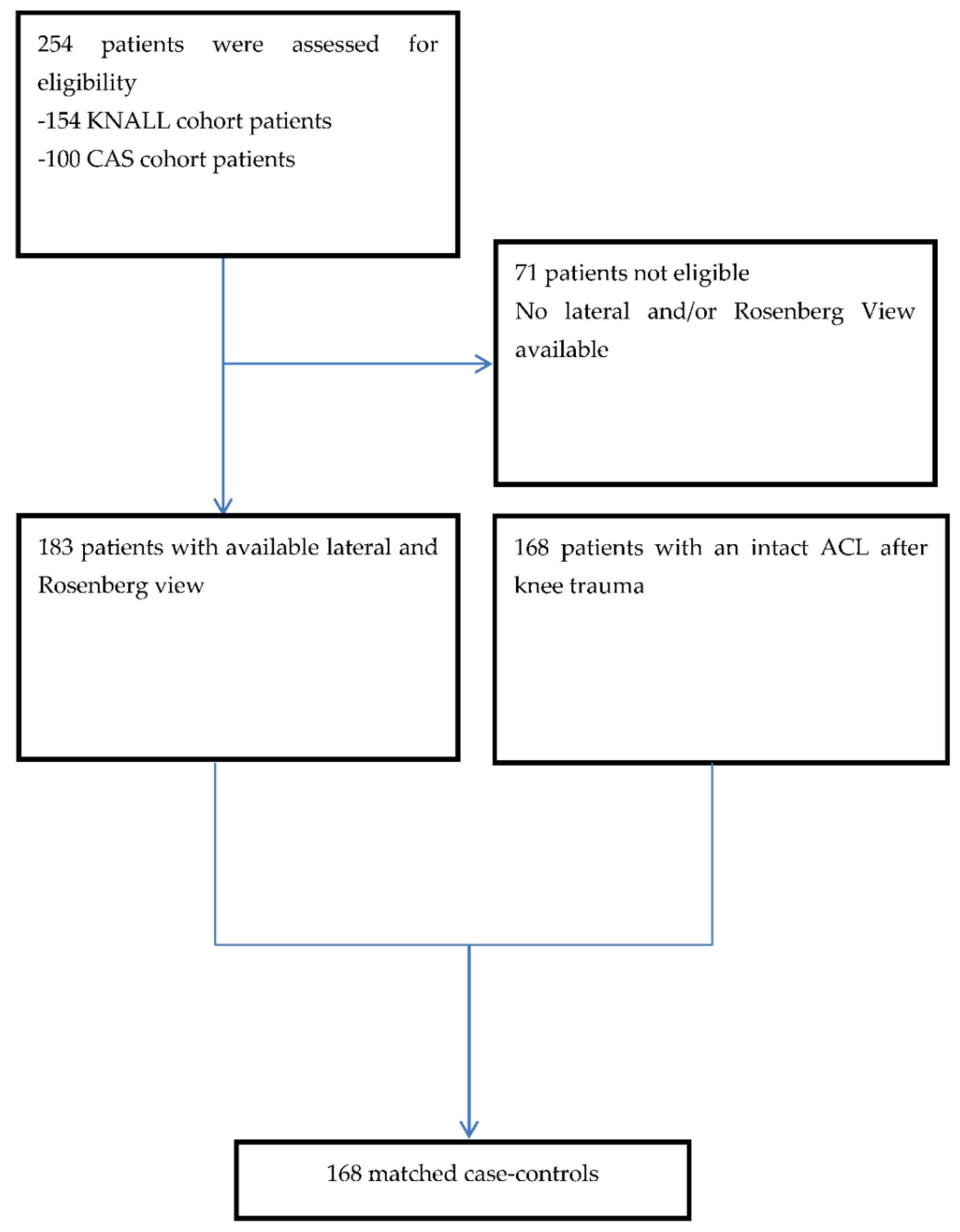
2.1. Cases
2.2. Controls
2.3. X-rays and Statistical Shape Modeling
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. SSM
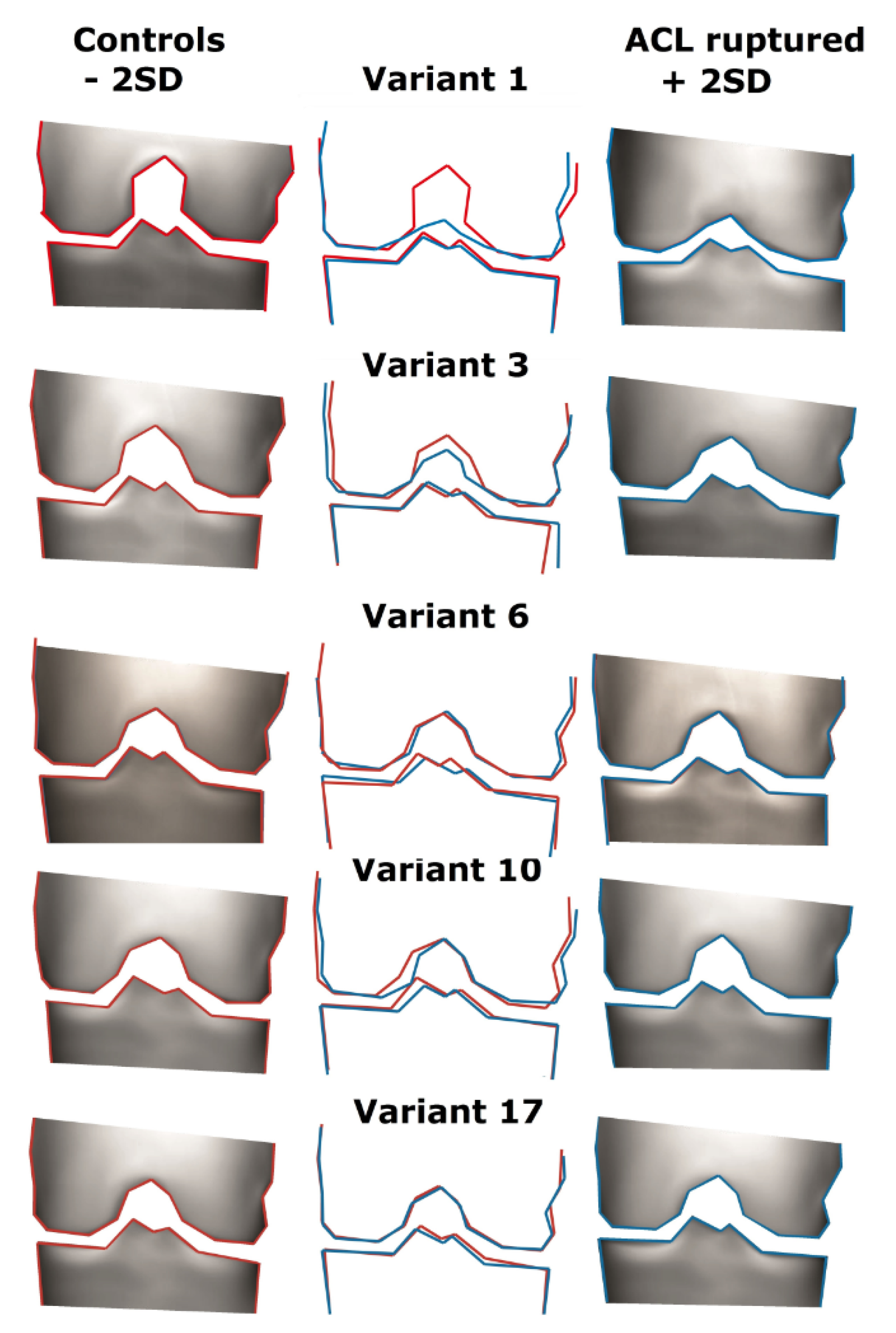
3.3. Description of the Variants
- Variant 1
- Variant 3
- Variant 6
- Variant 10
- Variant 17
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frobell, R.B.; Lohmander, L.S.; Roos, H.P.; Lohmander, L.S. Acute rotational trauma to the knee: Poor agreement between clinical assessment and magnetic resonance imaging findings. Scand. J. Med. Sci. Sports 2006, 17, 109–114. [Google Scholar] [CrossRef]
- Moses, B.; Orchard, J.; Orchard, J. Systematic review: Annual incidence of ACL injury and surgery in various populations. Res. Sports Med. 2012, 20, 157–179. [Google Scholar] [CrossRef]
- Nordenvall, R.; Bahmanyar, S.; Adami, J.; Stenros, C.; Wredmark, T.; Felländer-Tsai, L. A Population-Based Nationwide Study of Cruciate Ligament Injury in Sweden, 2001–2009. Am. J. Sports Med. 2012, 40, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Lindanger, L.; Strand, T.; Mølster, A.O.; Solheim, E.; Inderhaug, E. Return to Play and Long-term Participation in Pivoting Sports After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2019, 47, 3339–3346. [Google Scholar] [CrossRef]
- Ajuied, A.; Wong, F.; Smith, C.; Norris, M.; Earnshaw, P.; Back, D.; Davies, A. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: A systematic review and meta-analysis. Am. J. Sports Med. 2014, 42, 2242–2252. [Google Scholar] [CrossRef] [PubMed]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Whitehead, T.; Webster, K.E. Psychological Responses Matter in Returning to Preinjury Level of Sport After Anterior Cruciate Ligament Reconstruction Surgery. Am. J. Sports Med. 2013, 41, 1549–1558. [Google Scholar] [CrossRef]
- Niederer, D.; Engeroff, T.; Wilke, J.; Vogt, L.; Banzer, W. Return to play, performance, and career duration after anterior cruciate ligament rupture: A case-control study in the five biggest football nations in Europe. Scand. J. Med. Sci. Sports 2018, 28, 2226–2233. [Google Scholar] [CrossRef]
- Di Stasi, S.; Myer, G.D.; Hewett, T.E. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J. Orthop. Sports Phys. Ther. 2013, 43, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.J.; Jeong, J.H.; Chang, C.B.; Kim, Y.J.; Seo, B.K.; Song, M.K.; Kang, T.; Kang, S.-B. Revision surgery for failed anterior cruciate ligament reconstruction with extension deficiency. Scand. J. Med. Sci. Sports 2018, 28, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Robertsson, O.; Lidgren, L.; Sundberg, M.; W-Dahl, A. The Swedish Knee Ligament Registry. Annual Report 2018. 2018. Available online: www.alcregister.nu (accessed on 28 February 2021).
- Silvers, H.J.; Mandelbaum, B.R. Prevention of anterior cruciate ligament injury in the female athlete. Br. J. Sports Med. 2007, 41 (Suppl. 1), i52–i59. [Google Scholar] [CrossRef]
- Myklebust, G.; Engebretsen, L.; Braekken, I.H.; Skjolberg, A.; Olsen, O.E.; Bahr, R. Prevention of anterior cruciate ligament injuries in female team handball players: A prospective intervention study over three seasons. Clin. J. Sport Med. 2003, 13, 71–78. [Google Scholar] [CrossRef]
- Arundale, A.J.H.; Silvers-Granelli, H.; Marmon, A.R.; Zarzycki, R.; Dix, C.; Snyder-Mackler, L. Changes in biomechanical knee injury risk factors across two collegiate soccer seasons using the 11+ prevention program. Scand. J. Med. Sci. Sports 2018, 28, 2592–2603. [Google Scholar] [CrossRef]
- Fowler, P.J.; Messieh, S.S. Isolated posterior cruciate ligament injuries in athletes. Am. J. Sports Med. 1987, 15, 553–557. [Google Scholar] [CrossRef]
- Eggerding, V.; Meuffels, D.E.; Bierma-Zeinstra, S.M.; Verhaar, J.A.; Reijman, M. Factors Related to the Need for Surgical Reconstruction After Anterior Cruciate Ligament Rupture: A Systematic Review of the Literature. J. Orthop. Sports Phys. Ther. 2014, 45, 37–44. [Google Scholar] [CrossRef]
- Shen, L.; Jin, Z.-G.; Dong, Q.-R.; Li, L.-B. Anatomical Risk Factors of Anterior Cruciate Ligament Injury. Chin. Med. J. 2018, 131, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Wratten, C.J.; Tetsworth, K.; Hohmann, E. Three-Dimensional Femoral Notch Volume in Anterior Cruciate Ligament–Deficient Versus Anterior Cruciate Ligament–Intact Patients: A Matched Case-Control Study with Inter-gender Comparison. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 1117–1122. [Google Scholar] [CrossRef]
- Eggerding, V.; Van Kuijk, K.S.R.; Van Meer, B.L.; Bierma-Zeinstra, S.M.A.; Van Arkel, E.R.A.; Reijman, M.; Waarsing, J.H.; Meuffels, D.E. Knee shape might predict clinical outcome after an anterior cruciate ligament rupture. Bone Jt. J. 2014, 96, 737–742. [Google Scholar] [CrossRef] [PubMed]
- van Meer, B.L.; Oei, E.H.; Meuffels, D.E.; van Arkel, E.R.; Verhaar, J.A.; Bierma-Zeinstra, S.M.; Reijman, M. Degenerative Changes in the Knee 2 Years After Anterior Cruciate Ligament Rupture and Related Risk Factors: A Prospective Observational Follow-up Study. Am. J. Sports Med. 2016, 44, 1524–1533. [Google Scholar] [CrossRef]
- Eggerding, V.; Reijman, M.; Scholten, R.J.; Verhaar, J.A.; Meuffels, D.E. Computer-assisted surgery for knee ligament reconstruction. Cochrane Database Syst. Rev. 2014, 2014, CD007601. [Google Scholar]
- Meuffels, D.E.; Reijman, M.; Verhaar, J.A. Computer-assisted surgery is not more accurate or precise than conventional arthroscopic ACL reconstruction: A prospective randomized clinical trial. J. Bone Jt. Surg. Am. Vol. 2012, 94, 1538–1545. [Google Scholar] [CrossRef]
- Årøen, A.; Verdonk, P. Posterior cruciate ligament, exploring the unknown. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 996–997. [Google Scholar] [CrossRef]
- Cootes, T.F.; Cooper, D.H.; Graham, J. Active shape models: Their training and application. Comput. Vis. Image Underst. 1995, 61, 38–59. [Google Scholar] [CrossRef]
- Bollen, S. Epidemiology of knee injuries: Diagnosis and triage. Br. J. Sports Med. 2000, 34, 227–228. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Myer, G.D.; Silvers, H.J.; Samitier, G.; Romero, D.; Lázaro-Haro, C.; Cugat, R. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 2: A review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 859–879. [Google Scholar] [CrossRef]
- Domzalski, M.; Grzelak, P.; Gabos, P. Risk factors for Anterior Cruciate Ligament injury in skeletally immature patients: Analysis of intercondylar notch width using Magnetic Resonance Imaging. Int. Orthop. 2010, 34, 703–707. [Google Scholar] [CrossRef]
- van Diek, F.M.; Wolf, M.R.; Murawski, C.D.; van Eck, C.F.; Fu, F.H. Knee morphology and risk factors for developing an anterior cruciate ligament rupture: An MRI comparison between ACL-ruptured and non-injured knees. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Whitney, D.C.; Sturnick, D.R.; Vacek, P.M.; DeSarno, M.J.; Gardner-Morse, M.; Tourville, T.W.; Smith, H.C.; Slauterbeck, J.R.; Johnson, R.J.; Shultz, S.J.; et al. Relationship Between the Risk of Suffering a First-Time Noncontact ACL Injury and Geometry of the Femoral Notch and ACL: A Prospective Cohort Study With a Nested Case-Control Analysis. Am. J. Sports Med. 2014, 42, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Boden, B.P.; Dean, G.S.; Feagin, J.A., Jr.; Garrett, W.E., Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics 2000, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Dienst, M.; Schneider, G.; Altmeyer, K.; Voelkering, K.; Georg, T.; Kramann, B.; Kohn, D.M. Correlation of intercondylar notch cross sections to the ACL size: A high resolution MR tomographic in vivo analysis. Arch. Orthop. Trauma Surg. 2007, 127, 253–260. [Google Scholar] [CrossRef]
- Fridén, T.; Jonsson, A.; Erlandsson, T.; Jonsson, K.; Lindstrand, A. Effect of femoral condyle configuration on disability after an anterior cruciate ligament rupture: 100 patients followed for 5 years. Acta Orthop. Scand. 1993, 64, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Archbold, P.; Cucurulo, T.; Fayard, J.-M.; Bortolletto, J.; Thaunat, M.; Prost, T.; Chambat, P. The influence of the tibial slope and the size of the intercondylar notch on rupture of the anterior cruciate ligament. J. Bone Jt. Surg. Br. Vol. 2011, 93, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Stijak, L.; Herzog, R.F.; Schai, P. Is there an influence of the tibial slope of the lateral condyle on the ACL lesion? A case-control study. Knee Surg Sports Traumatol. Arthrosc. 2008, 16, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Van Eck, C.F.; Vyas, N.; Fu, F.H.; Otsuka, N.Y. Increased medial tibial slope in teenage pediatric population with open physes and anterior cruciate ligament injuries. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Shelbourne, K.D.; Facibene, W.A.; Hunt, J.J. Radiographic and intraoperative intercondylar notch width measurements in men and women with unilateral and bilateral anterior cruciate ligament tears. Knee Surg. Sports Traumatol. Arthrosc. 1997, 5, 229–233. [Google Scholar] [CrossRef]
| ACL Injured (n = 168) | Control Group (n = 168) | |
|---|---|---|
| Age, year | 31 ± 7.4 | 38 ± 12.0 |
| BMI, kg/m2 | 24.5 ± 3.4 | 24.7 ± 3.2 |
| Female n (%) | 49 (29.1) | 49 (29.1) |
| Mean time in months between trauma and radiograph | 1.0 | 6.9 |
| Alternative/additional diagnosis, n (%) | ||
| Medial Meniscus tear | 10 (6) | 57 (33.9) |
| Lateral meniscus tear | 12 (7) | 32 (19) |
| Cartilage lesion | 60 (35) | 15 (8.9) |
| Bone contusion | 50 (30) | 11 (6.5) |
| Collateral ligament lesion | 0 (0) | 7 (4.2) |
| No intra-articular lesions | 0 (0) | 46 (27.4) |
| Odds Ratio | 95% C.I. | Sig. | |
|---|---|---|---|
| Variant 1 | 2.2 | (1.7–2.8) | 0.001 |
| Variant 3 | 1.8 | (1.4–2.3) | 0.001 |
| Variant 6 | 2.1 | (1.6–2.7) | 0.001 |
| Variant 10 | 1.5 | (1.2–1.8) | 0.001 |
| Variant 17 | 1.4 | (1.1–1.8) | 0.0015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Kuijk, K.S.R.; Eggerding, V.; Reijman, M.; van Meer, B.L.; Bierma-Zeinstra, S.M.A.; van Arkel, E.; Waarsing, J.H.; Meuffels, D.E. Differences in Knee Shape between ACL Injured and Non-Injured: A Matched Case-Control Study of 168 Patients. J. Clin. Med. 2021, 10, 968. https://doi.org/10.3390/jcm10050968
van Kuijk KSR, Eggerding V, Reijman M, van Meer BL, Bierma-Zeinstra SMA, van Arkel E, Waarsing JH, Meuffels DE. Differences in Knee Shape between ACL Injured and Non-Injured: A Matched Case-Control Study of 168 Patients. Journal of Clinical Medicine. 2021; 10(5):968. https://doi.org/10.3390/jcm10050968
Chicago/Turabian Stylevan Kuijk, Koen S.R., Vincent Eggerding, Max Reijman, Belle L. van Meer, Sita M.A. Bierma-Zeinstra, Ewoud van Arkel, Jan H. Waarsing, and Duncan E. Meuffels. 2021. "Differences in Knee Shape between ACL Injured and Non-Injured: A Matched Case-Control Study of 168 Patients" Journal of Clinical Medicine 10, no. 5: 968. https://doi.org/10.3390/jcm10050968
APA Stylevan Kuijk, K. S. R., Eggerding, V., Reijman, M., van Meer, B. L., Bierma-Zeinstra, S. M. A., van Arkel, E., Waarsing, J. H., & Meuffels, D. E. (2021). Differences in Knee Shape between ACL Injured and Non-Injured: A Matched Case-Control Study of 168 Patients. Journal of Clinical Medicine, 10(5), 968. https://doi.org/10.3390/jcm10050968






