Comparison of Two Different Sedation Protocols during Transvaginal Oocyte Retrieval: Effects on Propofol Consumption and IVF Outcome: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population/Eligibly Criteria
2.2. Groups, Anesthesia Management and Assessments
2.3. IVF Protocol
2.4. Outcome Measures
- (1)
- Anesthesiological parameters: total dose of dexmedetomidine or remifentanil administered, hemodynamic parameters, dose of ephedrine, dose of atropine, BIS values, OAA/S score, adverse effects during anesthesia, time required to achieve the maximum OAA/S score, PONV, VAS, time required to achieve PADSS score ≥9 and the overall patients’ and gynecologist’ satisfaction.
- (2)
- IVF outcomes (definitions used were as reported in Zegers-Hochschild et al., 2017 [24]): number of oocytes retrieved at the day of the OR, number of fertilized embryos [number of oocytes with two nuclei (2PN) divided by the total number of oocytes retrieved], embryo quality and number of top quality embryos at day 3 (Veeck: 5-point scale: Grade 1 = excellent, Grade 2 = good, 3 = moderate, 4 = poor, 5 = unsustainable), cycle cancelation (in cases with premature ovulation, no oocytes retrieved or no embryos available for transfer), biochemical (defined as a positive pregnancy test), ectopic, clinical pregnancy (defined as the presence of fetal heart beat at 7 weeks gestation) and miscarriage rates (defined as pregnancy loss up to the 20th week of pregnancy per positive pregnancy test). Embryo quality was assessed according to morphological criteria based on the overall blastomere number, size, appearance and degree of fragmentation [25].
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bokhari, A.; Pollard, B.J. Anaesthesia for assisted conception: A survey of UK practice. Eur. J. Anaesthesiol. 1999, 16, 225–230. [Google Scholar] [CrossRef]
- Wilhelm, W.; Hammadeh, M.E.; White, P.F.; Georg, T.; Fleser, R.; Biedler, A. General anesthesia versus monitored anesthesia care with remifentanil for assisted reproductive technologies: Effect on pregnancy rate. J. Clin. Anesth. 2002, 14, 1–5. [Google Scholar] [CrossRef]
- Kwan, I.; Wang, R.; Pearce, E.; Bhattacharya, S. Pain relief for women undergoing oocyte retrieval for assisted reproduction. Cochrane Database Syst. Rev. 2018, 2018, CD004829. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, N.F.; Giannakikou, I.; Vlachos, A.; Vitoratos, N. Analgesia and anesthesia for assisted reproductive technologies. Int. J. Gynecol. Obstet. 2009, 105, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Matsota, P.; Sidiropoulou, T.; Batistaki, C.; Giannaris, D.; Pandazi, A.; Krepi, H.; Christodoulaki, K.; Kostopanagiotou, G. Analgesia with remifentanil versus anesthesia with propofol-alfentanil for transvaginal oocyte retrieval: A randomized trial on their impact on in vitro fertilization outcome. Middle East J. Anaesthesiol. 2012, 21, 23265031. [Google Scholar]
- Lier, M.C.; Douwenga, W.M.; Yilmaz, F.; Schats, R.; Hompes, P.G.; Boer, C.; Mijatovic, V. Patient-Controlled Remifentanil Analgesia as Alternative for Pethidine with Midazolam During Oocyte Retrieval in IVF/ICSI Procedures: A Randomized Controlled Trial. Pain Pr. 2014, 15, 487–495. [Google Scholar] [CrossRef]
- Hoy, S.M.; Keating, G.M. Dexmedetomidine: A review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 2011, 71, 1481–1501. [Google Scholar] [CrossRef]
- Candiotti, K.A.; Bergese, S.D.; Bokesch, P.M.; Feldman, M.A.; Wisemandle, W.; Bekker, A.Y. Monitored Anesthesia Care with Dexmedetomidine: A Prospective, Randomized, Double-Blind, Multicenter Trial. Anesth. Analg. 2010, 110, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs 2015, 75, 1119–1130. [Google Scholar] [CrossRef]
- Elnabtity, A.M.A.; Selim, M.F. A prospective randomized trial comparing dexmedetomidine and midazolam for conscious sedation during oocyte retrieval in an in vitro fertilization program. Anesth. Essays Res. 2017, 11, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Takizuka, A.; Minami, K.; Uezono, Y.; Horishita, T.; Yokoyama, T.; Shiraishi, M.; Sakurai, T.; Shigematsu, A.; Ueta, Y. Dexmedetomidine inhibits muscarinic type 3 receptors expressed in Xenopus oocytes and muscarine-induced intracellular Ca2+ elevation in cultured rat dorsal root ganglia cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 375, 293–301. [Google Scholar] [CrossRef]
- Do, S.-H.; Park, S.-J.; Shin, H.-J.; Paik, H.-S.; Zuo, Z.; Yoon, H.-J.; Ryu, J.-H. Dexmedetomidine increases the activity of excitatory amino acid transporter type 3 expressed in Xenopus oocytes: The involvement of protein kinase C and phosphatidylinositol 3-kinase. Eur. J. Pharmacol. 2014, 738, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, F.; Janssenswillen, C.; Verborgh, C.; Moerman, I.; Devroey, P.; Van Steirteghem, A.; Camu, F. Propofol concentrations in follicular fluid during general anaesthesia for transvaginal oocyte retrieval. Hum. Reprod. 1999, 14, 345–348. [Google Scholar] [CrossRef]
- Coetsier, T.; Dhont, M.; De Sutter, P.; Merchiers, E.; Versichelen, L.; Rosseel, M. Propofol anaesthesia for ultrasound guided oocyte retrieval: Accumulation of the anaesthetic agent in follicular fluid. Hum. Reprod. 1992, 7, 1422–1424. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Pridham, D.D.; Reshef, E.; Drury, K.; Cook, C.L.; Hurst, H.E.; Yussman, M.A. Follicular fluid Lidocaine levels during transvaginal oocyte retrieval. Fertil. Steril. 1990, 53, 171–173. [Google Scholar] [CrossRef]
- Endler, G.C.; Stout, M.; Magyar, D.M.; Hayes, M.F.; Moghissi, K.S.; Sacco, A.G. Follicular fluid concentrations of thiopental and thiamylal during laparoscopy for oocyte retrieval. Fertil. Steril. 1987, 48, 828–833. [Google Scholar] [CrossRef]
- Soussis, I.; Boyd, O.; Paraschos, T.; Duffy, D.; Bower, S.; Trougton, P.; Lowe, J.; Grounds, R. Follicular fluid levels of midazolam, fentanyl, and alfentanyl during transvaginal oocyte retrieval. Fertil. Steril. 1995, 64, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Matsota, P.; Kaminioti, E.; Kostopanagiotou, G. Anesthesia Related Toxic Effects on In Vitro Fertilization Outcome: Burden of Proof. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Depypere, H.; Dhont, M.; De Sutter, P.; Vandekerckhove, D. The influence of propofol on in vitro fertilization in mice. Hum. Reprod. 1991, 127, 151. [Google Scholar] [CrossRef]
- Janssenswillen, C.; Christiaens, F.; Camu, F.; Van Steirteghem, A. The effect of propofol on parthenogenetic activation, in vitro fertilization and early development of mouse oocytes. Fertil. Steril. 1997, 67, 769–774. [Google Scholar] [CrossRef]
- Hein, H.; Putman, J. Is propofol a proper proposition for reproductive procedures? J. Clin. Anesth. 1997, 9, 611–613. [Google Scholar] [CrossRef]
- Siristatidis, C.; Pouliakis, A.; Sergentanis, T.N. Special characteristics, reproductive, and clinical profile of women with unexplained infertility versus other causes of infertility: A comparative study. J. Assist. Reprod. Genet. 2020, 37, 1923–1930. [Google Scholar] [CrossRef]
- Siristatidis, C.; Askoxylaki, M.; Varounis, C.; Kassanos, D.; Chrelias, C. E-selectin, resistin and reactive oxygen species levels in GnRH -agonist and -antagonist protocols in IVF/ICSI: A prospective cohort study. J. Assist. Reprod. Genet. 2015, 32, 959–967. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; De Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017†‡§. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Ziebe, S.; Petersen, K.; Lindenberg, S.; Andersen, A.G.; Gabrielsen, A. Embryo morphology or cleavage stage: How to select the best embryos for transfer after in-vitro fertilization. Hum. Reprod. 1997, 12, 1545–1549. [Google Scholar] [CrossRef]
- Tatone, C.; Francione, A.; Marinangeli, F.; Lottan, M.; Varrassi, G.; Colonna, R. An evaluation of propofol toxicity on mouse oocytes and preimplantation embryos. Hum. Reprod. 1998, 13, 430–435. [Google Scholar] [CrossRef]
- Vincent, R.D., Jr.; Syrop, C.H.; Van Voorhis, B.J.; Chestnut, D.H.; Sparks, A.E.T.; McGrath, J.M.; Choi, W.W.; Bates, J.N.; Penning, D.H. An Evaluation of the Effect of Anesthetic Technique on Reproductive Success after Laparoscopic Pronuclear Stage Transfer. Propofol/nitrous oxide versus isoflurane/nitrous oxide. J. Am. Soc. Anesth. 1995, 82, 352–358. [Google Scholar] [CrossRef]
- Piroli, A.; Marci, R.; Marinangeli, F.; Paladini, A.; Di Emidio, G.; Artini, P.G.; Caserta, D.; Tatone, C. Comparison of different anaesthetic methodologies for sedation duringin vitrofertilization procedures: Effects on patient physiology and oocyte competence. Gynecol. Endocrinol. 2012, 28, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Omokanye, L.; Olatinwo, A.O.; Durowade, K.A.; Panti, A.A.; Salaudeen, G.A. Conscious sedation for oocyte retrieval: Experience at a tertiary health facility in North-Central, Nigeria. Trop. J. Obstet. Gynaecol. 2020, 37, 151. [Google Scholar] [CrossRef]
- Salas, M.; Hotman, A.; Stricker, B.H. Confounding by Indication: An Example of Variation in the Use of Epidemiologic Terminology. Am. J. Epidemiol. 1999, 149, 981–983. [Google Scholar] [CrossRef]
| Observer’s Assessment of Alertness/Sedation Scale (OAA/S) | ||
|---|---|---|
| Responds readily to name spoken in normal tone | 5 | |
| Lethargic response to name spoken in normal tone | 4 | |
| Responds only after name is called loudly and/or repeatedly | 3 | |
| Responds only after mild prodding or shaking | 2 | |
| Does not respond to mild prodding or shaking | 1 | |
| Visual Analogue Scale (VAS) | ||
| 0____________________________________10 | ||
| No pain | The worst pain | |
| Postoperative nausea/vomiting (PONV) assessment | ||
| VitalSigns | 2 = within 20% of preoperative value 1 = 20–40% of preoperative value 0 ≥ 40% preoperative value | |
| Activity and mental status | 2 = Oriented × 3 AND has a steady gait 1 = Oriented × 3 OR has a steady gait 0 = Neither | |
| Nausea and/or vomiting | 2 = Minimal 1 = Moderate, having required treatment 0 = Severe, requiring treatment | |
| Pain | 2 = Minimal 1 = Moderate, having required treatment 0 = Severe, requiring treatment | |
| Surgicalbleeding | 2 = Minimal 1 = Moderate 0 = Severe | |
| none | 0 | |
| Nausea | 1 | |
| <2 episodes of vomiting | 2 | |
| >2 episodes of vomiting | 3 | |
| Patients’ and gynecologist’s overall satisfaction assessment | ||
| Assessment is performed using the following two questions: 1st question: Are you satisfied with the anesthetic technique used? (Yes/No) 2nd question: Would you like to use the same anesthetic technique in the future again? (Yes/No) | ||
| Group DEX (n = 36) | Group REMI (n = 36) | p-Value | |
|---|---|---|---|
| Age (years) | 38.5 (8.0) | 37.5 (5.0) | 0.709 |
| BMI (kg/m2) | 24.3 ± 4.9 | 24.3 ± 4.1 | 0.986 |
| Smoking, n (%) | 9 (25%) | 15 (41.6%) | 0.211 |
| Alcohol (>4 cups/wk), n (%) | 5 (13.9%) | 4 (11.1%) | 0.721 |
| Age of menarche (years) | 12.58 ± 1.27 | 12.83 ±1.13 | 0.382 |
| AFC | 8.13 ± 2.21 | 8.1 ± 2.64 | 0.962 |
| AMH (ng/mL) | 2.19 ± 0.8 | 2.16 ± 0.7 | 0.867 |
| Basal FSH (IU/L) | 8.4 ± 1.7 | 8.1 ± 1.5 | 0.410 |
| Basal PRL (ng/mL) | 9.83 ± 3.3 | 9.1 ±2.58 | 0.299 |
| ASA class, n | |||
| 1 | 19 (52.7%) | 16 (44.4%) | 0.638 |
| 2 | 17 (47.2%) | 20 (55.5%) | |
| Infertility, n | |||
| Primary | 27 (75%) | 31 (86%) | 0.372 |
| Secondary | 9 (25%) | 5 (13.8%) | |
| Cause of infertility, n (%) | |||
| Unexplained | 17 (47%) | 19 (52%) | 0.637 |
| Male | 7 (19.4%) | 6 (16.7%) | 0.759 |
| Ovulatory | 9 (25%) | 6 (16.7%) | 0.384 |
| Tubal | 3 (8.3%) | 5 (13.9%) | 0.453 |
| Duration of infertility (years) | 2.12 ±0.64 | 2.25 ± 0.72 | 0.409 |
| Protocol, n | |||
| Long | 10 (27.7%) | 12 (33.3%) | 0.798 |
| Short | 26 (72.2%) | 24 (66.7%) | |
| MALE partner | |||
| BMI (kg/m2) | 25.2 ± 3.6 | 24.9 ± 3.5 | 0.720 |
| Smoking (current), n (%) | 14 (38.9%) | 17 (47.2%) | 0.475 |
| Alcohol (>4 cups/wk), n (%) | 9 (25%) | 8 (22.2%) | 0.781 |
| Varicocele/Cryptorchidism/Obstructions in reproductive tract, n (%) | 4 (11/1%) | 3 (8.3%) | 0.69 |
| Previous surgery/Infectious parotitis, n (%) | 3 (8.3%) | 3 (8.3%) | 1 |
| (a) | |||
| Group DEX (n = 36) | Group REMI (n = 36) | p-Value | |
| Intraoperative data | |||
| Anesthesia duration (min) | 22.0 (7.8) | 22.0 (7.5) | 0.599 |
| Surgery duration (min) | 11.5 (7.5) | 10.0 (7.8) | 0.739 |
| Cumulative Propofol consumption (mg) | 81.6 ± 64 | 17.2 ± 36.4 | <0.001 |
| Dexmedetomidine (μg) | 27.7 ± 9 | - | - |
| Remifentanil (μg) | - | 270 ± 78.5 | - |
| Airway obstruction, n | 1 (2.7%) | 5 (13.9%) | 0.199 |
| Need for assisted ventilation, n | 14 (38.9%) | 8 (22.2%) | 0.200 |
| Rigidity, n | 0 | 0 | - |
| Hypotension, n | 2 (5.5%) | 0 | 0.493 |
| Bradycardia, n | 1 (2.7%) | 0 | 1.0 |
| Postoperative data | |||
| OAA/S time to 5 (min) | 1.18 ± 1.46 | 1 ± 1.76 | 0.706 |
| Patient satisfaction | |||
| 1st question (Yes/No) | 31/5 | 36/0 | 0.054 |
| 2nd question (Yes/No) | 35/1 | 36/0 | 1.0 |
| Physician satisfaction | |||
| 1st question (Yes/No) | 32/4 | 36/0 | 0.120 |
| 2nd question (Yes/No) | 35/1 | 36/0 | 1.0 |
| VAS, 0–10 point | 0.27 ± 0.7 | 0.4 ± 1.15 | 0.552 |
| PONV, n | |||
| Nausea | 0 | 2 (5.5%) | 0.2 |
| Vomitus <2 episodes | 0 | 1 (2.7%) | |
| Hypotension, n | 0 | 0 | - |
| Bradycardia, n | 0 | 0 | - |
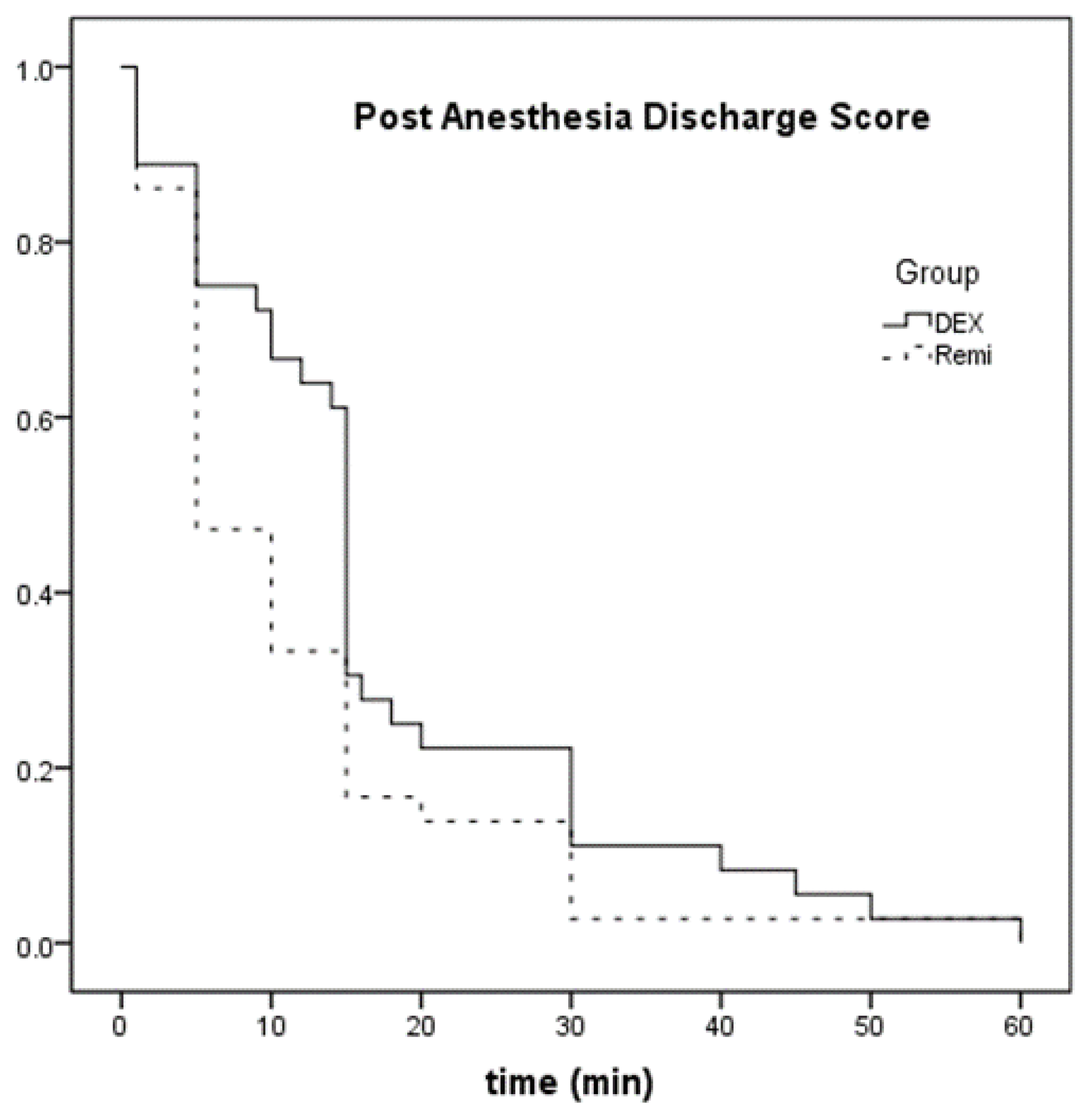
| Post Anesthesia Discharge Score (min) | 15.0 (13.5) | 5.0 (10.0) | 0.028 |
| (b) | |||
| Group DEX (n = 36) | Group REMI (n = 36) | p-Value | |
| Number of oocytes retrieved | 5 ± 2.3 | 5.5 ±3.2 | 0.462 |
| Embryo quality, n | |||
| 1 | 28 | 18 | 0.040 |
| 2 | 8 | 17 | |
| 2PN/total number of oocytes | 0.6 | 0.6 | 0.469 |
| Top quality Day 3 | 20.7 (5.4) | 23.4 (4.7) | 0.052 |
| Positive HCG test, n | 7 (19.4%) | 10 (27.7%) | 0.580 |
| Clinical pregnancy, n | 7 (19.4%) | 10 (27.7%) | |
| Miscarriage, n | 3 (8.3%) | 0 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsota, P.; Sidiropoulou, T.; Vrantza, T.; Boutsikou, M.; Midvighi, E.; Siristatidis, C. Comparison of Two Different Sedation Protocols during Transvaginal Oocyte Retrieval: Effects on Propofol Consumption and IVF Outcome: A Prospective Cohort Study. J. Clin. Med. 2021, 10, 963. https://doi.org/10.3390/jcm10050963
Matsota P, Sidiropoulou T, Vrantza T, Boutsikou M, Midvighi E, Siristatidis C. Comparison of Two Different Sedation Protocols during Transvaginal Oocyte Retrieval: Effects on Propofol Consumption and IVF Outcome: A Prospective Cohort Study. Journal of Clinical Medicine. 2021; 10(5):963. https://doi.org/10.3390/jcm10050963
Chicago/Turabian StyleMatsota, Paraskevi, Tatiana Sidiropoulou, Tereza Vrantza, Maria Boutsikou, Elena Midvighi, and Charalampos Siristatidis. 2021. "Comparison of Two Different Sedation Protocols during Transvaginal Oocyte Retrieval: Effects on Propofol Consumption and IVF Outcome: A Prospective Cohort Study" Journal of Clinical Medicine 10, no. 5: 963. https://doi.org/10.3390/jcm10050963
APA StyleMatsota, P., Sidiropoulou, T., Vrantza, T., Boutsikou, M., Midvighi, E., & Siristatidis, C. (2021). Comparison of Two Different Sedation Protocols during Transvaginal Oocyte Retrieval: Effects on Propofol Consumption and IVF Outcome: A Prospective Cohort Study. Journal of Clinical Medicine, 10(5), 963. https://doi.org/10.3390/jcm10050963








