Difficult Cases of Paroxysmal Nocturnal Hemoglobinuria: Diagnosis and Therapeutic Novelties
Abstract
1. Introduction
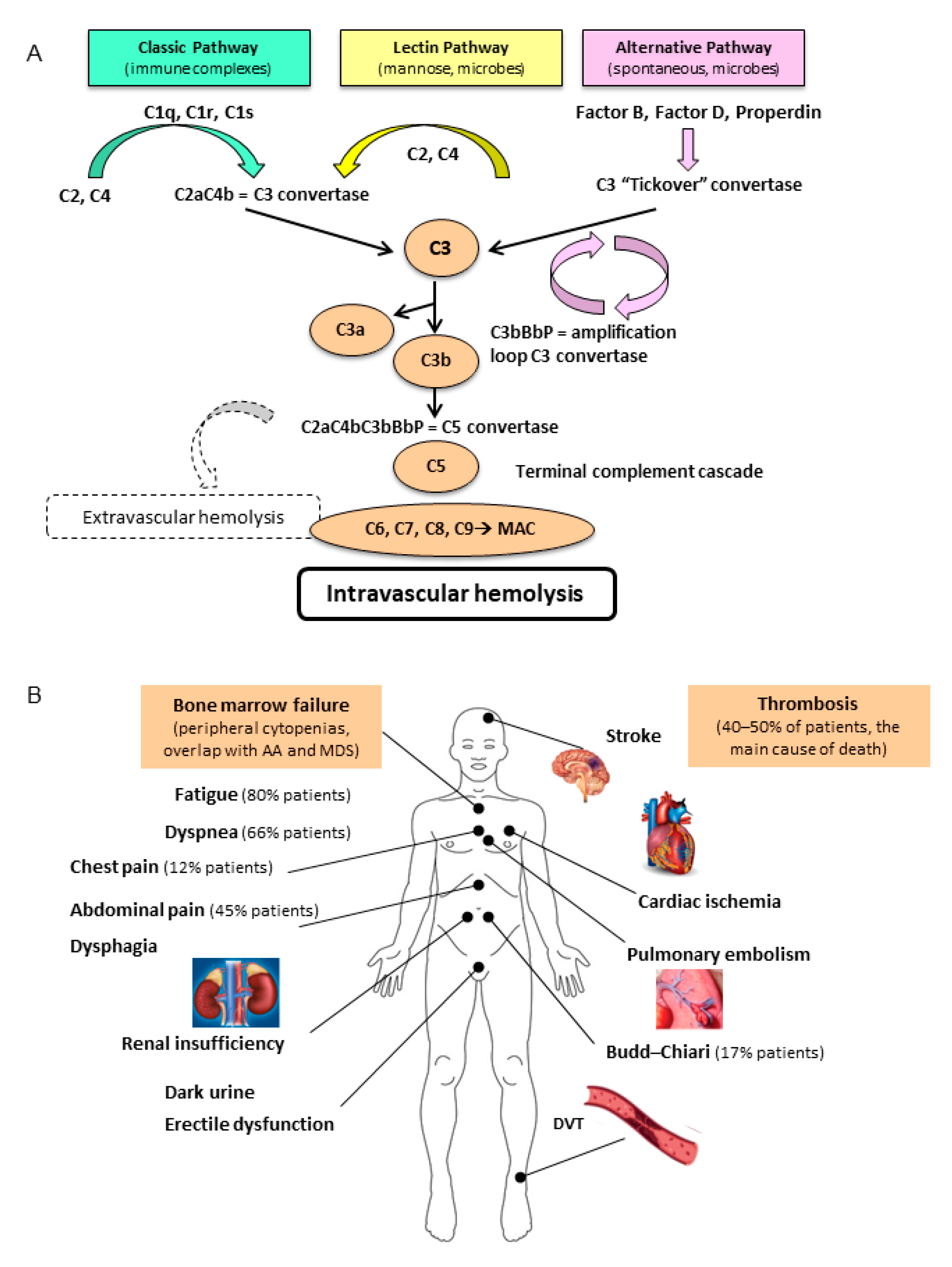
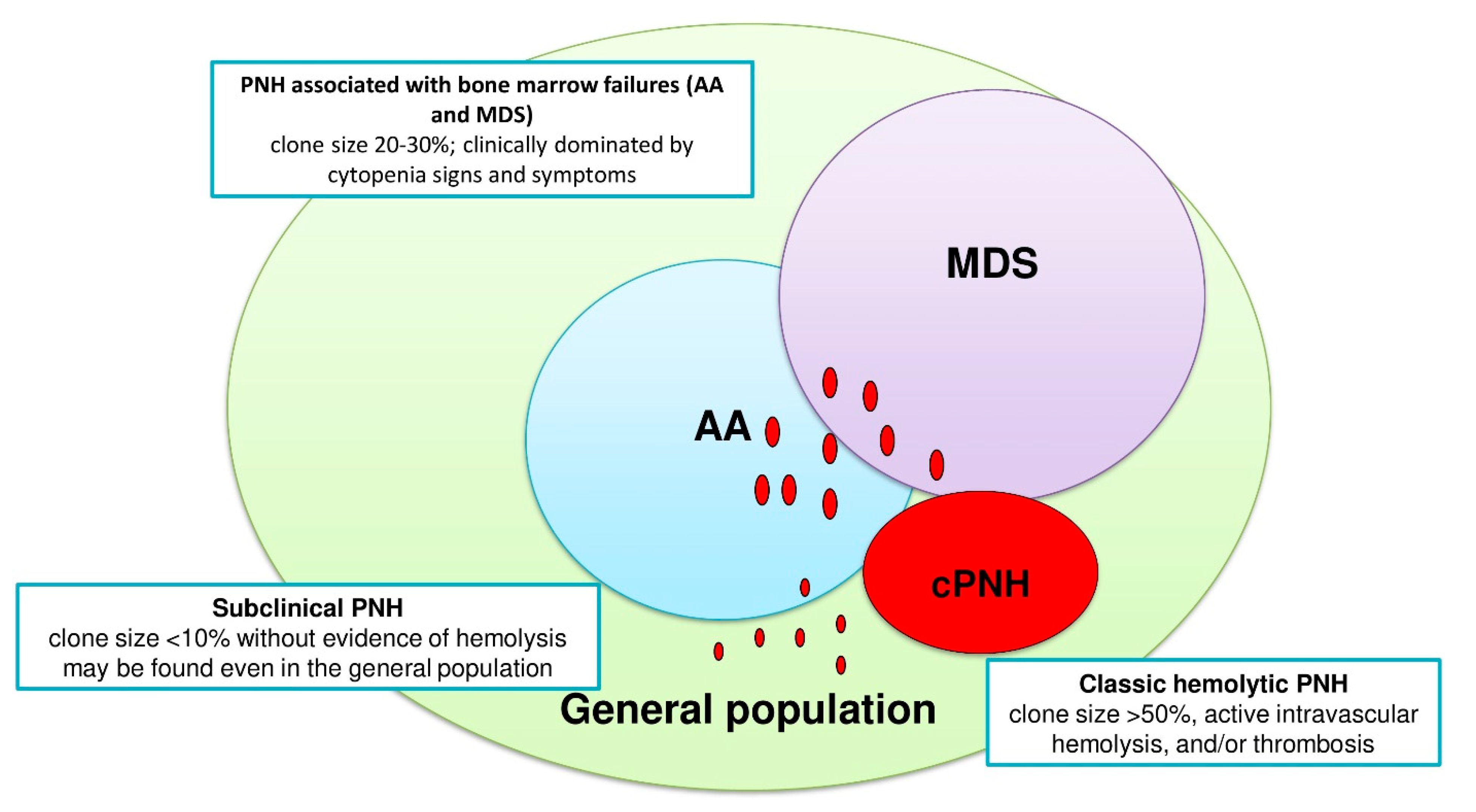
2. Pathogenesis
3. Clinical Presentation and Diagnosis
3.1. Clinical Vignette 1
3.2. Clinical Vignette 2
3.3. Clinical Vignette 3
4. PNH Therapy
4.1. Clinical Vignette 4
4.2. Clinical Vignette 5
4.3. Clinical Vignette 5 (Second Part)
5. Management of Infectious and Thrombotic Risks
5.1. Clinical Vignette 6
5.2. Clinical Vignette 7
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parker, C.J. Update on the diagnosis and management of paroxysmal nocturnal hemoglo binuria. Hematol. Am. Soc. Hematol. Educ. Program. 2016, 1, 208–216. [Google Scholar] [CrossRef]
- Donohue, R.E.; Marcogliese, A.N.; Sasa, G.S.; Elghetany, M.T.; Redkar, A.A.; Bertuch, A.A.; Curry, C.V. Standardized high-sensitivity flow cytometry testing for paroxysmal nocturnal hemoglobinuria in children with acquired bone marrow failure disorders: A single center US study. Cytometry Part B 2018, 94B, 699–704. [Google Scholar] [CrossRef]
- Hillmen, P.; Hall, C.; Marsh, J.C.W.; Elebute, M. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2004, 350, 552–559. [Google Scholar] [CrossRef]
- Caruso, A.; Vollmer, J.; Machacek, M.; Kortvely, E. Modeling the activation of the alternative complement pathway and its effects on hemolysis in health and disease. PLoS Comput. Biol. 2020, 16, e1008139. [Google Scholar] [CrossRef] [PubMed]
- Nicholson-Weller, A.; March, J.P.; Rosenfeld, S.I.; Austen, K.F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc. Natl. Acad. Sci. USA 1983, 80, 5066–5070. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, Y.; Merk, B.; McIntosh, J.; Marsh, J.C.; Schrezenmeier, H.; Rutherford, T.R.; BIOMED II Pathophysiology and Treatment of Aplastic Anaemia Study Group. The spectrum of PIG-A gene mutations in aplastic anemia/paroxysmal nocturnal hemoglobinuria (AA/PNH): A high incidence of multiple mutations and evidence of a mutational hot spot. Blood 2003, 101, 2833–2841. [Google Scholar] [CrossRef]
- Hill, A.; Kelly, R.J.; Hillmen, P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 2013, 121, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Devalet, B.; Mullier, F.; Chatelain, B.; Dogné, J.M.; Chatelain, C. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: A review. Eur. J. Haematol. 2015, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Dunlop, A.; Ireland, R.M.; Kassam, S.; Consonni, D.; Yallop, D.; Mufti, G.J.; Marsh, J.; Zanella, A.; Barcellini, W.; et al. Prognostic and Predictive Impact of Small PNH Clones in a Large Cohort of Patients with Myelodysplastic Syndromes and Aplastic Anemia: A Single-Center Experience. Blood 2018, 132, 3870. [Google Scholar] [CrossRef]
- Fattizzo, B.; Giannotta, J.; Zaninoni, A.; Kulasekararaj, A.; Cro, L.; Barcellini, W. Small Paroxysmal Nocturnal Hemoglobinuria Clones in Autoimmune Hemolytic Anemia: Clinical Implications and Different Cytokine Patterns in Positive and Negative Patients. Front. Immunol. 2020, 11, 1006. [Google Scholar] [CrossRef]
- Parker, C.; Omine, M.; Richards, S.; Nishimura, J.; Bessler, M.; Ware, R.; Hillmen, P.; Luzzatto, L.; Young, N.; Kinoshita, T.; et al. International PNH Interest Group. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005, 106, 3699–3709. [Google Scholar] [CrossRef]
- Raza, A.; Ravandi, F.; Rastogi, A.; Bubis, J.; Lim, S.H.; Weitz, I.; Castro-Malaspina, H.; Galili, N.; Jawde, R.A.; Illingworth, A. A prospective multicenter study of paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure. Cytometry B Clin. Cytom. 2014, 86, 175–182. [Google Scholar] [CrossRef]
- Brodsky, R.A. How I treat paroxysmal nocturnal hemoglobinuria. Blood 2009, 113, 6522–6527. [Google Scholar] [CrossRef]
- Kelly, R.J.; Höchsmann, B.; Szer, J.; Kulasekararaj, A.; de Guibert, S.; Röth, A.; Weitz, I.C.; Armstrong, E.; Risitano, A.M.; Patriquin, C.J.; et al. Eculizumab in Pregnant Patients with Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J Med. 2015, 373, 1032–1039. [Google Scholar] [CrossRef]
- Risitano, A.M.; Marotta, S.; Ricci, P.; Marano, L.; Frieri, C.; Cacace, F.; Sica, M.; Kulasekararaj, A.; Calado, R.T.; Scheinberg, P.; et al. Anti-complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper from the SAAWP of the EBMT. Front. Immunol. 2019, 10, 1157. [Google Scholar] [CrossRef]
- Nishimura, J.; Yamamoto, M.; Hayashi, S.; Ohyashiki, K.; Ando, K.; Brodsky, A.L.; Noji, H.; Kitamura, K.; Eto, T.; Takahashi, T.; et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 2014, 370, 632–639. [Google Scholar] [CrossRef]
- Brodsky, R.A.; de Latour, R.P.; Rottinghaus, S.T.; Röth, A.; Risitano, A.M.; Weitz, I.C.; Hillmen, P.; Maciejewski, J.P.; Szer, J.; Lee, J.W.; et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica 2021, 106, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Notaro, R.; Marando, L.; Serio, B.; Ranaldi, D.; Seneca, E.; Ricci, P.; Alfinito, F.; Camera, A.; Gianfaldoni, G.; et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood 2009, 113, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Höchsmann, B.; Leichtle, R.; von Zabern, I.; Kaiser, S.; Flegel, W.A.; Schrezenmeier, H. Paroxysmal nocturnal haemoglobinuria treatment with eculizumab is associated with a positive direct antiglobulin test. Vox Sang. 2012, 102, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Kulasekararaj, A.G. Second-Generation C5 Inhibitors for Paroxysmal Nocturnal Hemoglobinuria. BioDrugs 2020, 34, 149–158. [Google Scholar] [CrossRef]
- Risitano, A.M.; Marotta, S. Toward complement inhibition 2.0: Next generation anticomplement agents for paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 2018, 93, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Robert, D.; Mahon, F.X.; Richard, E.; Etienne, G.; de Verneuil, H.; Moreau-Gaudry, F. A SIN lentiviral vector containing PIGA cDNA allows long-term phenotypic correction of CD34+-derived cells from patients with paroxysmal nocturnal hemoglobinuria. Mol. Ther. 2003, 7, 304–316. [Google Scholar] [CrossRef]
- Kulasekararaj, A.G.; Hill, A.; Rottinghaus, S.T.; Langemeijer, S.; Wells, R.; Gonzalez-Fernandez, F.A.; Gaya, A.; Lee, J.W.; Gutierrez, E.O.; Piatek, C.I.; et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: The 302 study. Blood 2019, 133, 540–549. [Google Scholar] [CrossRef]
- Schubart, A.; Anderson, K.; Mainolfi, N.; Sellner, H.; Ehara, T.; Adams, C.M.; Mac Sweeney, A.; Liao, S.M.; Crowley, M.; Littlewood-Evans, A.; et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 7926–7931. [Google Scholar] [CrossRef]
- Maibaum, J.; Liao, S.M.; Vulpetti, A.; Ostermann, N.; Randl, S.; Rüdisser, S.; Lorthiois, E.; Erbel, P.; Kinzel, B.; Kolb, F.A.; et al. Small-molecule factor D inhibitors targeting the alternative complement pathway. Nat. Chem. Biol. 2016, 12, 1105–1110. [Google Scholar] [CrossRef]
- Borodovsky, A.; Yucius, K.; Sprague, A.; Butler, J.; Fishman, S.; Nguyen, T.; Vaishnaw, A.K.; Maier, M.; Kallanthottathil, R.; Kuchimanchi, S.; et al. Development of RNAi therapeutics targeting the complement pathway. Blood 2013, 122, 2471. [Google Scholar] [CrossRef]
- Roumenina, L.T. Terminal complement without C5 convertase? Blood 2021, 137, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Mannes, M.; Dopler, A.; Zolk, O.; Lang, S.J.; Halbgebauer, R.; Höchsmann, B.; Skerra, A.; Braun, C.K.; Huber-Lang, M.; Schrezenmeier, H.; et al. Complement inhibition at the level of C3 or C5: Mechanistic reasons for ongoing terminal pathway activity. Blood 2021, 137, 443–455. [Google Scholar] [CrossRef]
- Brodsky, R.A. How I Treat Paroxysmal nocturnal hemoglobinuria. Blood 2021, 112, 6522–6527. [Google Scholar] [CrossRef]
- Langereis, J.D.; van den Broek, B.; Franssen, S.; Joosten, I.; Blijlevens, N.M.A.; de Jonge, M.I.; Langemeijer, S. Eculizumab impairs Neisseria meningitidis serogroup B killing in whole blood despite 4CMenB vaccination of PNH patients. Blood Adv. 2020, 4, 3615–3620. [Google Scholar] [CrossRef]
- Hillmen, P.; Muus, P.; Dührsen, U.; Risitano, A.M.; Schubert, J.; Luzzatto, L.; Schrezenmeier, H.; Szer, J.; Brodsky, R.A.; Hill, A.; et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood 2007, 110, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, F.; Chiarotti, F.; Lombardi, L.; Iori, A.P.; Cafolla, A. Anticoagulant therapy with rivaroxaban in a young patient with paroxysmal nocturnal hemoglobinuria. Clin. Case Rep. 2015, 3, 790–792. [Google Scholar] [CrossRef]
| TEST | RESULTS IN PNH | COMMENTS |
|---|---|---|
| Hb | ↓↓↓ | Anemia from mild to very severe, usually macrocytic normocromic |
| MCV | ↓ to ↑↑ | If reduced, consider coexisting iron deficiency |
| Reticulocyte | ↓ to ↑↑ | If reduced, consider coexisting nutrients/iron deficiencies or associated BMF |
| LDH | ↑↑↑ | Consider possible confounders (liver/tissue damage, folate/B12 deficiency) |
| Haptoglobin | ↓↓↓ | Possibly reduced in case of liver insufficiency or hereditary hypohaptoglobinemia |
| Bilirubin | ↑ | Usually unconjugated; conjugated bilirubin may increase in Budd–Chiari syndrome |
| PLT | = to ↓ | If reduced, consider associated BMF; if increased, consider iron deficiency or rare association with MPN |
| WBC | = to ↓ | If reduced, consider associated BMF |
| Hemosiderinuria | ↑ to ↑↑↑ | Not routinely performed |
| Schistocytes | Absent | If present, consider alternative diagnosis (e.g., microangiopathies and intravascular devices) |
| Direct antiglobulin test | ↓ | If positive, consider AIHA; may be positive for C3d upon ECU treatment |
| Extravascular hemolysis | ↑ to ↑↑ | May be present, especially upon ECU treatment |
| Thrombosis | ↑↑ (atypical sites) | Test for hereditary or acquired thrombophilia |
| Infections | ↑↑ | May be present, especially upon treatment with ECU or due to BMF; vaccines are indicated prior to ECU |
| Flow cYtometry | ↑ to ↑↑↑ | Clone size usually related with PNH subtype, anemia/hemolysis, and thrombotic risk |
| Target of Inhibition | Drug | Company | Mechanism of Action | AD. | Phase of Study | Registered Number |
|---|---|---|---|---|---|---|
| c5 | ABP959 | Amgen (Thousand Oaks, CA, USA) | C5 Ab (Biosimilar) | IV | phase III | NCT03818607 |
| c5 | SB12 | Samsung Bioepis | C5 Ab (Biosimilar) | IV | phase III | NCT04058158 |
| C5 | BCD-148 | Biocad | C5 Ab (Biosimilar) | IV | phase III | NCT04060264 |
| c5 | Elizaria | Genirium | C5 Ab (Biosimilar) | IV | - | NCT04671810 |
| c5 | Ravulizumab, Ultomiris, ALXN1210 | Alexion | C5 Ab (increased halflife) | IV and sc | Approved FDA; EMEA phase III for sc drug | NCT02946463 NCT03056040 |
| c5 | Crovalimab, SKY59, RO7112689 | Roche | C5 Ab (increased halflife) | IV and sc | III | NCT04654468 NCT04432584 NCT04434092 |
| c5 | Tesidolumab, LFG316 | Novartis | C5 Ab | IV | phase II | NCT02534909 |
| c5 | Pozelimab, REGN3918 | Regeneron | C5 Ab | IV and sc | phase II | NCT03946748 |
| c5 | Zilucoplan, RA101495 | Ra Pharma | C5 Small peptide | sc | phase II; oral formulation under development | NCT03225287 |
| c5 | Nomacopan, VA576, Coversin | Akari | C5 Small peptide | sc | phase III | NCT03829449 |
| c5 | Cemdisiran, ALN-CC5 | Alnylam | C5RNAi | sc | phase I/II | NCT02352493 |
| C3 | Pegcetacoplan, APL-2 | Apellis | Pegylated compstatin | sc | phase II/III | NCT04085601 |
| Factor D | Danicopan, ACH-4471 | Achillion/Alexion | Small peptide | oral | phase IIphase III | NCT03472885 NCT03181633 NCT04170023 NCT04469465 |
| Factor D | BCX9930 | Biocryst | Small peptide | oral | phase I | NCT04330534 |
| Factor D | Iptacopan, LNP023 | Novartis | Small peptide | oral | phase IIphase III | NCT03439839 NCT04558918 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattizzo, B.; Serpenti, F.; Giannotta, J.A.; Barcellini, W. Difficult Cases of Paroxysmal Nocturnal Hemoglobinuria: Diagnosis and Therapeutic Novelties. J. Clin. Med. 2021, 10, 948. https://doi.org/10.3390/jcm10050948
Fattizzo B, Serpenti F, Giannotta JA, Barcellini W. Difficult Cases of Paroxysmal Nocturnal Hemoglobinuria: Diagnosis and Therapeutic Novelties. Journal of Clinical Medicine. 2021; 10(5):948. https://doi.org/10.3390/jcm10050948
Chicago/Turabian StyleFattizzo, Bruno, Fabio Serpenti, Juri Alessandro Giannotta, and Wilma Barcellini. 2021. "Difficult Cases of Paroxysmal Nocturnal Hemoglobinuria: Diagnosis and Therapeutic Novelties" Journal of Clinical Medicine 10, no. 5: 948. https://doi.org/10.3390/jcm10050948
APA StyleFattizzo, B., Serpenti, F., Giannotta, J. A., & Barcellini, W. (2021). Difficult Cases of Paroxysmal Nocturnal Hemoglobinuria: Diagnosis and Therapeutic Novelties. Journal of Clinical Medicine, 10(5), 948. https://doi.org/10.3390/jcm10050948







