Abstract
Background: Most data in carotid stenosis treatment arise from randomized control trials (RCTs) and cohort studies. The aim of this meta-analysis was to compare 30-day outcomes in real-world practice from centers providing both modalities. Methods: A data search of the English literature was conducted, using PubMed, EMBASE and CENTRAL databases, until December 2019, using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA) guidelines. Only studies reporting on 30-day outcomes from centers, where both techniques were performed, were eligible for this analysis. Results: In total, 15 articles were included (16,043 patients). Of the patients, 68.1% were asymptomatic. Carotid artery stenting (CAS) did not differ from carotid endarterectomy (CEA) in terms of stroke (odds ratio (OR) 0.98; 0.77–1.25; I2 = 0%), myocardial ischemic events (OR 1.03; 0.72–1.48; I2 = 0%) and all events (OR 1.0; 0.82–1.21; I2 = 0%). Pooled stroke incidence in asymptomatic patients was 1% (95% CI: 0–2%) for CEA and 1% for CAS (95% CI: 0–2%). Pooled stroke rate in symptomatic patients was 3% (95% CI: 1–4%) for CEA and 3% (95% CI: 1–4%) for CAS. The two techniques did not differ in either outcome both in asymptomatic and symptomatic patients. Conclusion: Carotid revascularization, performed in centers providing both CAS and CEA, is safe and effective. Both techniques did not differ in terms of post-procedural neurological and cardiac events, both in asymptomatic and symptomatic patients. These findings reiterate the importance of a tailored therapeutic strategy and that “real-world” outcomes may only be valid from centers providing both treatments.
1. Introduction
As stroke is one of the main disabling and fatal causes in the developed world, carotid atherosclerosis management enforces its role in daily clinical practice [1,2]. The number of strokes will increase from 1.1 million per year in 2000 to more than 1.5 million per year in the next five years [2]. The beneficial role of carotid endarterectomy (CEA) in preventing stroke, mostly to symptomatic and to a lesser extent to asymptomatic patients, has been well established [1]. In the era of less invasive procedures, carotid artery stenting (CAS) has emerged as an alternative therapeutic modality. Current guidelines recommend CEA as the standard treatment in symptomatic and asymptomatic patients, while CAS is suggested as an alternative approach in high-risk patients with adverse medical co-morbidities or anatomical restrictions [1].
The risk of peri-procedural events, including stroke, myocardial infarction (MI) and death, is reasonable to consider in the decision making. CAS offers comparable survival as CEA, while the risk of stroke remains higher in CAS, and of MI in CEA [3]. Although randomized controlled trials (RCTs) of the previous decade are essential for providing level I evidence, and current recommendations have been extracted based on them, the results from “real-world” practice do not always reflect the outcomes reported in the RCTs. In daily clinical practice, the experience is not balanced, as in many cases the reports come from centers providing only one of the treatment modalities; thus, patient selection and reporting biases are inevitable.
In order to assess the 30-day outcomes of both CEA and CAS in “real-world” practice, we conducted the present meta-analysis including only studies from centers providing both treatment modalities.
2. Methods
2.1. Eligibility Criteria
The aim of the present meta-analysis was to investigate real-world evidence in carotid artery disease treatment. Real-world evidence means evidence obtained from real-world data (RWD), which are observational data obtained outside the context of RCTs and generated during routine clinical practice. The objectives, methodology of the systematic review, analysis and inclusion criteria for study enrollment were pre-specified and registered to the PROSPERO (CRD 42020153756) [4]. The present meta-analysis was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA) guidelines [5]. Data extraction was performed by two independent reviewers (P.N., G.K.) using a non-blinded standardized form, and any discrepancies were resolved by consulting a third reviewer (K.S.). No informed consent or institutional review board approval was required, as no patients were used for the conduction of this analysis. Studies considered for inclusion and full-text review fulfilled the following criteria: (1) to report on patients that underwent carotid revascularization for atherosclerotic carotid stenosis, (2) to provide 30-day outcomes as new neurological events, myocardial ischemic events and/or death, (3) to present outcomes of both CAS and CEA techniques, and (4) to report experience from single centers providing both treatment modalities. Randomized controlled trials; data from national registries; studies reporting on less than 50 cases, without follow-up outcomes; technical characteristics different from transcarotid stenting; and studies providing outcomes other than the aforementioned were excluded. The primary selection was based on title and abstract and a secondary scrutiny on the full text.
2.2. Search Strategy
A data search of the English medical literature was conducted using PubMed, EMBASE and CENTRAL databases, until 31 December 2019. The P.I.C.O. (patient; intervention; comparison; outcome) model was used to define the clinical questions and select relevant articles (Supplementary Table S1) [6]. The following search terms including Expanded Medical Subject Headings (MeSH) were used in various combinations: ‘‘carotid stent”, “carotid angioplasty”, “carotid endarterectomy”, “cohort studies”, “comparative study”.
2.3. Data Extraction and Quality Assessment
A standardized data extraction Microsoft Excel file was developed. Data were retrieved from the text or tables. Extracted data included study characteristics such as author, date of publication and study nature. Furthermore, the following clinical information was collected: baseline demographics (age, sex), symptomatic or asymptomatic carotid disease, post-operative neurological events (including stroke and transient ischemic attack), myocardial ischemic events and death, as well as the composite events (neurological event/myocardial ischemia/death) during the early follow-up. Individual studies were assessed for clarity of reporting using the Robins I tool for non-randomized studies (Supplementary Table S2) [7]. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to evaluate the quality of evidence assessment and the summary of findings for each of the included outcomes, to ensure that the effectuated judgments are systematic and transparent [8]. Supplemental Table S3 summarizes the results of the evidence quality assessment using the GRADE approach, while Supplementary Table S4 represents a summary of the evidence. Neurological events included stroke (major and minor) and transient ischemic attack during the 30-day post-operative period. Death included all-cause mortality during the same period.
2.4. Statistical Analysis
The outcomes were summarized as proportion incidence and odds ratio along with their 95% confidence intervals (CIs), through a proportion meta-analysis and a paired meta-analysis, respectively. The inter-study heterogeneity was evaluated using the significance of the Cochran’s Q-metric (pQ) and quantified by the Higgins I² statistics. Significance was set at p < 0.05, and we used continuity correction equal to 0.5 for metrics associated with zero events. The pooled estimate was assessed using the random effects model in the presence of inter-study heterogeneity (I² > 50%), or else with the fixed effects model. Publication bias was eyeballed by trim-and-fill funnel plots. All statistical analyses were executed using the packages “meta” and “metaphor” of the R-statistical environment [9,10].
3. Results
The initial search identified 3252 articles potentially suitable for inclusion. After exclusion of articles whose titles had no relevance to the topic, the full texts of 26 articles were retrieved and assessed for eligibility. The final analysis included 15 articles published between 2003 and 2019, which included a total of 16,043 patients (Figure 1). The study cohorts ranged from 65 to 6940 patients. All articles presented the results of observational, retrospective, comparative studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The studies’ characteristics are shown in Table 1. In nine studies the operators were vascular surgeons exclusively, in three studies vascular surgeons and radiologists, in two studies vascular surgeons and cardiologists, while in one study CAS was performed by cardiologists, radiologists and neurosurgeons. Only four studies reported on long-term follow-up outcome (mean follow-up 24, 65, 120, 39.6 months, respectively).
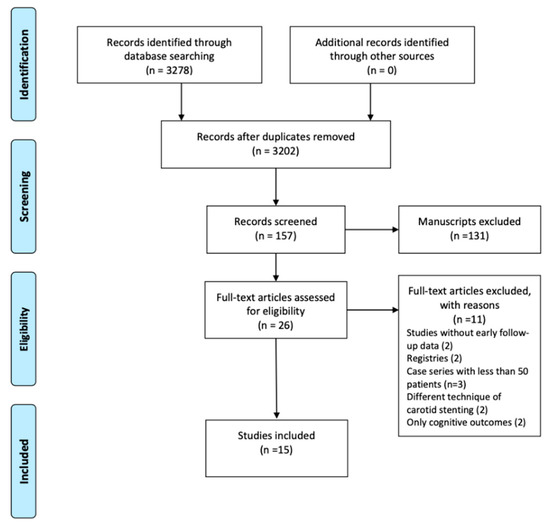
Figure 1.
The present meta-analysis was conducted using the PRISMA guidelines and included 15 articles with 16,043 patients.

Table 1.
The studies’ characteristics.
Patients underwent carotid revascularization using CAS or CEA for symptomatic or asymptomatic carotid disease. Males were 72.7%, and the mean age was estimated at 71.5 years (range 64–86.9 years), with a mean age at 69.7 years in the CAS and 69.5 years in the CEA group. Coronary artery disease was the most common comorbidity among CEA 84% (95% CI 78–88%) and CAS 85% (95% CI 79–90%) patients, respectively. The distribution of comorbidities is presented in Table 2. There were no significant differences between the two groups in either comorbidity factor.

Table 2.
Patients’ comorbidities in each group.
Asymptomatic patients represented 68.1% of the whole cohort. The distribution of asymptomatic carotid disease was higher in both groups (79.8% in CAS and 64.8% in CEA). In CAS, embolic protection was used in 3911 patients (90.1%), while in four studies the use of a filter or other protection device was not recorded [11,19,22,25].
3.1. Synthesis of Results and Outcome
Thirteen studies reported on the occurrence of neurological events during the 30-day postoperative period, resulting in an estimated pooled proportion incidence of 2.4% (95% CI: 1.69–3.4%) and 2.75% (2.01–3.76%) for CEA and CAS, respectively. The two techniques did not differ in terms of post-procedural new neurological events (odds ratio (OR) 0.98; 0.77–1.25; I2 = 0%). The results were robust to major changes (Q 0.69; df 3; p 0.841) after the stratification of the evidence according to the preoperative neurological status (asymptomatic vs. symptomatic). Meanwhile, the relevant funnel plot was not indicative of significant publication bias (Figure 2).
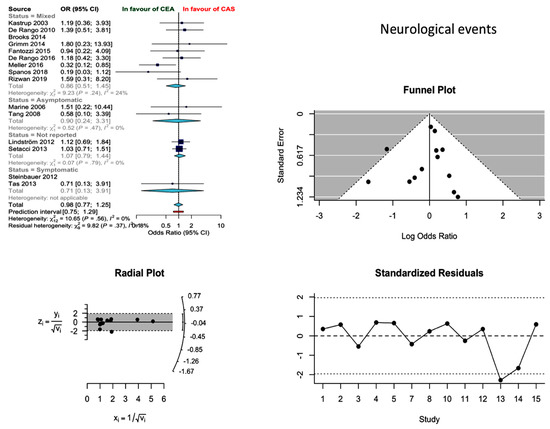
Figure 2.
Differences in neurological event rate and related funnel plot between the CEA and CAS groups [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Based on 13 studies, the pooled proportion incidence of MI after CEA and CAS was 1.66% (1.44–1.93%) and 1.16% (0.08–1.55%), respectively. In the absence of publication bias, the difference between the two approaches was not statistically significant (OR 1.03; 0.72–1.25; I2 = 0%) (Figure 3).
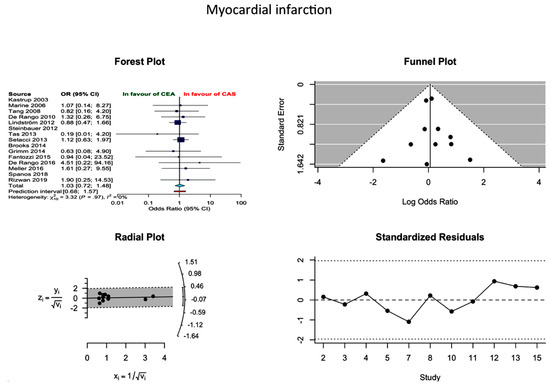
Figure 3.
Differences in MI rate and related funnel plot between the CEA and CAS groups [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
Incidence of all events (neurological events/MI/death) was reported in twelve studies, without establishing a statistical difference between the two modalities (OR 1.0 0.82–1.21, I2 = 0%). More specifically, the proportion of incidences of all events in CEA and CAS were 3.96 (2.95–5.30) and 4.23 (3.11–5.74), respectively (Figure 4). The probability of publication bias was low.
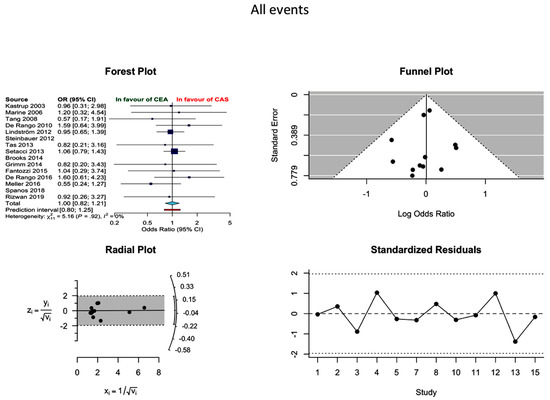
Figure 4.
Differences in all events rate and related funnel plot between the CEA and CAS groups [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
3.2. Asymptomatic Patients
Data on asymptomatic patients were available from nine studies (2850 patients; 1707 CEA, 1143 CAS) [11,12,13,14,15,20,22,23,24]. In asymptomatic patients undergoing CEA the pooled incidence of neurological event, MI and death was 1% (95% CI 0–2%), 0% (95% CI 0–1%) and 0% (95% CI 0–1%), respectively. The pooled incidence of neurological events, MI and death after CAS was 1% (95% CI 0–2%), 1% (95% CI 0–1%) and 1% (95% CI 0–1%), respectively. In asymptomatic patients the two techniques did not differ in terms of neurological events (OR −0.05 (−0.93, 0.83), p = 0.907), MI (OR −0.85 (−2.07, 0.38), p = 0.177) and death (−0.61 (−1.59, 0.36), p = 0.218) (Figure 5).
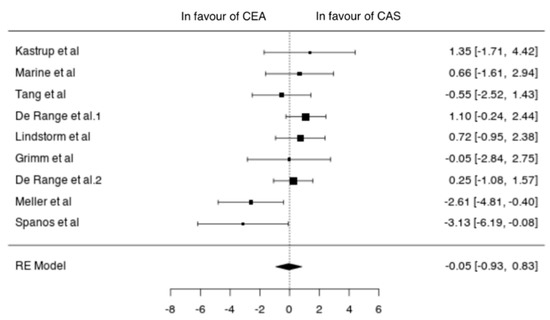
Figure 5.
Differences in neurological events between the CEA and CAS groups in asymptomatic patients [11,12,13,14,15,20,22,23,24].
3.3. Symptomatic Patients
Eight studies reported outcomes in symptomatic patients (1671 patients; 1151 CEA, 520 CAS) [11,14,15,17,20,22,23,24]. In symptomatic patients undergoing CEA the pooled incidence of neurological events, MI and death was 3% (95% CI 1–4%), 0% (95% CI 0–1%) and 1% (95% CI 0–1%), respectively. The pooled incidence of neurological events, MI and death after CAS was 3% (95% CI 1–4%), 1% (95% CI 0–1%) and 1% (95% CI 0–2%), respectively. In symptomatic patients the two techniques showed no differences in terms of neurological events (OR −0.13 (−0.73, 0.48), p = 0.681), MI (OR −0.03 (−1.38, 1.33), p = 0.968) and death (−0.18 (−1.17, 0.81), p = 0.721) (Figure 6).
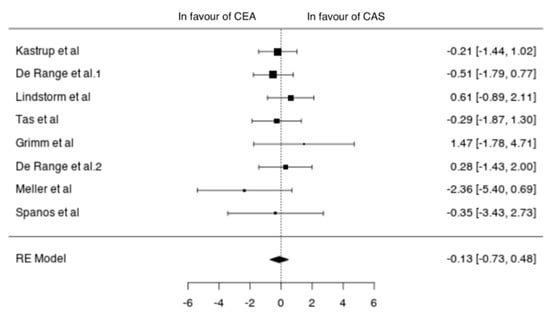
Figure 6.
Differences in neurological events between the CEA and CAS groups in symptomatic patients [11,12,13,14,15,20,22,23,24].
4. Discussion
Several RCTs comparing CEA versus CAS have demonstrated that CEA remains the standard of care in stroke prevention in symptomatic and asymptomatic patients with high-grade carotid stenosis [1,26,27,28]. However, as CAS has evolved through years, recent reports have shown that both techniques may be used with comparable outcomes, after a detailed evaluation of carotid plaque characteristics and patients’ specific risk factors [29]. The present meta-analysis has demonstrated that among centers providing both CEA and CAS, no differences were found in 30-day outcomes. The incidence of 30-day neurologic events presented a tendency in favor of CEA (CEA: 2.1% and CAS: 2.6%). However, CAS-related neurologic events were lower than those reported in RCTs. In CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial), which had the most austere enrollment protocol, the periprocedural stroke rate was 4.1% for the CAS and 2.3% for the CEA group [30]. In the rest of the RCTs, EVA-3S (Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis) was stopped prematurely for safety and futility reasons, as the 30-day incidence of any stroke or death was 3.9% after CEA vs 9.6% after CAS, while SPACE trial (Stent-Protected Angioplasty versus Carotid Endarterectomy) failed to prove the non-inferiority of CAS compared to CEA in terms of periprocedural complication rate [26,27]. Along this line, ICSS (International Carotid Stenting Study) concluded that CEA should remain the treatment of choice due to a 4.0% of disabling stroke or death event rate in CAS versus 3.2% in CEA [28]. Furthermore, all events (stroke/death/MI) were significantly higher in CAS in this trial [28].
RCTs have been subject to criticism because of their strict inclusion and exclusion for patients’ and centers’ eligibility criteria, raising concerns whether their results could be extrapolated to the daily clinical practice [31]. In RCTs, by using inclusion and exclusion (“high-risk”) criteria, a significant portion of eligible carotid patients were eventually excluded. Each technique is associated with specific high-risk features. CEA can be considered as high-risk in patients with severe cardiac comorbidity, while plaque morphology (type IV, V and VI) and unfavorable vessel anatomy can also influence the outcomes of CAS. A retrospective analysis has shown that aortic arch anatomy and a careful pre-operative imaging assessment is very important for the prevention of embolic events during catheter manipulations in the aortic lumen [32]. Therefore, some patients may be more suitable candidates for CEA but not for CAS and vice versa [31]. This strategy of an individualized treatment selection can only be offered in centers with adequate experience in both CEA and CAS, and this is the reason why, in this systematic review, only such experience was included in the analysis.
Detailed evaluation and careful patient selection, operative planning and technical details in the pre- and intra-operative setting seem to be mandatory in improving outcomes for both techniques [31]. However, patients’ risk assessment during pre-operative risk stratification would be incomplete without a thoughtful assessment of interventionists’ experience. Poor outcomes were detected in the initial experience with CAS [33,34], and rationally the learning curve of each interventionist and each center providing CAS has affected its outcomes. At the same time, technological innovations related to stent design and cerebral protection methods, surgeon credentialing and rigorous training through recent years have inevitably affected the efficacy of CAS contributing to the improved reported outcomes [31]. Operators participating in the most recent RCTs had to perform a specific number of procedures to prove their expertise before being allowed to participate in the trial. In most RCTs, the interventionists’ experience needed to enroll patients was limited to less than 25 CAS cases [30,35]. Furthermore, the majority of RCTs conducted during the previous decade were inherent to such influence of the limited experience in CAS. In the current systematic review, the majority of the studies have included many patients treated during the last decade when the endovascular treatment had rapidly evolved and many of the current technological advancements have been already adopted. Unfortunately, the effect of CAS evolution on patients’ outcomes cannot be proved in the current analysis, as most studies have a wide time period spanning an average of seven years.
RCTs have shown significant differences between CEA and CAS in symptomatic patients. European Society for Vascular Surgery (ESVS) guidelines recommend a potential role for both CEA and CAS, but the levels of evidence are slightly lower for CAS than for CEA, mostly because 30-day risks of death/stroke in the RCTs were significantly higher after CAS than after CEA [1,36]. Furthermore, in a systematic review, Paraskevas et al. found that 13/18 administrative dataset registries (72%) reported 30-day death/stroke rates in excess of the recommended 6% risk threshold following CAS in symptomatic patients, while 5/18 (28%) reported stroke rates in excess of 10% [37]. On the contrary, only 1/18 registries reported 30-day death/stroke rates exceeding 6% in patients undergoing CEA [37]. In this analysis, no difference in a 30-day outcome was found in symptomatic patients treated with either modality, while stroke rate for both CEA and CAS was 3%. This finding is creating concerns that the results obtained in RCTs and registries may not be generalizable into routine clinical practice.
For asymptomatic patients, levels of evidence are also slightly less for CAS than for CEA [1]. Thirty-day risks of death/stroke in the largest RCTs, which used experienced CAS interventionists, were only just within the accepted 3% risk threshold [38,39]. In the same systematic review, Paraskevas et al. found that 9/21 administrative dataset registries (43%) reported 30-day death/stroke rates in excess of the recommended 3% risk threshold after CAS in asymptomatic patients, while 7/21 (33%) reported stroke rates in excess of 4% [37]. On the contrary, only 1/21 registries reported 30-day death/stroke rates exceeding 3% in patients undergoing CEA. Data for asymptomatic patients from centers providing both CEA and CAS are different. In the present analysis, stroke rates for both treatment modalities were 1% in asymptomatic patients. Those rates are lower than those reported in RCTs and registries, showing that results can be improved when a tailored therapeutic strategy is followed.
CEA and CAS may be performed not only by vascular surgeons but also by neurosurgeons, cardiologists or radiologists. Each specialty has unique education and capabilities. Meller et al. compared the 30-day CAS results between the three different specialties and found the interventional cardiology group demonstrated the lowest rates of the composite endpoint (2.8%), and the interventional radiology group the highest (12.1%) [23]. Although this analysis may be biased by significant selection criteria, as the interventional cardiologists performed the vast majority of the CAS procedures and had larger experience, we should admit that profound logistics and the requirement of a multidisciplinary approach of related specialties should be fulfilled for a hospital to be able to provide both procedures with equal results.
RCTs’ results do not guide management in all patients with carotid artery stenosis in daily clinical practice. It is not always suitable to generalize the results of cautiously conducted RCTs with specific inclusion and exclusion criteria to the general population and in all centers, regardless of their experience. In the present analysis we included centers with significant experience in both procedures, where each patient’s treatment could be tailored according to specific indications and anatomic characteristics. Certain centers of excellence may accomplish better results than those reported in RCTs, whereas other less experienced institutions may achieve significantly worse outcomes. These innate weaknesses limit the generalizability of the results of RCTs to every individual patient encountered in everyday clinical practice. Additionally, it is rational that outside RCTs, in every center there might be a number of physicians with different levels of expertise; thus, each could perform the procedure that is more familiar to them, while in the RCTs the physicians that were included performed both procedures. That is the idea behind an expertise-based approach to trial design, where health professionals only deliver an intervention in which they have expertise, and this has been proposed as an alternative [40].
The aim of the present analysis is not to directly compare RCTs’ outcomes versus single-center studies’ outcomes but to highlight the discrepancy between the accumulated results. Should we rely solely on RCTs because they are less exposed to bias, or should we seek for more real-world evidence as it might deliver some of the information we really need to guide decision strategy in daily practice? The future may not be about RCTs vs. real-world evidence but about RCTs and real-world evidence—not just for the assessment of safety but also of efficacy. Whereas, fully recognizing the need to improve the feasibility of RCTs, we need to explore methods of synthesizing randomized and nonrandomized data.
Limitations
This systematic review included data across cohort studies to estimate the 30-day outcomes of CAS and CEA in real-world experience. Centers providing both techniques were selected in order to eliminate patient selection and reporting biases. The main limitation of this review is that all studies were of a retrospective nature. The methodological quality of them varied considerably. Furthermore, in technical terms, specific patient selection criteria, type of stent or embolic protection device used, as well as the morphological characteristics of the carotid plaque (synthesis, location and length) were not available in all studies. Observational studies, even from centers with large experience on carotid artery disease treatment, usually lack a systematic, independent neurological examination due to their retrospective nature. Under-reporting of minor neurologic events or chemical MIs may become evident, although those usually do not a have a significant clinical impact on patients’ post-operative course. Although transient neurologic symptoms and subclinical MIs may become underreported, in well-conducted retrospective studies from experienced centers the report of robust endpoints as major stroke, MI with clinical symptoms or biochemical and electrocardiographic markers and deaths remain valid. Furthermore, although this study focusses on 30-day outcomes after CEA/CAS, data on long-term follow up are lacking in the studies included; therefore, the effect of both procedures on late complications could not be assessed.
5. Conclusions
Carotid revascularization performed in centers providing both CAS and CEA is safe and effective. Both techniques did not differ in terms of post-procedural neurological and cardiac events, both in asymptomatic and symptomatic patients. These findings reiterate the importance of a tailored therapeutic strategy and that “real-world” outcomes may only be valid from centers providing both treatments.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/5/935/s1, Table S1: P.I.C.O. (patient; intervention; comparison; outcome) model was used to define the clinical questions and clinically relevant evidence in the literature, Table S2: Grading of the retrieved articles with regard to the quality of evidence, Table S3: Summary of evidence, Table S4: The risk of bias of each study included in the analysis was assessed using the Robins I tool for non-randomized trial.
Author Contributions
P.N.: Methodology, Research of the literature, Data extraction, Writing—Original Draft Preparation, Revision; G.K.: Conceptualization, Writing—Original Draft Preparation, Revision; A.B.: Statistical analysis, Revision; K.S.: Research of the literature, Revision; E.D.: Revision; M.M.: Revision; A.G.: Revision, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naylor, A.R.; Ricco, J.B.; de Borsta, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.K.; Lepidi, S.; et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European society for vascular surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef]
- Truelsen, T.; Piechowski-Jozwiak, B.; Bonita, R.; Mathers, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in Europe: A review of available data. Eur. J. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.-J.; Zhu, S.-H.; Xu, B.; Wang, L. Long-term efficacy and safety of carotid artery stenting versus endarterectomy: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0180804. [Google Scholar] [CrossRef]
- PROSPERO. International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 14 April 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- The University Illinois at Chicago, What Is the PICO Model? Available online: http://researchguides.uic.edu/c.php?g=252338&p=1683349nb (accessed on 15 October 2019).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. Introduction to GRADE Handbook. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html#h.dce0ghnajwsm (accessed on 15 October 2019).
- Schwarzer, G. Meta: An R package for meta-analysis. R News 2007, 7, 40–59. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metaphor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Kastrup, A.; Skalejb, M.; Krapf, H.; Nägele, T.; Dichgans, J.; Schulz, J.B. Early outcome of carotid angioplasty and stenting versus carotid endarterectomy in a single academic center. Cerebrovasc. Dis. 2003, 15, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Marine, L.A.; Rubin, B.G.; Reddy, R.; Sanchez, L.A.; Parodi, J.C.; Sicard, G.A. Treatment of asymptomatic carotid artery disease: Similar early outcomes after carotid stenting for high-risk patients and endarterectomy for standard-risk patients. J. Vasc. Surg. 2006, 43, 953–958. [Google Scholar] [CrossRef][Green Version]
- Tang, G.L.; Matsumura, J.S.; Morasch, M.D.; Pearce, W.H.; Nguyen, A.; Amaranto, D.; Eskandari, M.K. Carotid angioplasty and stenting vs carotid endarterectomy for treatment of asymptomatic disease: Single-center experience. Arch. Surg. 2008, 143, 653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Rango, P.; Parlani, G.; Caso, V.; Verzini, F.; Giordano, G.; Cieri, E.; Cao, P. A comparative analysis of the outcomes of carotid stenting and carotid endarterectomy in women. J. Vasc. Surg. 2010, 51, 337–344. [Google Scholar] [CrossRef][Green Version]
- Lindström, D.; Jonsson, M.; Formgren, J.; Delle, M.; Rosfors, S.; Gillgren, P. Outcome after 7 years of carotid artery stenting and endarterectomy in Sweden—single centre and national results. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 499–503. [Google Scholar] [CrossRef]
- Steinbauer, M.G.; Pfister, K.; Greindl, M.; Schlachetzki, F.; Borisch, I.; Schuirer, G.; Feuerbach, S.; Kasprzak, P.M. Alert for increased long-term follow-up after carotid artery stenting: Results of a prospective, randomized, single-center trial of carotid artery stenting vs carotid endarterectomy. J. Vasc. Surg. 2008, 48, 93–98. [Google Scholar] [CrossRef]
- Tas, M.H.; Simşek, Z.; Colak, A.; Koza, Y.; Demir, P.; Demir, R.; Kaya, U.; Tanboga, I.H.; Gundogdu, F.; Sevimli, S. Comparison of carotid artery stenting and carotid endarterectomy in patients with symptomatic carotid artery stenosis: A single center study. Adv. Ther. 2013, 30, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Setacci, F.; Sirignano, P.; Galzerano, G.; de Donato, G.; Cappelli, A.; Setacci, C. Carotid restenosis after endarterectomy and stenting: A critical issue? Ann. Vasc. Surg. 2013, 27, 888–893. [Google Scholar] [CrossRef]
- Brooks, W.H.; Jones, M.R.; Gisler, P.; McClure, R.R.; Coleman, T.C.; Breathitt, L.; Spear, C. Carotid angioplasty with stenting versus endarterectomy: 10-year randomized trial in a community hospital. JACC Cardiovasc. Interv. 2014, 7, 163–168. [Google Scholar] [CrossRef]
- Grimm, J.C.; Arhuidese, I.; Beaulieu, R.J.; Qazi, U.; Perler, B.A.; Freischlag, J.A.; Malas, M.B. Surgeon’s 30-day outcomes supporting the carotid revascularization endarterectomy versus stenting trial. JAMA Surg. 2014, 149, 1314–1318. [Google Scholar] [CrossRef]
- Fantozzi, C.; Taurino, M.; Rizzo, L.; Stella, N.; Persiani, F. Carotid endarterectomy or stenting in octogenarians in a monocentric experience. Ann. Vasc. Surg. 2016, 33, 132–137. [Google Scholar] [CrossRef] [PubMed]
- De Rango, P.; Simonte, G.; Farchioni, L.; Cieri, E.; Manzone, A.; Parlani, G.; Lenti, M.; Verzini, F. Safety of carotid revascularization in symptomatic patients with less than 70 years. Ann. Vasc. Surg. 2016, 32, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.M.; Al-Damluji, M.S.; Gutiérrez, A.; Stilp, E.; Mena-Hurtado, C. Carotid stenting versus endarterectomy for the treatment of carotid artery stenosis: Contemporary results from a large single center study. Catheter. Cardiovasc. Interv. 2016, 88, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Karathanos, C.; Lachanas, V.A.; Drakou, A.; Stamoulis, K.; Koutsias, S.; Giannoukas, A.D. Real-world experience of extracranial carotid artery interventions for atherosclerotic disease during a 10-year period. Int. Angiol. 2018, 37, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Aridi, H.D.; Dang, T.; Alshwaily, W.; Nejim, B.; Malas, M.B. Long-term outcomes of carotid endarterectomy and carotid artery stenting when performed by a single vascular surgeon. Vasc. Endovasc. Surg. 2019, 53, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Mas, J.-L.; Chatellier, G.; Beyssen, B.; Branchereau, A.; Moulin, T.; Becquemin, J.-P.; Larrue, V.; Lièvre, M.; Leys, D.; Bonneville, J.-F.; et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 2006, 355, 1660–1671. [Google Scholar] [CrossRef]
- SPACE Collaborative Group; Ringleb, P.A.; Allenberg, J.; Brückmann, H.; Eckstein, H.H.; Fraedrich, G.; Hartmann, M.; Hennerici, M.; Jansen, O.; Klein, G.; et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: A randomised non-inferiority trial. Lancet 2006, 368, 1239–1247. [Google Scholar]
- International Carotid Stenting Study Investigators; Ederle, J.; Dobson, J.; Featherstone, R.L.; Bonati, L.H.; van der Worp, H.B.; de Borst, G.J.; Lo, T.H.; Gaines, P.; Dorman, P.J.; et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): An interim analysis of a randomised controlled trial. Lancet 2010, 375, 985–997. [Google Scholar] [PubMed]
- Mannheim, D.; Falah, B.; Karmeli, R. Endarterectomy or stenting in severe asymptomatic carotid stenosis. Isr. Med. Assoc. J. IMAJ 2017, 19, 289–292. [Google Scholar] [PubMed]
- Brott, T.G.; Hobson, R.W., 2nd; Howard, G.; Roubin, G.S.; Clark, W.M.; Brooks, W.; Mackey, A.; Hill, M.D.; Leimgruber, P.P.; Sheffet, A.J.; et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 2010, 363, 11–23. [Google Scholar] [CrossRef]
- Yoshida, K.; Miyamoto, S. Evidence for management of carotid artery stenosis. Neurol. Medico-Chir. 2015, 55, 230–240. [Google Scholar] [CrossRef]
- Moresoli, P.; Habib, B.; Reynier, P.; Secrest, M.H.; Eisenberg, M.J.; Filion, K.B. Carotid stenting versus endarterectomy for asymptomatic carotid artery stenosis: A systematic review and meta-analysis. Stroke 2017, 48, 2150–2157. [Google Scholar] [CrossRef]
- Lin, P.H.; Barshes, N.R.; Annambhotla, S.; Huynh, T.T. Prospective randomized trials of carotid artery stenting versus carotid endarterectomy: An appraisal of the current literature. Vasc. Endovasc. Surg. 2008, 42, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Noiphithak, R.; Liengudom, A. Recent update on carotid endarterectomy versus carotid artery stenting. Cerebrovasc. Dis. 2016, 43, 68–75. [Google Scholar] [CrossRef]
- Safian, R.D. Carotid artery stenting: Optimizing patient selection and technique. Catheter. Cardiovasc. Interv. 2015, 86, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Bolia, A.; Abbott, R.J.; Pye, I.F.; Smith, J.; Lennard, N.; Lloyd, A.J.; London, N.J.; Bell, P.R. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: A stopped trial. J. Vasc. Surg. 1998, 28, 326–334. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Kalmykov, E.; Naylor, A. Stroke/death rates following carotid artery stenting and carotid endarterectomy in contemporary administrative dataset registries: A systematic review. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 3–12. [Google Scholar] [CrossRef]
- Halliday, A.; Harrison, M.; Hayter, E.; Kong, X.; Mansfield, A.; Marro, J.; Pan, H.; Peto, R.; Potter, J.; Rahimi, K.; et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 2010, 376, 1074–1084. [Google Scholar] [CrossRef]
- Naylor, A.R. Endarterectomy versus stenting for stroke prevention. Stroke Vasc. Neurol. 2018, 3, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.A.; Elders, A.; Boachie, C.; Bassinga, T.; Fraser, C.M.; Altman, D.G.; Boutron, I.; Ramsay, C.R.; MacLennan, G.S. A systematic review of the use of an expertise-based randomised controlled trial design. Trials 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).